Screen-Printed Sensors for Colorimetric Detection of Hydrogen Sulfide in Ambient Air
Abstract
:1. Introduction
2. Materials and Methods
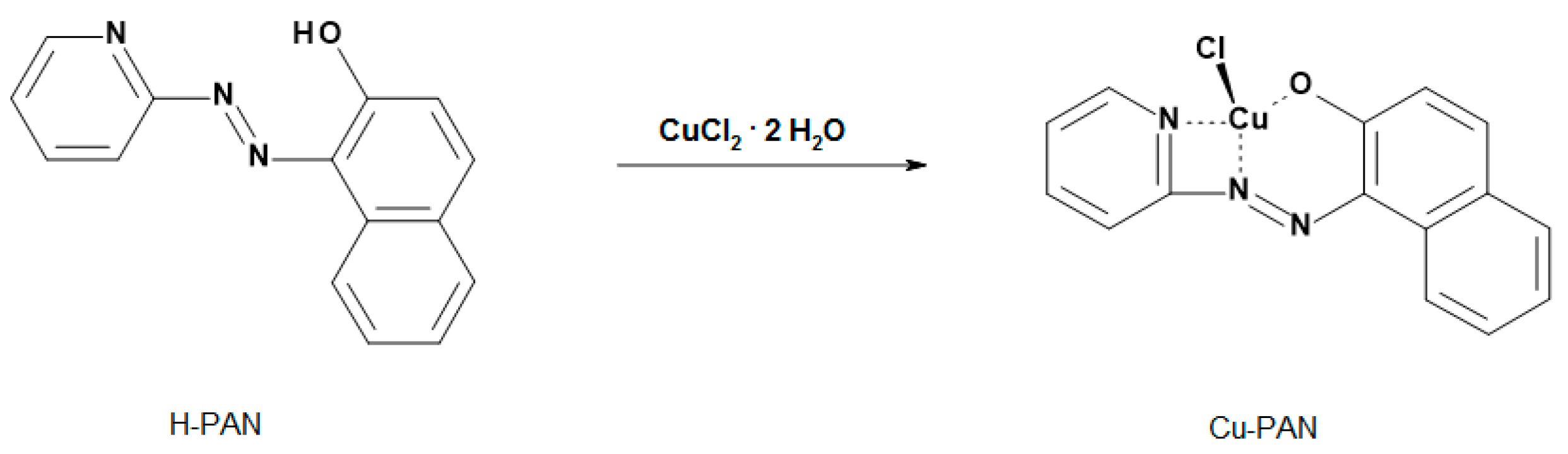
2.1. Synthesis and Characterization of