2.1. Electrode Base Fabrication
A wide number of different electrode designs and configurations are possible via 3D printing, and in this work, we consider three
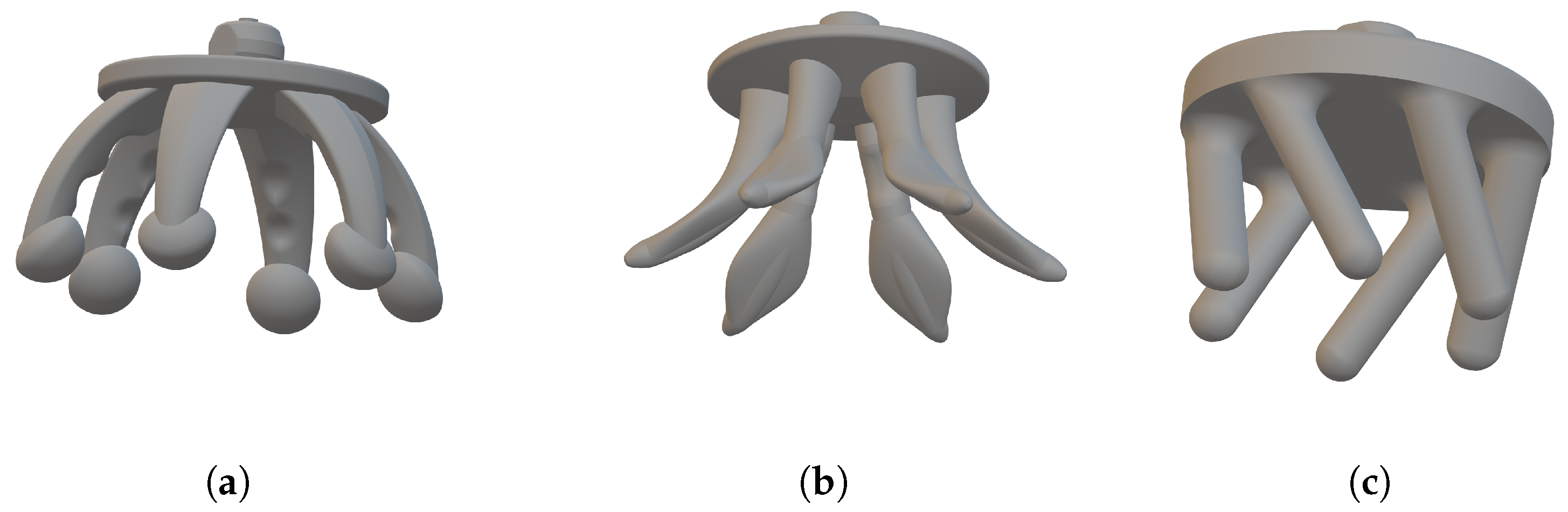
base options as shown in
Figure 3, all similar in starting shape to the Cognionics flexible electrode [
7], g.tec g.SAHARA [
8] and Neuroelectrics Drytrode [
9] with a single ring of
fingers around the outside. These base options are then manufactured with a range of finger numbers to get different levels of performance. All electrodes start with a flat base, which on one side has a 1.5-mm snap connector printed for connecting the electrode to standard electro-physiological recording equipment. The electrodes then differ in the shapes of the fingers that connect to the other side of the base.
Figure 3a shows the
spider electrode type with six fingers. Here, the fingers are convex such that the finger tips spread outwards when they are pushed down, pushing hair out of the way. This is intended to be similar to the shape of the Cognionics electrode [
7], which is available commercially. Our electrode design has a new ball shape at the end of each finger to increase the potential contact area available. The finger also has small parts taken out of it along the length, such that the cross-sectional area is not constant, in order to increase the mechanical flexibility after printing.
Figure 3b shows the
anti-spider electrode type where fingers are concave such that when pushed down, the inner side of the leg makes contact with the scalp. This inner surface has been flattened in our design compared to having a circular cross-section in order to increase the potential contact area available.
