Hydrogel-Based Plasmonic Sensor Substrate for the Detection of Ethanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the Sensor Substrate
2.3. Hydrogel Synthesis and Immobilization on the Sensor Substrate
2.4. Studies of the Free Swelling of the Hydrogel
2.5. Plasmonic Transducer and Sensor Setup
3. Results and Discussion
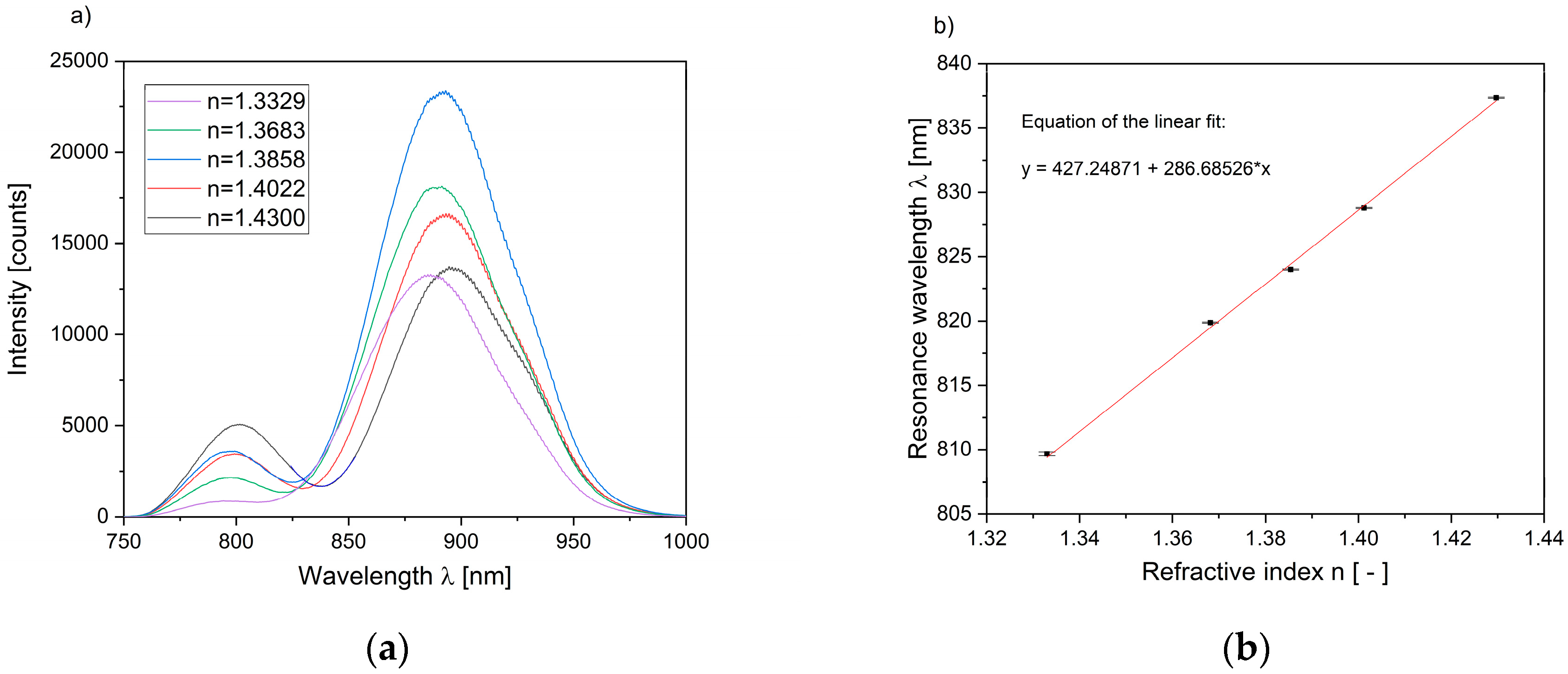
3.1. Characterization of the Sensor Substrate
3.2. Optical Interrogation of Hydrogel Swelling States Induced by Ethanol
3.2.1. Reversible Free Swelling of the Hydrogel
3.2.2. Optical Detection of Different Hydrogel Swelling States
3.2.3. Swelling Kinetics and Response Time
3.2.4. Long-Term Stability of the Sensor Substrate
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonzalez-Rodrıguez, J.; Perez-Juan, P.; Luque de Castro, M.D. Determination of ethanol in beverages by flow injection, pervaporation and density measurements. Talanta 2003, 59, 691–696. [Google Scholar] [CrossRef]
- Cavinto, A.G.; Mayes, D.M.; Ge, Z.; Callis, J.B. Noninvasive Method for Monitoring Ethanol in Fermentation Processes Using Fiber-Optic Near-Infrared Spectroscopy. Anal. Chem. 1990, 62, 1977–1982. [Google Scholar] [CrossRef]
- Michel, K.; Bureau, B.; Boussard-Plédel, C.; Jouan, T.; Adam, J.L.; Staubmann, K.; Baumann, T. Monitoring of pollutant in waste water by infrared spectroscopy using chalcogenide glass optical fibers. Sens. Actuators 2004, 101, 252–259. [Google Scholar] [CrossRef]
- Osorio, D.; Pérez-Correa, J.R.; Agosin, E.; Cabrera, M. Soft-sensor for on-line estimation of ethanol concentrations in wine stills. J. Food Eng. 2008, 87, 571–577. [Google Scholar] [CrossRef]
- Guenther, M.; Gerlach, G.; Wallmersperger, T.; Avula, M.N.; Cho, S.H.; Xie, X.; Devener, B.V.; Solzbacher, F.; Tathireddy, P.; Magda, J.J.; et al. Smart Hydrogel-based biochemical microsensor array for medical diagnostics. Adv. Sci. Tech. 2013, 85, 47–52. [Google Scholar] [CrossRef]
- Guenther, M.; Wallmersperger, T.; Gerlach, G. Piezoresistive chemical sensors based on functionalized hydrogels. Chemosensors 2014, 2, 145–170. [Google Scholar] [CrossRef]
- Gerlach, G.; Guenther, M.; Suchaneck, G.; Sorber, J.; Arndt, K.-F.; Richter, A. Application of sensitive hydrogels in chemical and pH sensors. Macromol. Symp. 2004, 210, 403–410. [Google Scholar] [CrossRef]
- Erfkamp, J.; Guenther, M.; Gerlach, G. Hydrogel-based piezoresistive sensor for the detection of ethanol. J. Sens. Sens. Syst. 2018, 7, 219–226. [Google Scholar] [CrossRef]
- Wang, J.; Ward, M.D.; Ebersole, R.C.; Foss, R.P. Piezoelectric pH Sensors: AT-cut quartz resonators with amphoteric polymer films. Anal. Chem. 1993, 65, 2553–2562. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.J. Kinetics of swelling of gels. J. Chem. Phys. 1979, 70, 1214. [Google Scholar] [CrossRef]
- Yang, S.-C.; Hou, J.-L.; Finn, A.; Kumar, A.; Ge, Y.; Fischer, W.-J. Synthesis of multifunctional plasmonic nanopillar array using soft thermal nanoimprint lithography for highly sensitive refractive index sensing. Nanoscale 2015, 7, 5760–5766. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.L.; Chang, C.-C.; You, M.-L.; Pan, M.-Y.; Wei, P.-K. Enhancing the surface sensitivity of metallic nanostructures using oblique-angle-induced fano resonances. Sci. Rep. 2016, 6, 33126. [Google Scholar] [CrossRef] [PubMed]
