Classification Method for Viability Screening of Naturally Aged Watermelon Seeds Using FT-NIR Spectroscopy
Abstract
1. Introduction
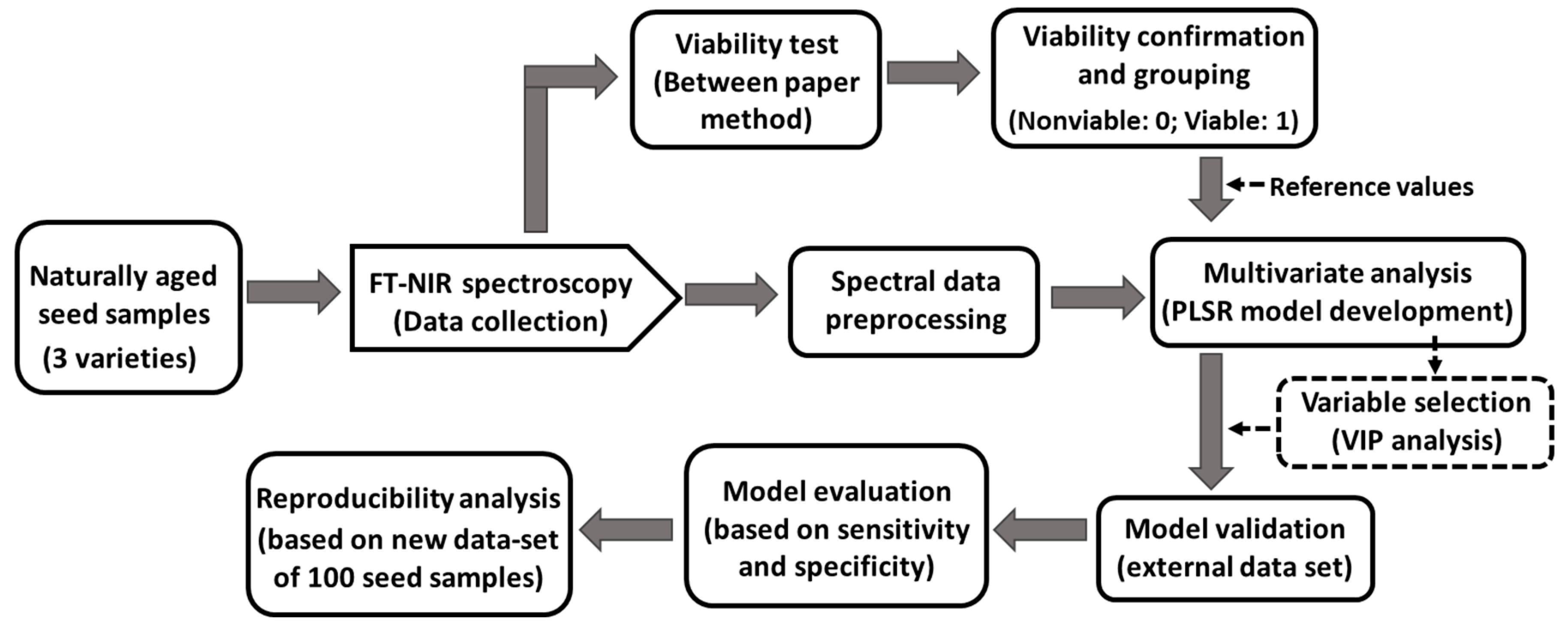
2. Materials and Methods
2.1. Seed Samples
2.2. FT-NIR Spectra Acquisition
2.3. Between Paper Germination Test
2.4. Data Preprocessing and Multivariate Analysis
2.5. Optimal Variable Selection
3. Result and Discussion
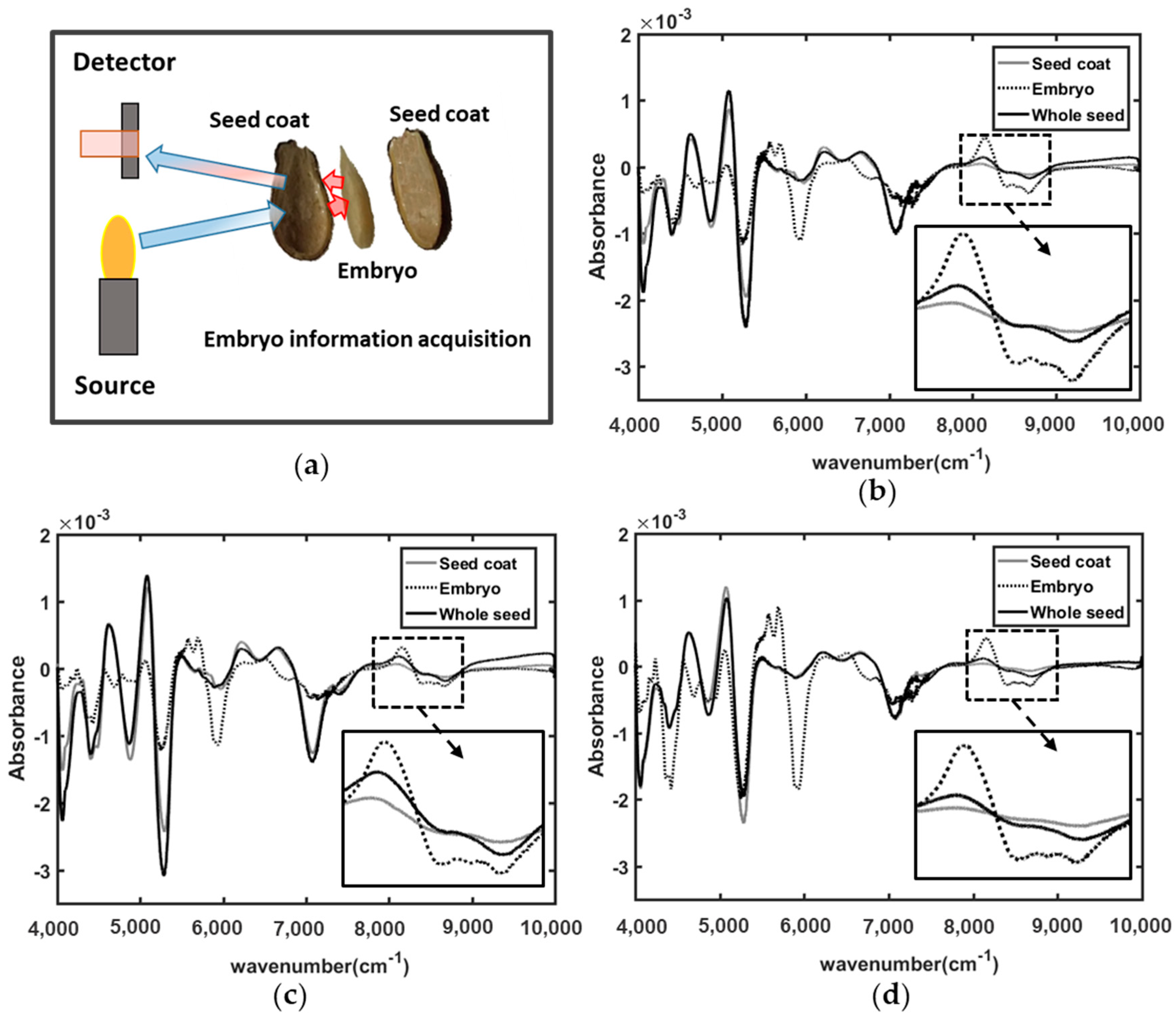
3.1. Embryo Information Acquisition
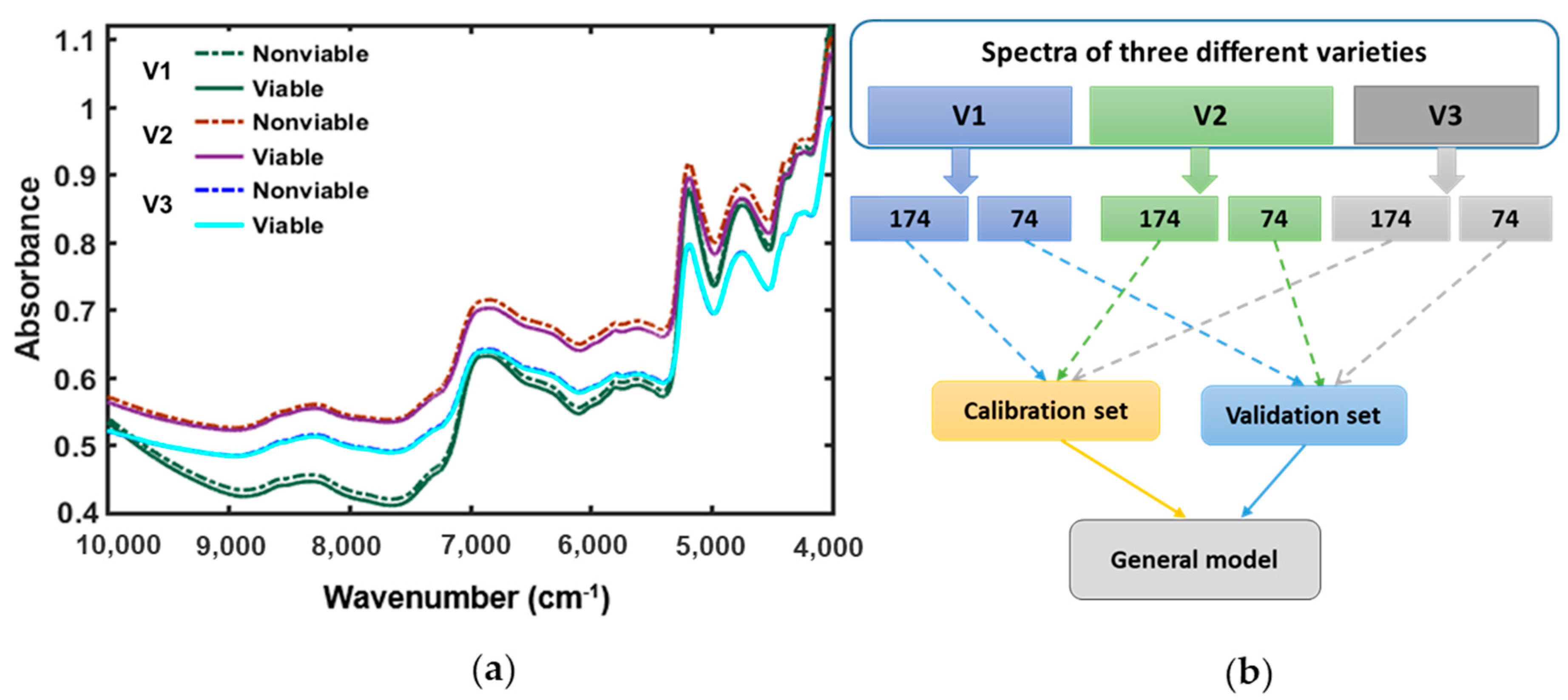
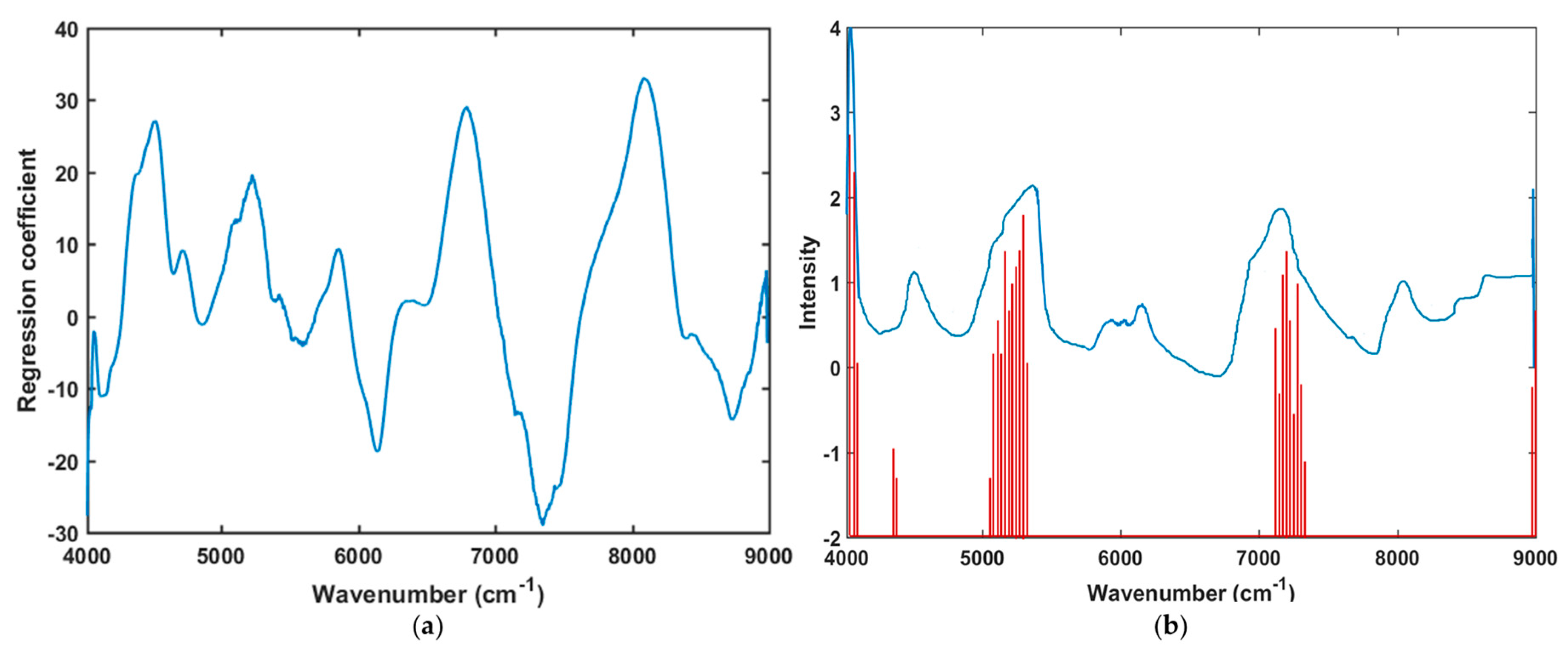
3.2. Spectral Interpretation
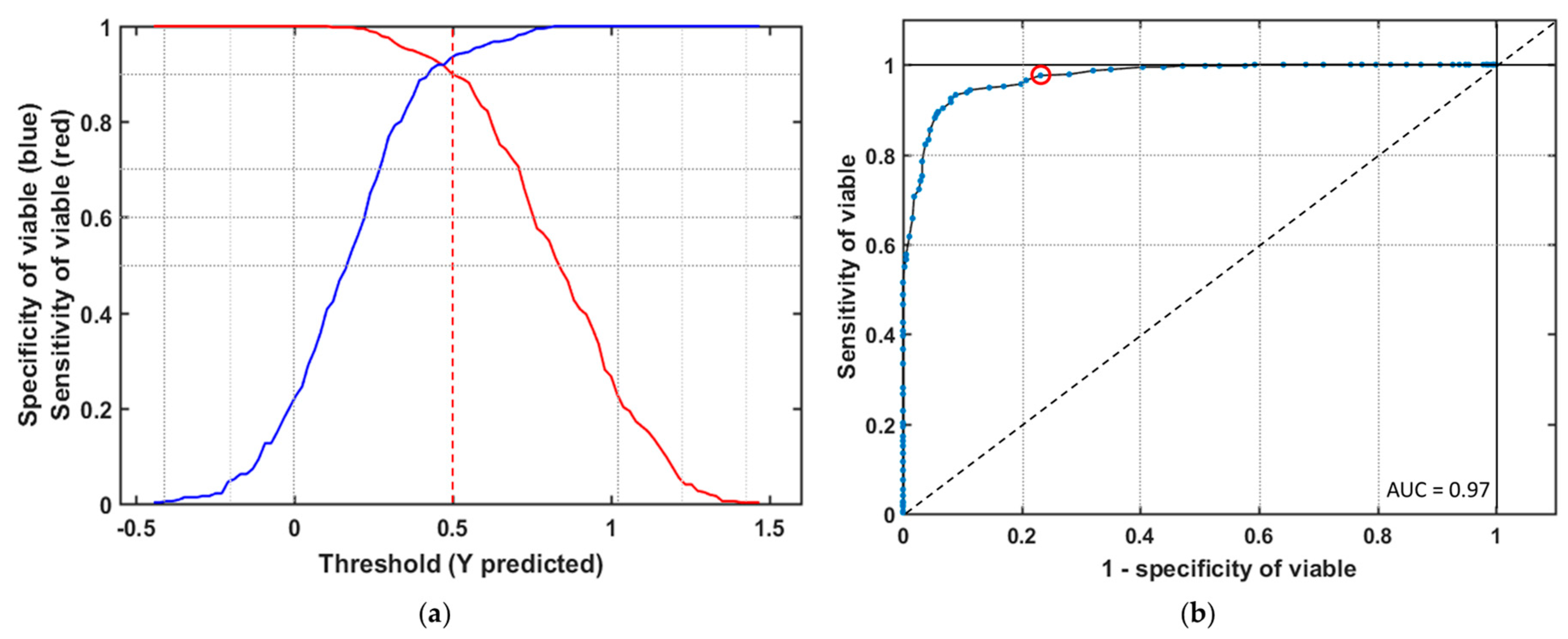
3.3. PLS-DA Model
3.4. Model Validation with New Data Set
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marcos, F.J. Deterioração de sementes. Fisiologia de sementes de plantas cultivadas. Piracicaba FEALQ 2005, 12, 291–352. [Google Scholar]
- Nikolaeva, M.G. Factors Controlling the Seed Dormancy Pattern; North-Holland: Amsterdam, The Netherlands, 1977; ISBN 0444804234. [Google Scholar]
- Demir, I.; Mavi, K.; Oztokat, C. Changes in germination and potential longevity of watermelon (Citrullus lanatus) seeds during development. N. Z. J. Crop Hortic. Sci. 2004, 32, 139–145. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Types of Seeds and Kinds of Seed Dormancy. In Seeds; Elsevier: Amsterdam, The Netherlands, 2014; pp. 37–77. [Google Scholar]