Figure 3c shows the
spiny electrode type where fingers are cylindrical, projecting out at an angle of 30 degrees from the base. When this electrode is pressed down, the fingers do not spread, but rather, the electrode collapses in on itself, so the electrodes act as a mechanical buffer.
These three base shapes were printed using a desktop-grade 3D printer, a Lulzbot Mini 3D printer with settings: height = 0.24 mm, temperature = 223, printing speed 12 and travel speed 200. The electrodes were printed with the prongs facing upwards, with an automatically-generated support structure added to the printing/filament profile as a 15% infill support structure. A commercially-available flexible TPU (thermoplastic Polyurethane) printing filament (NinjaFlex Semiflex) was used, and after printing, a lighter was lightly passed near to the electrodes as they were removed from the printer to remove residual stringing.
A picture showing the base printed electrodes is given in
Figure 4. This shows the three electrode types, each printed with 5, 6 and 7 fingers, giving 9 electrode configurations in total to test. For each configuration, the finger shape was the same, the only difference being the angular spacing of the fingers on the base, which decreased uniformly as the number of fingers increased.
2.2. Electrode Coating
The base electrodes shown in
Figure 4 are non-conductive and so are not directly suitable for measuring EEG and first need activating by depositing a conductive layer on top. Previously, we used silver ink for this [
6], as it is readily available and inexpensive in small quantities and has previously been used for EEG electrodes in [
3]. However, silver has poor long-term stability as a skin–electrode interface [
3], with silver/silver-chloride giving less noise and better stability [
3]. For improved sensing performance, we used silver/silver-chloride ink available from Creative Materials [