the Cu-PAN Complex
2.2. Sensing Mechanism of the Cu-PAN Complex
2.3. Preparation of Cu-PAN on Silica
2.4. Preparation of the Cu-PAN Test Strips
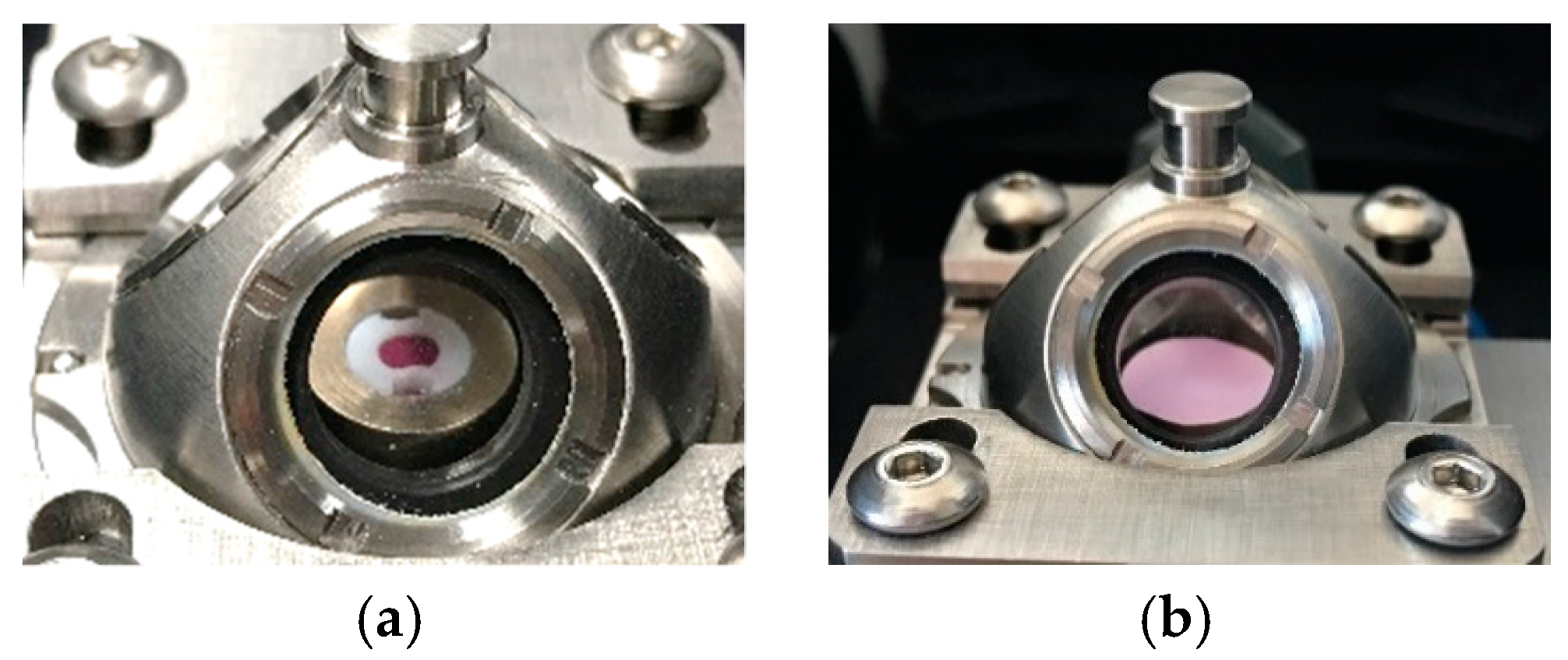
2.5. H2S Gas Measurements