- Walsh, D.G.; Waldren, S.; Martin, J.R. Monitoring seed viability of fifteen species after storage in the Irish threatened plant genebank. JSTOR 2003, 103, 59–67. [Google Scholar] [CrossRef]
- Mazliak, P. Plant membrane lipids: Changes and alterations during aging and senescence. In Post-Harvest Physiology and Crop Preservation; Springer US: Boston, MA, USA, 1983; pp. 123–140. [Google Scholar]
- McDonald, M. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. Technol. 1999, 27, 177–237. [Google Scholar]
- Ahmed, M.R.; Yasmin, J.; Collins, W.; Cho, B.K. X-ray CT image analysis for morphology of muskmelon seed in relation to germination. Biosyst. Eng. 2018, 175, 183–193. [Google Scholar] [CrossRef]
- Marcos, F.J. Vigor de sementes: Conceitos e testes. In Vigor de sementes; Krzyzanowski, F.C.K., Ed.; Abrates: Londrina, Brazil, 1999; pp. 1–21. [Google Scholar]
- Esteve Agelet, L.; Ellis, D.D.; Duvick, S.; Goggi, A.S.; Hurburgh, C.R.; Gardner, C.A. Feasibility of near infrared spectroscopy for analyzing corn kernel damage and viability of soybean and corn kernels. J. Cereal Sci. 2012, 55, 160–165. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, Y.; Lee, Y.; Lee, G. Large-scale screening of intact tomato seeds for viability using near infrared reflectance spectroscopy (NIRS). Sustainability 2017, 9, 618. [Google Scholar] [CrossRef]
- Juhász, R.; Gergely, S.; Gelencsér, T.; Salgó, A. Relationship Between NIR Spectra and RVA Parameters During Wheat Germination. Cereal Chem. 2005, 82, 488–493. [Google Scholar] [CrossRef]
