A Method for Estimating Time-Dependent Corrosion Depth of Carbon and Weathering Steel Using an Atmospheric Corrosion Monitor Sensor
Abstract
:1. Introduction
2. Prediction Method for Mean Corrosion Depth Based on Corrosion Current
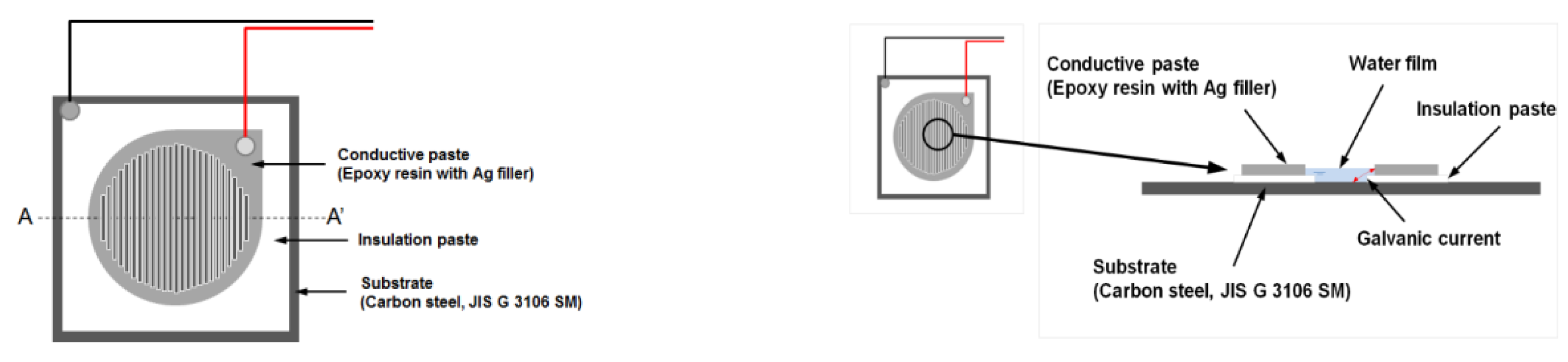
2.1. Measurement of Corrosion Current Using an ACM Sensor
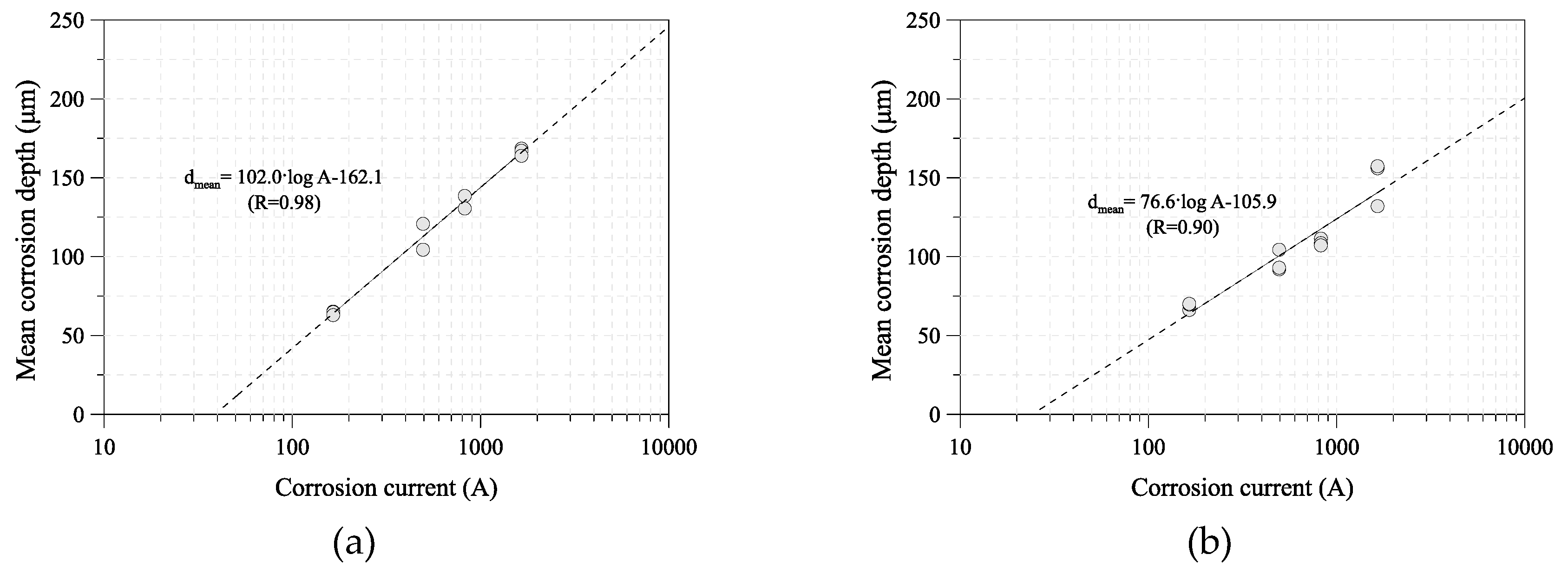
2.2. Prediction Method of Corrosion Damage
3. Accelerated Corrosion Test and Results
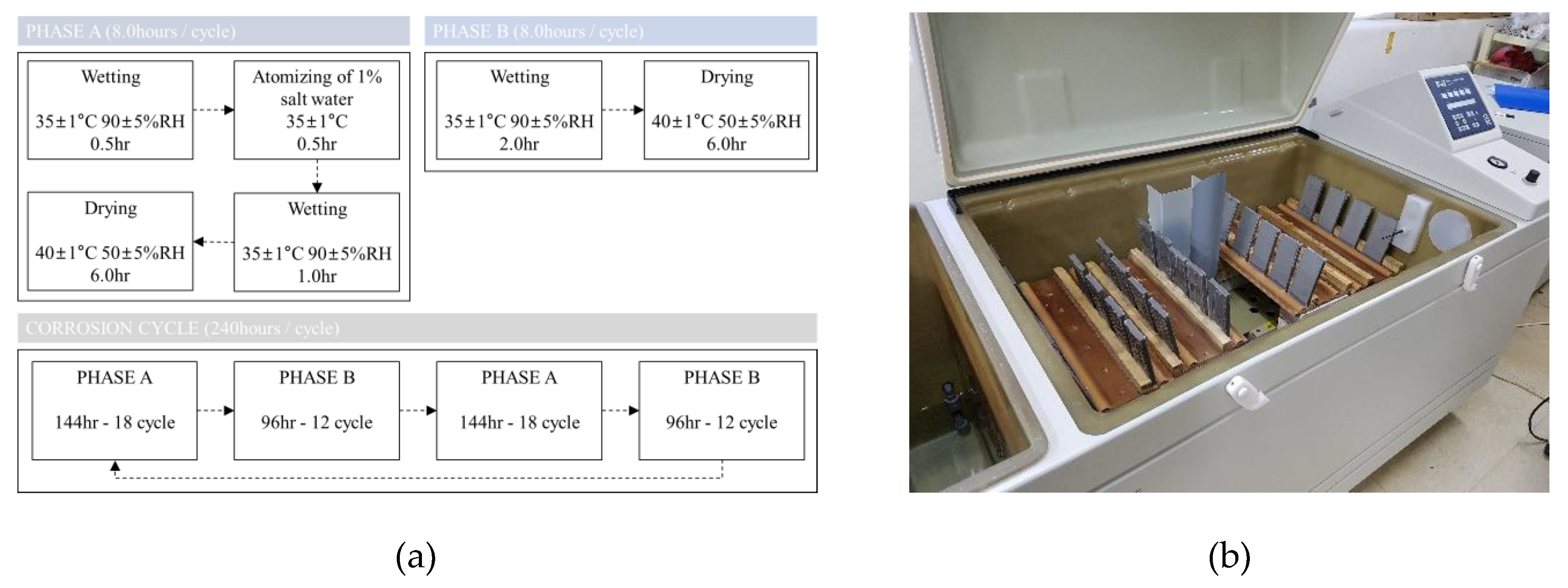
3.1. Acceleration Corrosion Test Condition and Results
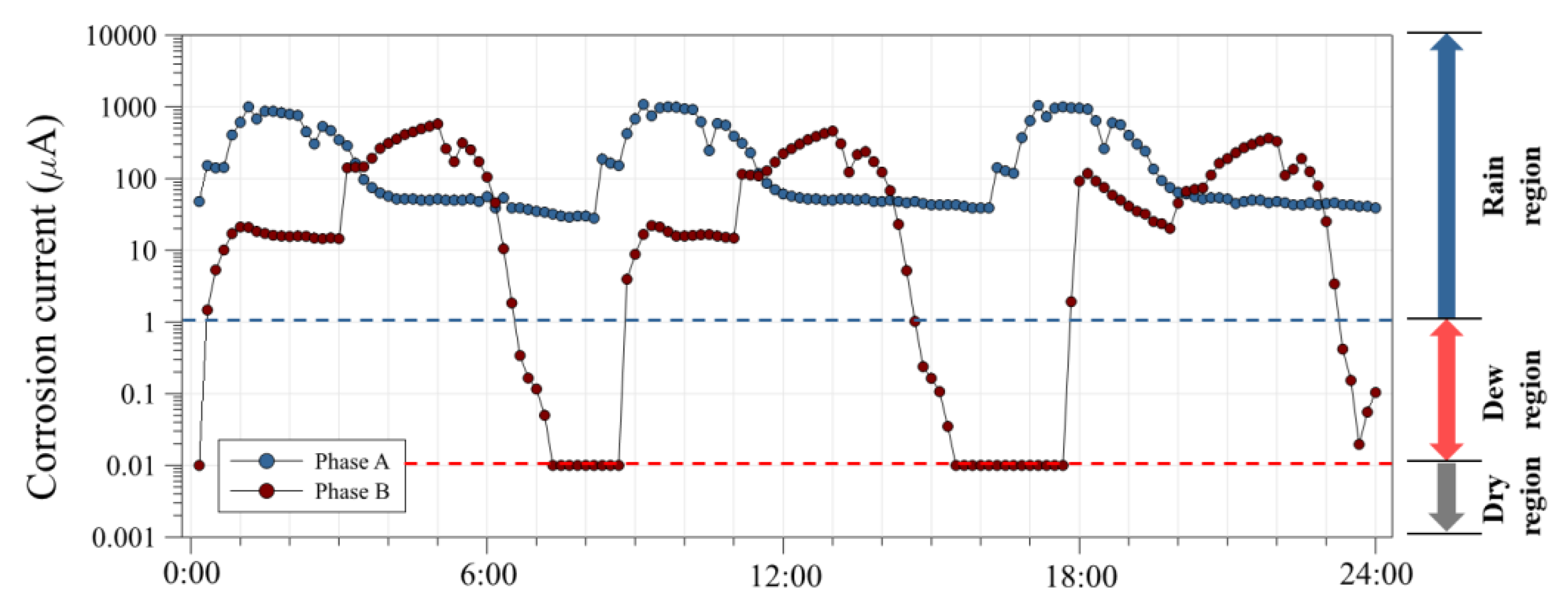
3.2. Corrosion Current Using an ACM Sensor
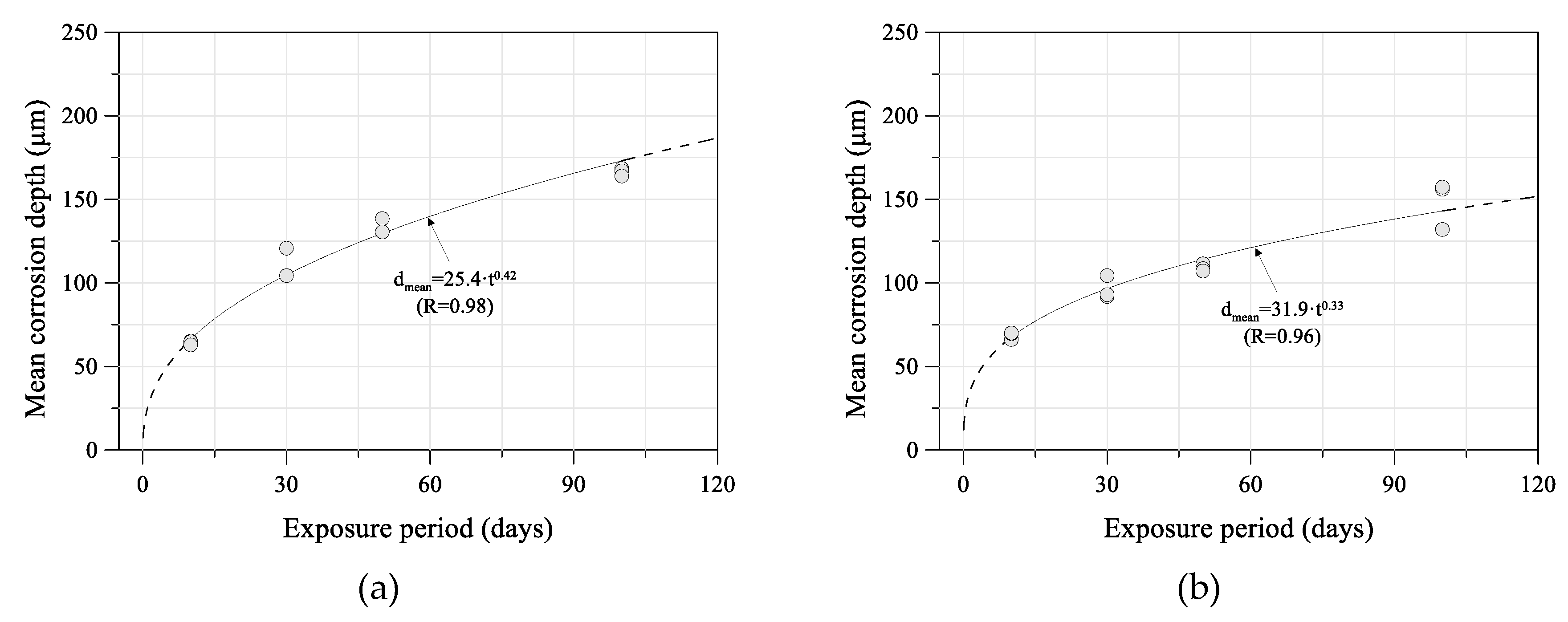
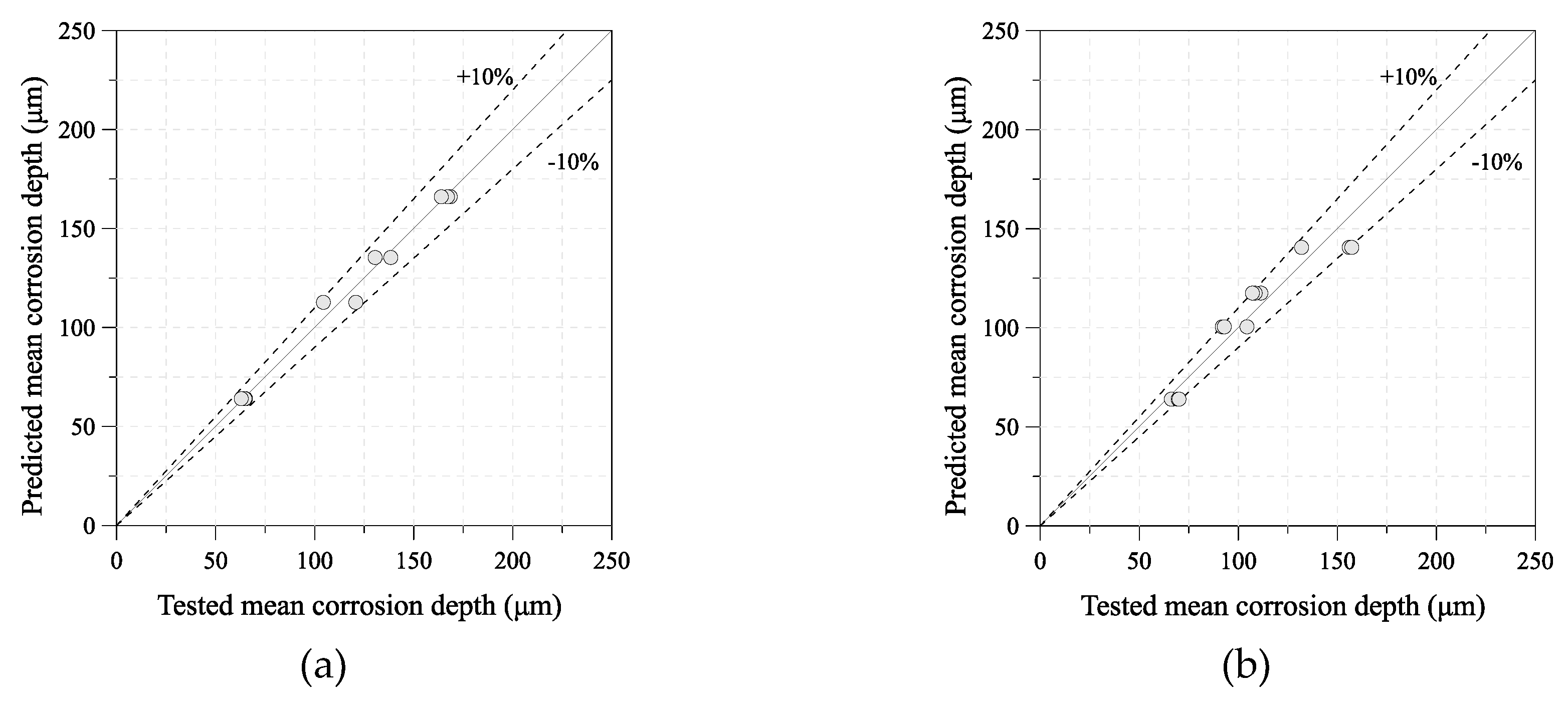
4. Estimated Time-Dependent Mean Corrosion Depth
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Natori, T.; Nishikawa, K.; Murakoshi, J.; Ohno, T. Study on characteristics of corrosion damages in steel bridge members. Doboku Gakkai Ronbunshu 2001, 668, 299–311. [Google Scholar] [CrossRef]
- Sakiya, K.; Sugiyama, Y.; Yoshiyuki, T. Crack penetration of orthotropic steel bridge deck by corrosion in Nishinomiya Osaka line. In Proceedings of the 42nd Hanshin Expressway Technical Research Conference, Osaka, Japan; 2007; pp. 233–240. [Google Scholar]