3. Results and Discussion
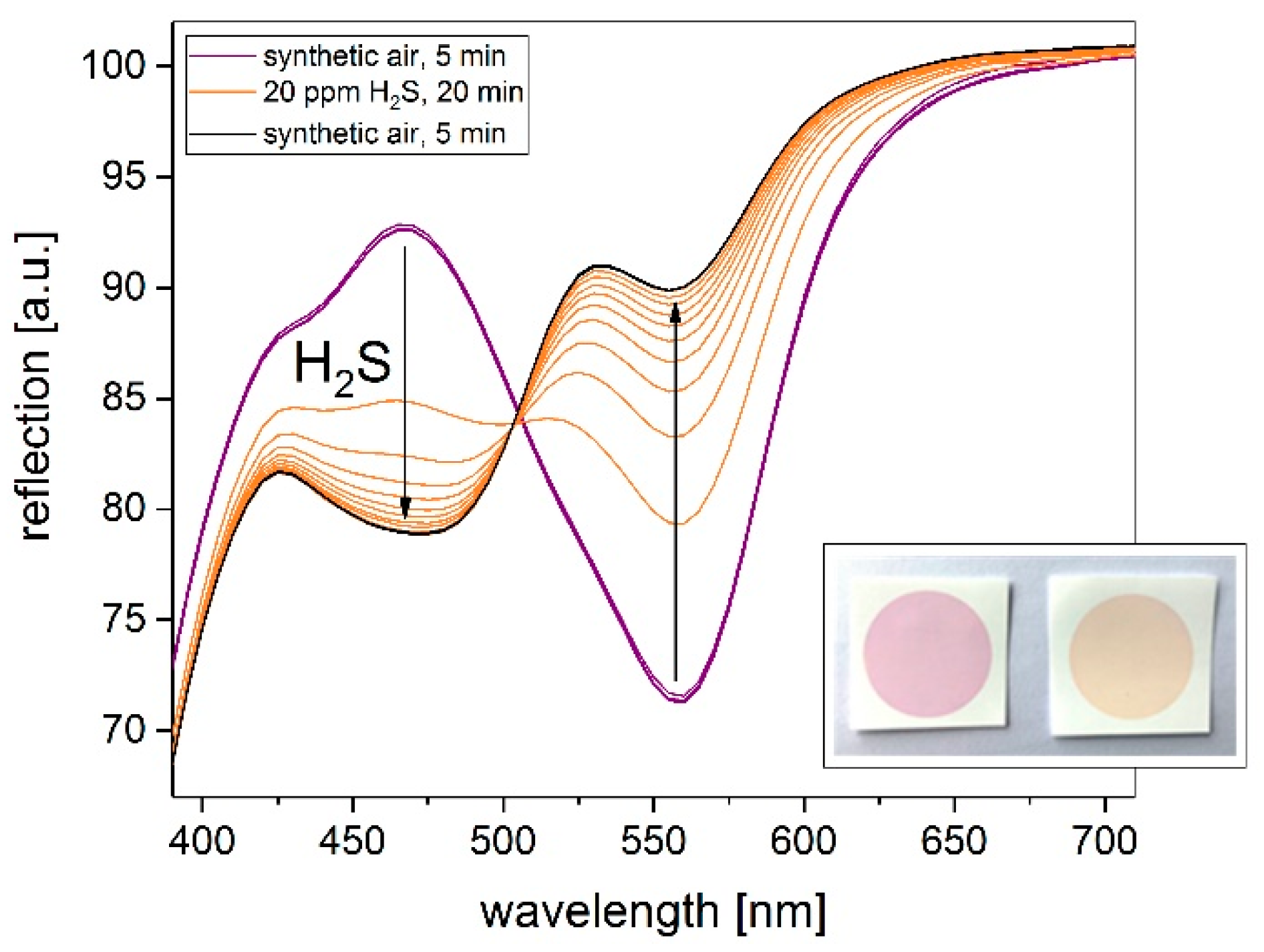
3.1. Cu-PAN on Silica
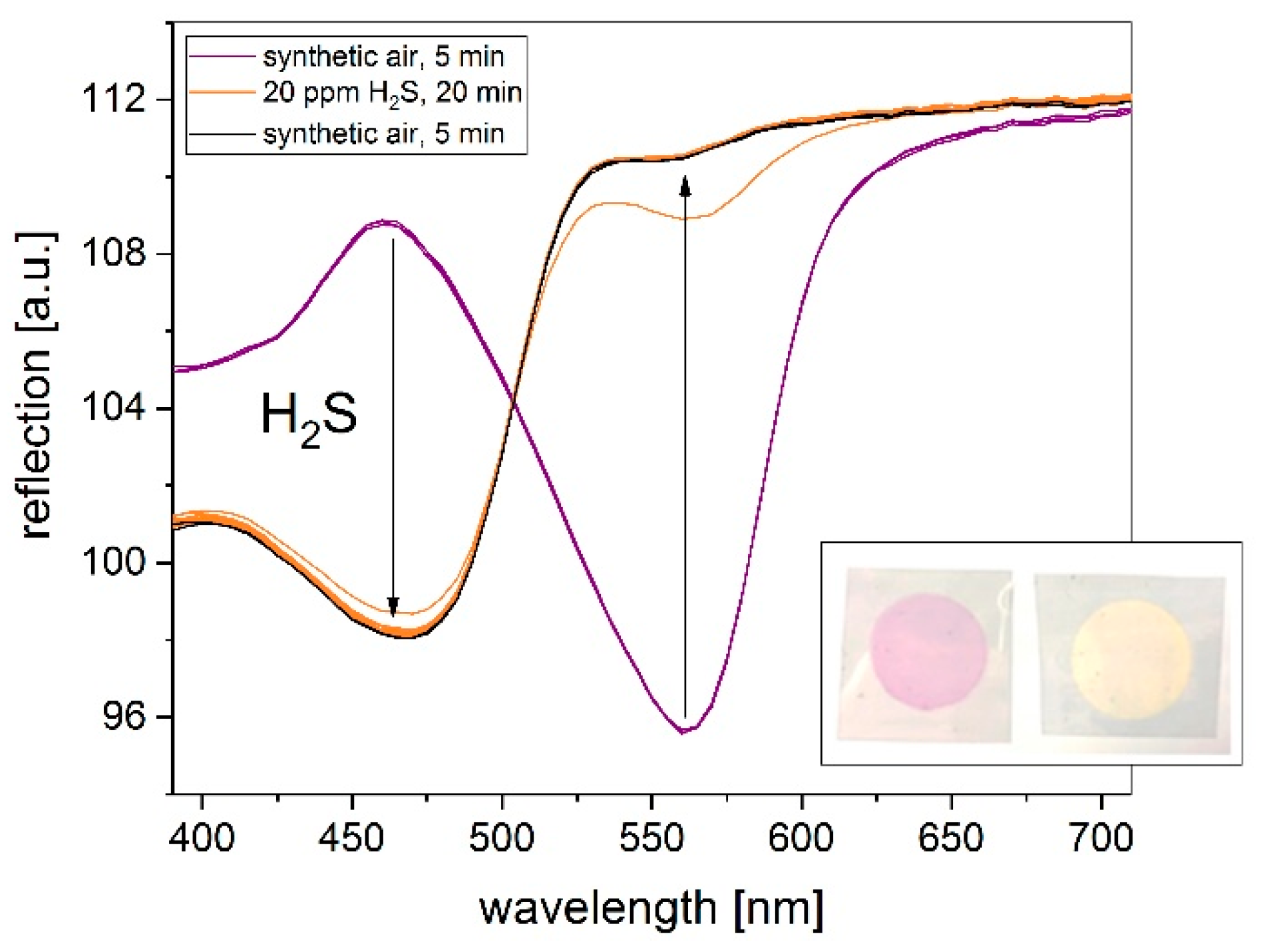