- Tigabu, M.; Odén, P.C. Discrimination of viable and empty seeds of Pinus patula Schiede & Deppe with near-infrared spectroscopy. New For. 2003, 25, 163–176. [Google Scholar] [CrossRef]
- Tai-Gi, M.; Kang, W.-S. Nondestructive classification of viable and nonviable radish (Raphanus sativus L.) seeds using single seed near infrared spectroscopy. Hortic. Environ. Biotechnol. 2008, 49, 42–46. [Google Scholar]
- Lohumi, S.; Mo, C.; Kang, J.S.; Hong, S.J.; Cho, B.K. Nondestructive Evaluation for the Viability of Watermelon (Citrullus lanatus) Seeds Using Fourier Transform Near Infrared Spectroscopy. J. Biosyst. Eng. 2013, 38, 312–317. [Google Scholar] [CrossRef]
- Mulugeta, D.; Stoltenberg, D.E. Increased weed emergence and seed bank depletion by soil disturbance in a no-tillage system. Weed Sci. 1997, 45, 234–241. [Google Scholar] [CrossRef]
- Giurizatto, M.I.; Ferrarese-Filho, O.; Ferrarese, M.D.; Robaina, A.D.; Gonçalves, M.C.; Cardoso, C.A. α-Tocopherol levels in natural and artificial aging of soybean seeds. Acta Sci. Agron. 2012, 34, 339–343. [Google Scholar] [CrossRef]
- Petruzzelli, L.; Carella, G. The Effect of Ageing Conditions on Loss of Viability in Wheat (T. durum). J. Exp. Bot. 1983, 34, 221–225. [Google Scholar] [CrossRef]
- Fantazzini, T.B.; Rosa, D.V.; Pereira, C.C.; Pereira, D.D.; Cirillo, M.Â.; Ossani, P.C. Association between the artificial aging test and the natural storage of coffee seeds. J. Seed Sci. 2018, 40, 164–172. [Google Scholar] [CrossRef]