- Katayama, H.; Tay, Y.C.; Viloria, A.S.; Nishikata, A.; Tsuru, T. Corrosion monitoring of Zn and Zn–Al coated steels under wet-dry cyclic conditions using AC impedance method. Mater. Trans. 1997, 38, 1089–1094. [Google Scholar] [CrossRef]
- Nishikata, A.; Suzuki, F.; Tsuru, T. Corrosion monitoring of nickel-containing steels in marine atmospheric environment. Corros. Sci. 2005, 47, 2578–2588. [Google Scholar] [CrossRef]
- Wall, F.D.; Martinez, M.A.; Missert, N.A.; Copeland, R.G.; Kilgo, A.C. Characterizing corrosion behavior under atmospheric conditions using electrochemical techniques. Corros. Sci. 2005, 47, 17–32. [Google Scholar] [CrossRef]
- Nishikata, A.; Zhu, Q.; Tada, E. Long-term monitoring of atmospheric corrosion at weathering steel bridges by an electrochemical impedance method. Corros. Sci. 2014, 87, 80–88. [Google Scholar] [CrossRef]
- Motoda, S.; Suzuki, Y.; Shinohara, T.; Kojima, Y.; Tsujikawa, S.; Oshikawa, W.; Itomura, S.; Fukushima, T.; Izumo, S. ACM (Atmospheric Corrosion Monitor) type corrosion sensor to evaluate corrosivity of marine atmosphere. Corros. Eng. (Translation of Zairyo-to-Kankyo) 1994, 43, 583–594. [Google Scholar]