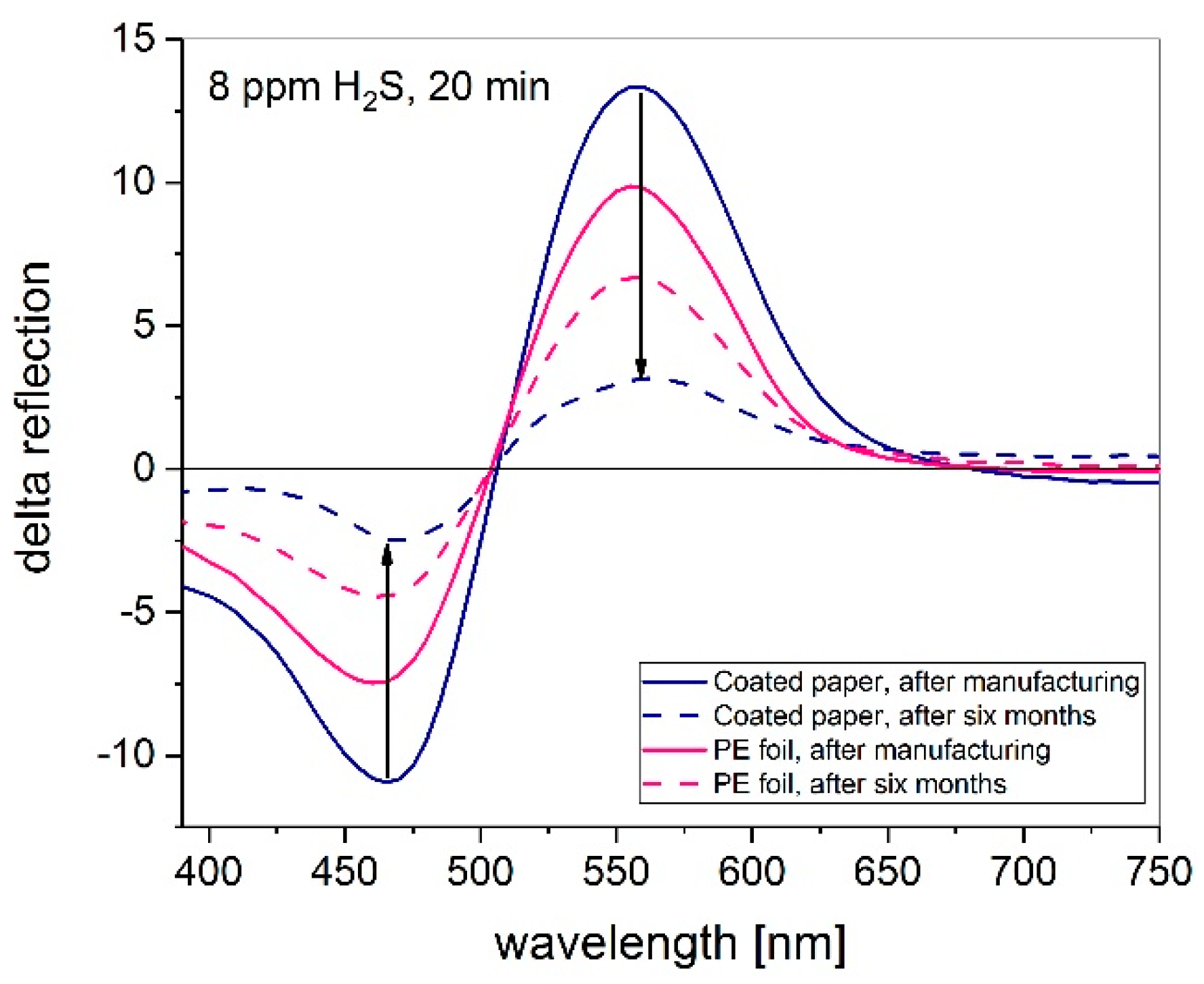
3.2. Influence on Printing Substrate