- Maggio, R.M.; Kaufman, T.S.; Del Carlo, M.; Cerretani, L.; Bendini, A.; Cichelli, A.; Compagnone, D. Monitoring of fatty acid composition in virgin olive oil by Fourier transformed infrared spectroscopy coupled with partial least squares. Food Chem. 2009, 114, 1549–1554. [Google Scholar] [CrossRef]
- International Seed Testing Association (ISTA). International Rules for Seed Testing, Chapter 5: The Germination Test; International Seed Testing Association: Bassersdorf, Switzerland, 1996. [Google Scholar]
- Lohumi, S.; Joshi, R.; Kandpal, L.M.; Lee, H.; Kim, M.S.; Cho, H.; Mo, C.; Seo, Y.W.; Rahman, A.; Cho, B.K. Quantitative analysis of Sudan dye adulteration in paprika powder using FTIR spectroscopy. Food Addit. Contam. Part A 2017, 34, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Kandpal, L.; Lee, S.; Kim, M.; Bae, H.; Cho, B.K. Short wave infrared (SWIR) hyperspectral imaging technique for examination of aflatoxin B1 (AFB1) on corn kernels. Food Control 2015, 51, 171–176. [Google Scholar] [CrossRef]
- Sun, J.L.; Nakagawa, H.; Karita, S.; Ohmiya, K.; Hattori, T. Rice embryo globulins: Amino-terminal amino acid sequences, cDNA cloning and expression. Plant Cell Physiol. 1996, 37, 612–620. [Google Scholar] [CrossRef]