10], matching the material used in conventional EEG electrodes. This silver/silver-chloride ink is medical grade and specifically designed for the collection of electro-physiological bio-signals. We coated the whole of the electrode, unlike approaches such as the Cognionics electrode [
7] which only has the tip coated, as our desktop-grade, flexible, 3D printer element was not conductive in its own right. This comes at the cost of needing much more Ag/AgCl coating than approaches that only cover the electrode tip.
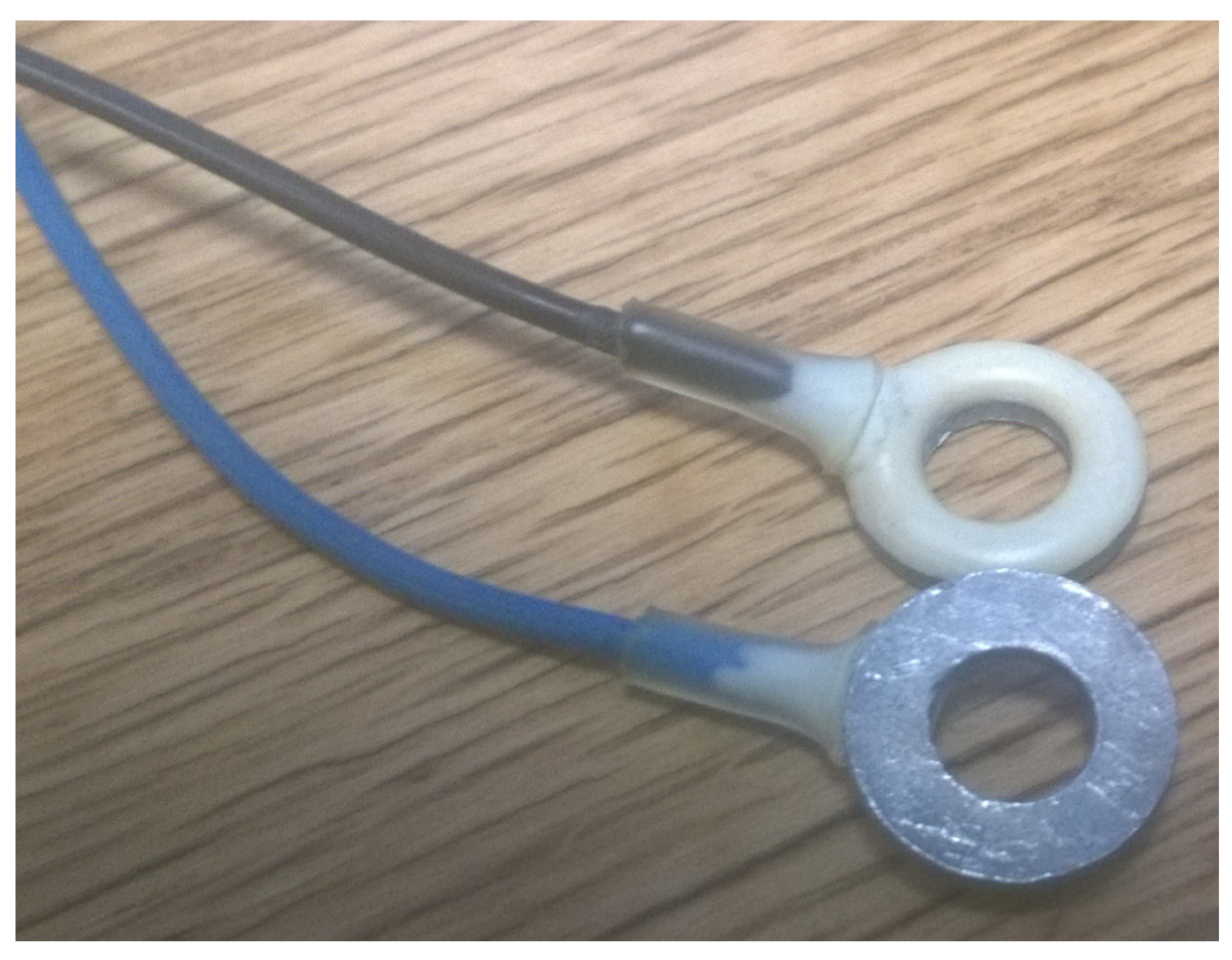
Figure 5 shows two sets of electrodes that have been coated in two different inks: Ag/AgCl (mixture 113-09 from Creative Materials) and Silicone Ag/AgCl (mixture 126-49 from Creative Materials for highly-flexible substrates). These were applied in a 50/50 mix of paint and thinner, using dip coating to ensure that the electrode was fully covered. The electrodes were then placed on the curing bed seen in
Figure 5 for 12 h after the ink application. Fifteen minutes of curing was then performed. For silicone Ag/AgCl, the curing was done at 160 degrees Celsius, keeping the temperature below the 168 degrees Celsius melting point of the NinjaFlex Semiflex filament, and the Ag/AgCl curing was done at 100 degrees Celsius. The silicone Ag/AgCl was found to have a poor adhesion to the 3D-printed filament and would require many hours to dry fully even after the curing process, leaving a poor quality surface on the finished electrode. Based on this, results in
Section 3 only consider the use of the Ag/AgCl ink.
2.3. Electrode Summary and Test Methods
The final electrodes used for testing are summarised in
Table 1. This includes estimates of the total contact surface area available from each design when they are fully pressed down, which affects the performance of the electrode [
11]. For comparison to other electrodes, we also include measured results from a commercially-available passive disc Ag/AgCl electrode from EasyCap (Herrsching, Germany) and the
Foretrode dry EEG electrode for use on the forehead from Neuroelectrics (Barcelona, Spain).