- Motoda, S.; Suzuki, Y.; Shinohara, T.; Tsujikawa, S.; Oshikawa, W.; Itomura, S.; Fukushima, T.; Izumo, S. Corrosive factors of a marine atmosphere analyzed by ACM sensor for 1 year. Corros. Eng. (Translation of Zairyo-to-Kankyo) 1995, 44, 253–265. [Google Scholar]
- Cheng, Y.F.; Luo, J.L.; Zheng, L.Q.; Du, Y.L.; Cao, C.N. Monitoring of galvanic current of dual electrode ACM covered with thin electrolyte film. Bull. Electrochem. 1997, 13, 280–283. [Google Scholar]
- Saitou, M.; Oshikawa, W.; Itomura, S.; Tsuzikawa, S. Fractal behavior of galvanic current of dual electrode type of atmospheric corrosion monitor. J. Chem. Phys. 1999, 110, 12122–12124. [Google Scholar] [CrossRef]
- Shinohara, T. Evaluation and monitoring methods for atmospheric corrosion. Corros. Eng. (Translation of Zairyo-to-Kankyo) 2015, 64, 26–33. [Google Scholar] [CrossRef]
- He, Y.; Tian, G.; Zhang, H.; Alamin, M.; Simm, A.; Jackso, P. Steel corrosion characterization using pulsed eddy current systems. IEEE Sens. J. 2012, 12, 2113–2120. [Google Scholar] [CrossRef]
- He, Y.; Tian, G.Y.; Pan, M.; Chen, D.; Zhang, H. An Investigation into eddy current pulsed thermography for detection of corrosion blister. Corros. Sci. 2014, 87, 1–6. [Google Scholar] [CrossRef]