- Thijssen, M.H.; Spoelstra, P.; Emons, A.M.C. Immunodetection and immunolocalization of globulin storage proteins during zygotic and somatic embryo development in Zea mays. Physiol. Plant. 1996, 98, 539–549. [Google Scholar] [CrossRef]
- Burgess, S.R.; Shewry, P.R. Identification of homologous globulins from embryos of wheat, barley, rye and oats. J. Exp. Bot. 1986, 37, 1863–1871. [Google Scholar] [CrossRef]
- Shenk, J.S.; Burns, D.A.; Ciurczak, E.W. Application of NIR Spectroscopy to Agricultural Products. In Handbook of Near-Infrared Spectroscopy; M. Dekker: New York, NY, USA, 2001; pp. 419–474. ISBN 1420002570. [Google Scholar]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Ballabio, D.; Consonni, V. Classification tools in chemistry. Part 1: Linear models. PLS-DA. Anal. Methods 2013, 5, 3790–3798. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W. Rapid Quantification Analysis and Visualization of Escherichia coli Loads in Grass Carp Fish Flesh by Hyperspectral Imaging Method. Food Bioprocess Technol. 2015, 8, 951–959. [Google Scholar] [CrossRef]
- Osborne, B.; Fearn, T.; Hindle, P. Practical NIR Spectroscopy with Applications in Food and Beverage Analysis, 2nd ed.; Longman Scientific and Technical: Harlow, UK, 1993. [Google Scholar]
- Shaban, M. Biochemical aspects of protein changes in seed physiology and germination. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 885–898. [Google Scholar]