- Piliarik, M.; Šípová, H.; Kvasnička, P.; Galler, N.; Krenn, J.R.; Homola, J. High-resolution biosensor based on localized surface plasmons. Opt. Express 2012, 20, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Zalyubovskiy, S.J.; Bogdanova, M.; Deinega, A.; Lozovik, Y.; Pris, A.D.; An, K.H.; Hall, W.P.; Potyrailo, R.A. Theoretical limit of localized surface plasmon resonance sensitivity to local refractive index change and its comparison to conventional surface plasmon resonance sensor. J. Opt. Soc. Am. 2012, 29, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Arndt, K.-F.; Kuckling, D.; Richter, A. Application of sensitive hydrogels in flow control. Polym. Adv. Technol. 2000, 11, 496–505. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Satoh, M. Swelling behavior of poly(acrylic acid) gels in aqueous ethanol—effects of counterion species and ionic strength. Macromol. Rapid Commun. 2000, 21, 174–177. [Google Scholar] [CrossRef]
- Bai, W.; Gariano, N.A.; Spivak, D.A. Macromolecular amplification of binding response in superaptamer hydrogels. J. Am. Chem. Soc. 2013, 135, 6977–6984. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, A. Nanoplasmonic Sensors; Springer: New York, NY, USA, 2012. [Google Scholar]
- Svedendahl, M.; Chen, S.; Dmitriev, A.; Kall, M. Refractometric sensing using propagating versus localized surface plasmons: A direct comparison. Nano Lett. 2009, 9, 4428–4433. [Google Scholar] [CrossRef] [PubMed]
- Çaykara, T.; Dogmus, M. The effect of solvent composition on swelling and shrinking properties of poly(acrylamideco-itaconic acid) hydrogels. Eur. Polym. J. 2004, 40, 2605–2609. [Google Scholar] [CrossRef]
- Guenther, M.; Gerlach, G. Hydrogels for Chemical Sensors. In Hydrogel Sensors and Actuators—Engineering and Technology, Springer Series on Chemical Sensors and Biosensors 6; Springer: Berlin/Heidelberg, Germany, 2009; pp. 165–195. [Google Scholar]






| Solution | Refractive Index |
|---|---|
| 100 v% H2O | 1.3329 |
| 66 v% H2O, 33 v% EG | 1.3683 |
| 50 v% H2O, 50 v% EG | 1.3858 |
| 33 v% H2O, 66 v% EG | 1.4022 |
| 100 v% EG | 1.4300 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kroh, C.; Wuchrer, R.; Steinke, N.; Guenther, M.; Gerlach, G.; Härtling, T. Hydrogel-Based Plasmonic Sensor Substrate for the Detection of Ethanol. Sensors 2019, 19, 1264. https://doi.org/10.3390/s19061264
Kroh C, Wuchrer R, Steinke N, Guenther M, Gerlach G, Härtling T. Hydrogel-Based Plasmonic Sensor Substrate for the Detection of Ethanol. Sensors. 2019; 19(6):1264. https://doi.org/10.3390/s19061264
Chicago/Turabian StyleKroh, Christoph, Roland Wuchrer, Nadja Steinke, Margarita Guenther, Gerald Gerlach, and Thomas Härtling. 2019. "Hydrogel-Based Plasmonic Sensor Substrate for the Detection of Ethanol" Sensors 19, no. 6: 1264. https://doi.org/10.3390/s19061264
APA StyleKroh, C., Wuchrer, R., Steinke, N., Guenther, M., Gerlach, G., & Härtling, T. (2019). Hydrogel-Based Plasmonic Sensor Substrate for the Detection of Ethanol. Sensors, 19(6), 1264. https://doi.org/10.3390/s19061264