- Kainuma, S.; Sugitani, K.; Itoh, Y.; Kim, I.T. Evaluation method for time-dependent corrosion behavior of carbon steel plate using atmospheric corrosion monitoring sensor. Key Eng. Mater. 2010, 417–418, 417–420. [Google Scholar] [CrossRef]
- Kainuma, S.; Yamamoto, Y.; Itoh, Y.; Oshikawa, W. Prediction method for mean corrosion depth of uncoated carbon steel plate subjected to rainfall effect using Fe/Ag galvanic couple ACM-Type corrosion sensor. Corros. Eng. (Translation of Zairyo-to-Kankyo) 2011, 60, 498–503. [Google Scholar]
- Kainuma, S.; Yamamoto, Y.; Hayashi, H.; Itoh, Y.; Oshikawa, W. Practical method for estimating time-dependent corrosion depth of uncoated carbon steel plate under atmospheric environment using Fe/Ag galvanic couple ACM-type corrosion sensor. Corros. Eng. (Translation of Zairyo-to-Kankyo) 2013, 63, 1–8. [Google Scholar]
- Kainuma, S.; Yamamoto, Y.; Itoh, Y.; Hayashi, H.; Oshikawa, W. Evaluation method for corrosion depth of uncoated carbon steel and its time-dependence using thickness of corrosion product layer. Corros. Eng. (Translation of Zairyo-to-Kankyo) 2012, 61, 344–358. [Google Scholar]