In order to measure the electrical and mechanical properties of EEG electrodes in a controlled way, the work in [
3] introduced the use of a conductive agar as a phantom head model, which replicates the ionic conductors present in the head and scalp. This was extended in [
12] to use ballistic-grade gelatine, and we made use of this approach with the tests in
Section 3, using a phantom head model previously reported in [
6,
11,
13]. A 30% gelatine to 70% water mixture was used, and unlike [
11,
13], where the gelatine was set in the shape of a physical head, in this study, the gelatine was set as 6 × 6 cm cuboids, allowing them to be placed into a mechanical test structure; see
Figure 6. A conventional silver/silver-chloride electrode was set into the gelatine as shown in
Figure 6b to act as a reference electrode, with the test 3D-printed electrode placed on the surface of the gelatine cuboid. This could then be placed in a mechanical test set, with one configuration shown in
Figure 6c, where weights could be added to change the pressure/loading with which the test electrode was pressed against the gelatine piece. The mass used to load the test electrode was varied between 150 g and 400 g, with the resulting pressure exerted on the electrode–gelatine (electrode–skin) interface, which will depend on the contact area of the electrode, given in
Table 1. This range of masses, and resulting pressures, was selected to match the range of comfortable pressures for EEG electrodes in on-person tests reported in [
14] with our estimated contact areas in
Table 1.
Contact impedance of the different electrodes was measured using an Agilent 4284A Impedance Analyzer, between 20 and 1000 Hz, with the 3D-printed electrode as one terminal and the electrode inside the gelatine model as the other terminal. A 135-g mass was used for all tests. These contact impedance magnitude and phase results were compared against the electrical model of a wet silver/silver-chloride electrode from [
15] and shown in
Figure 7. This is made up of a series resistor (
) with a value of 120 Ω, a parallel resistor (
) with a value between 10 kΩ and 2 MΩ and a parallel capacitor (
) with a value between 10 nF and 40 nF [
15].
If the base electrode material is flexible to increase comfort, it is essential that the conductivity of the electrode material remain approximately constant under different amounts of compression/tension [
16]. Without this, slightly different signals will be collected depending on how the EEG is put on; if the cap slightly tighter or slightly looser, changing the amount of compression present. To study this, three different tests of mechanical performance are reported here. Firstly, pictures are given of the physical deformation of the electrodes when pressed against a fixed surface to show how the legs spread. Secondly, the DC bulk impedance (resistance) of the electrodes is shown as they are loaded at 50 g, then 350 g, and then 50 g again to show any hysteresis effects. Finally, a test of the contact impedance magnitude and phase at 35 Hz is performed, matching the frequency used by the SIGGI II EEG impedance meter (Easycap, Germany) for on-person contact impedance measurements, as the loading mass is swept from 0–400 g. For brevity here, this last result is reported only for the spider electrode with seven fingers. In all cases where the mass was varied, the electrode was allowed to settle for five minutes before a reading was taken to remove any transient effects.
The contact noise of each electrode was measured by performing a two-electrode EEG measurement with a camNtech actiwave EEG recorder (camNtech, Cambridge, UK), with the 3D-printed electrode and silver/silver-chloride electrode inside the gelatine acting as the two contact points. This records the residual electrical noise when no EEG signal is present. The recorded signal is thus only the electrode contact noise and the instrumentation noise that will be common to all tests. A reference recording using a conventional EEG electrode was included to quantify this common noise. In all cases, 10-bit, 1024-Hz sampling was used, with three-minute recordings band-limited to 100 Hz. The average RMS of the recorded signals in the 0.3–100 Hz range, with a 150-g loading mass, and popcorn noise greater than 15 μV removed, is then reported. As short-term recordings were used, we did not extract the drift rate of the electrodes from this noise test and did not consider how the drift rate varies for the different types of electrodes under different amounts of pressure. This should be considered as a limitation of the current work.