- Mbofung, G.C.Y.; Goggi, A.S.; Leandro, L.F.S.; Mullen, R.E. Effects of storage temperature and relative humidity on viability and vigor of treated soybean seeds. Crop. Sci. 2013, 53, 1086–1095. [Google Scholar] [CrossRef]
- Sekar, S.; Ahamed, R.; Natesan, S.; Nagappan, A.; Hyeon Soo, P.; Kalaiselvi, S.; Umarani, R.; Raveendran, M.; Bhaskaran, M.; Kim, G.S. Proteomic analysis of ageing in black gram (‘Vigna mungo’L.) seeds and its relation to seed viability. Plant Omics 2015, 8, 201–211. [Google Scholar]
- Machado, N.B.; Custódio, C.C.; Takaki, M. Evaluation of naturally and artificially aged seeds of Phaseolus vulgaris L. Seed Sci. Technol. 2001, 29, 137–149. [Google Scholar] [CrossRef]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.C.; Belghazi, M.; Job, C.; Job, D. Proteome-wide characterization of seed aging in Arabidopsis: A comparison between artificial and natural aging protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. Seed moisture content, storage, viability and vigour. Seed Sci. Res. 1991, 1, 275–279. [Google Scholar] [CrossRef]
- Lohumi, S.; Lee, S.; Cho, B.K. Optimal variable selection for Fourier transform infrared spectroscopic analysis of starch-adulterated garlic powder. Sens. Actuators B Chem. 2015, 216, 622–628. [Google Scholar] [CrossRef]


| Model | Samples Used for Model Making | Preprocessing | 1 LVs | Calibration | Validation | ||||
|---|---|---|---|---|---|---|---|---|---|
| Viable | Non-Viable | Total | Viable | Non-Viable | Total | ||||