- Kainuma, S.; Yamamoto, Y.; Hayashi, H.; Itoh, Y. Practical method for estimating corrosion depth of uncoated carbon steel using thickness of the corrosion-product layer. Key Eng. Mater. 2014, 577–578, 201–204. [Google Scholar] [CrossRef]
- Shinohara, T.; Tahara, A.; Hosoya, Y. Datasheets of atmospheric corrosion behaviors of low alloyed steels with corrosivities at exposure test sites. In Proceedings of the 3rd International Conference on Advanced Structural Steels, Gyeongju, Korea, 22–24 August 2006. [Google Scholar]
- Lee, Y.B.; Jeong, Y.S.; Seong, T.R.; Ahn, J.H. Estimation of Mean Corrosion Depth of Structural Steel Using Corrosion Product. J. Korean Soc. Steel Constr. 2018, 30, 153–161. (In Korean) [Google Scholar] [CrossRef]
- Japan Weathering Test Center. The Method of Accelerated Cyclic Corrosion Test—1% Salt Spray; JWTCS: Tokyo, Japan, 2009; Volume 1001, Available online: http://www.jwtc.or.jp/kikaku/JWTCS1001-2009.pdf (accessed on 1 February 2002).
- Korean Standards. Rolled Steels for Bridge Structures; KS D 3868; Korea Industrial Standards Commission: Seoul, Korea, 2016. [Google Scholar]