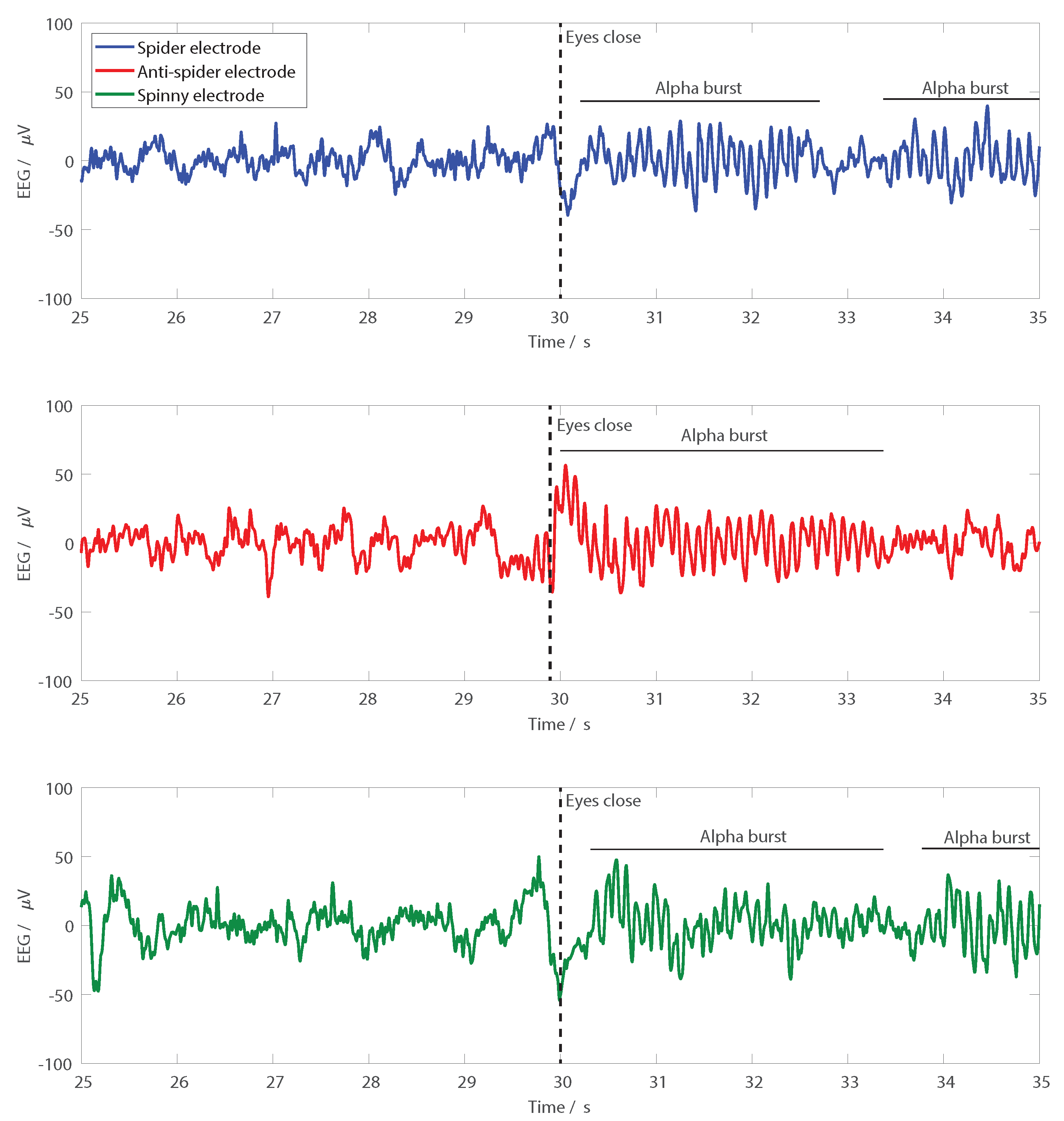
Finally, to demonstrate EEG recordings, a functional test where the electrodes were used on a person is presented. Pairs of electrodes were set up in turn, with one seven-finger electrode over FCz and one six-finger electrode of the same type over Oz, with the electrodes held down using a standard EEG cap. A two-electrode EEG recording using the camNtech actiwave EEG recorder (camNtech, Cambridge, UK) was then performed. All signals were sampled at 1024 Hz, 10-bit resolution and band-limited from 1–30 Hz for presentation. To give recognisable signals in the time domain traces, the participant sat stationary and was asked to shut his/her eyes after 30 s, allowing spontaneous alpha activity to be observed at the back of the head. All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. The research was approved by the University of Manchester research ethics committee, Number 2018-4015-5913.