| General model made of three varieties | 744 | Raw | 13 | 198/260 | 182/260 | 73.1% | 85/112 | 74/112 | 72.1% |
| Savitzky-Golay 1st | 8 | 253/260 | 250/260 | 96.7% | 103/112 | 99/112 | 90.1% | ||
| Test Set Variety | Samples Used | NER/Accuracy | ER | Sensitivity | Specificity | PREC | FPR |
|---|---|---|---|---|---|---|---|
| Random | 100 | 0.85 | 0.15 | 0.877 | 0.82 | 0.83 | 0.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasmin, J.; Raju Ahmed, M.; Lohumi, S.; Wakholi, C.; Kim, M.S.; Cho, B.-K. Classification Method for Viability Screening of Naturally Aged Watermelon Seeds Using FT-NIR Spectroscopy. Sensors 2019, 19, 1190. https://doi.org/10.3390/s19051190
Yasmin J, Raju Ahmed M, Lohumi S, Wakholi C, Kim MS, Cho B-K. Classification Method for Viability Screening of Naturally Aged Watermelon Seeds Using FT-NIR Spectroscopy. Sensors. 2019; 19(5):1190. https://doi.org/10.3390/s19051190
Chicago/Turabian StyleYasmin, Jannat, Mohammed Raju Ahmed, Santosh Lohumi, Collins Wakholi, Moon S. Kim, and Byoung-Kwan Cho. 2019. "Classification Method for Viability Screening of Naturally Aged Watermelon Seeds Using FT-NIR Spectroscopy" Sensors 19, no. 5: 1190. https://doi.org/10.3390/s19051190
APA StyleYasmin, J., Raju Ahmed, M., Lohumi, S., Wakholi, C., Kim, M. S., & Cho, B.-K. (2019). Classification Method for Viability Screening of Naturally Aged Watermelon Seeds Using FT-NIR Spectroscopy. Sensors, 19(5), 1190. https://doi.org/10.3390/s19051190