- Public Work Research Institute-Ministry of Construction; The Kozai Club (Iron and Steel Mill Products Association). Report on Application of Carbon Steel to Highway Bridges (XX); Japan Association of Steel Bridge Construction: Tokyo, Japan, 1993. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.-H.; Jeong, Y.-S.; Kim, I.-T.; Jeon, S.-H.; Park, C.-H. A Method for Estimating Time-Dependent Corrosion Depth of Carbon and Weathering Steel Using an Atmospheric Corrosion Monitor Sensor. Sensors 2019, 19, 1416. https://doi.org/10.3390/s19061416
Ahn J-H, Jeong Y-S, Kim I-T, Jeon S-H, Park C-H. A Method for Estimating Time-Dependent Corrosion Depth of Carbon and Weathering Steel Using an Atmospheric Corrosion Monitor Sensor. Sensors. 2019; 19(6):1416. https://doi.org/10.3390/s19061416
Chicago/Turabian StyleAhn, Jin-Hee, Young-Soo Jeong, In-Tae Kim, Seok-Hyeon Jeon, and Chan-Hee Park. 2019. "A Method for Estimating Time-Dependent Corrosion Depth of Carbon and Weathering Steel Using an Atmospheric Corrosion Monitor Sensor" Sensors 19, no. 6: 1416. https://doi.org/10.3390/s19061416
APA StyleAhn, J.-H., Jeong, Y.-S., Kim, I.-T., Jeon, S.-H., & Park, C.-H. (2019). A Method for Estimating Time-Dependent Corrosion Depth of Carbon and Weathering Steel Using an Atmospheric Corrosion Monitor Sensor. Sensors, 19(6), 1416. https://doi.org/10.3390/s19061416