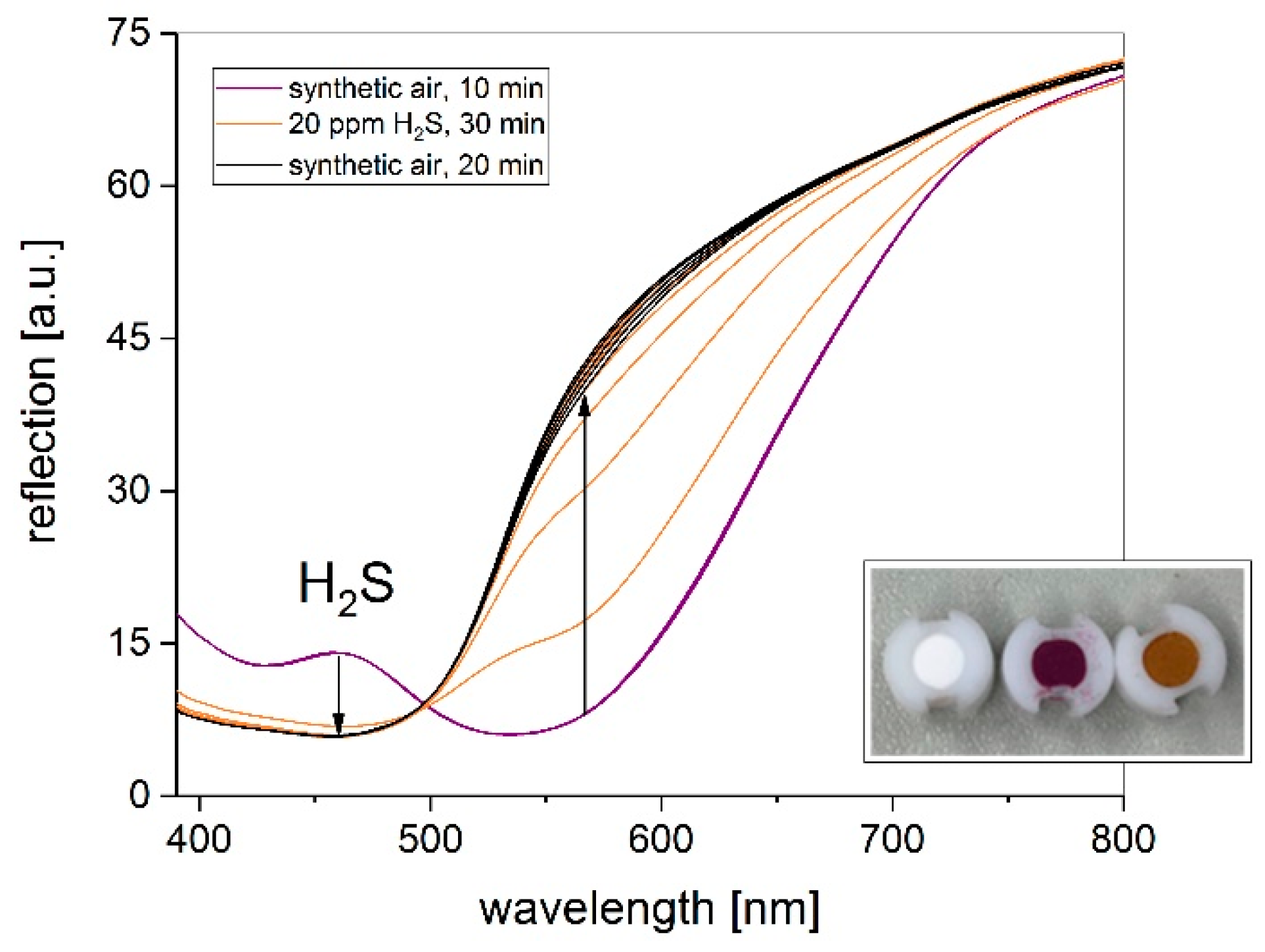
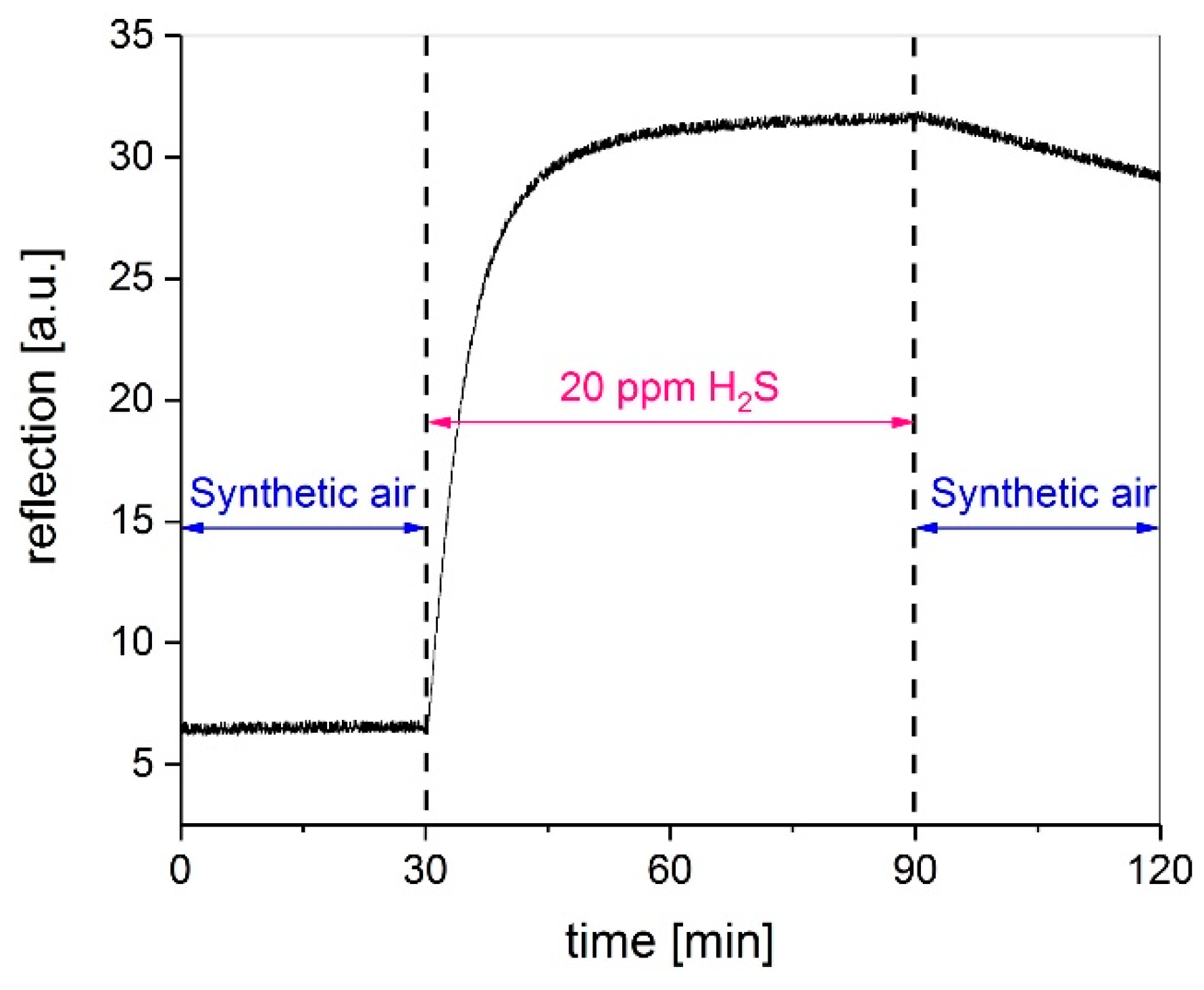
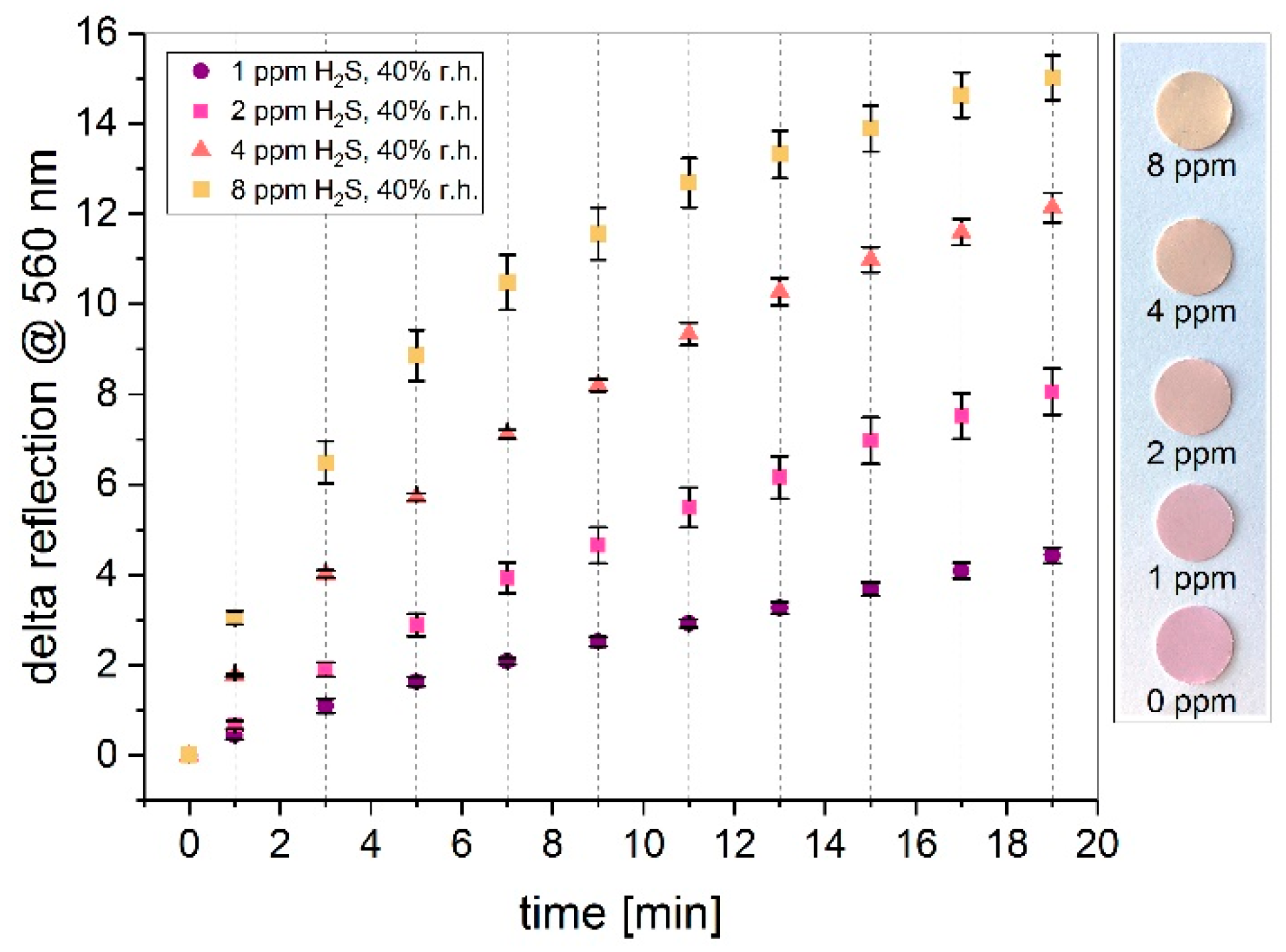
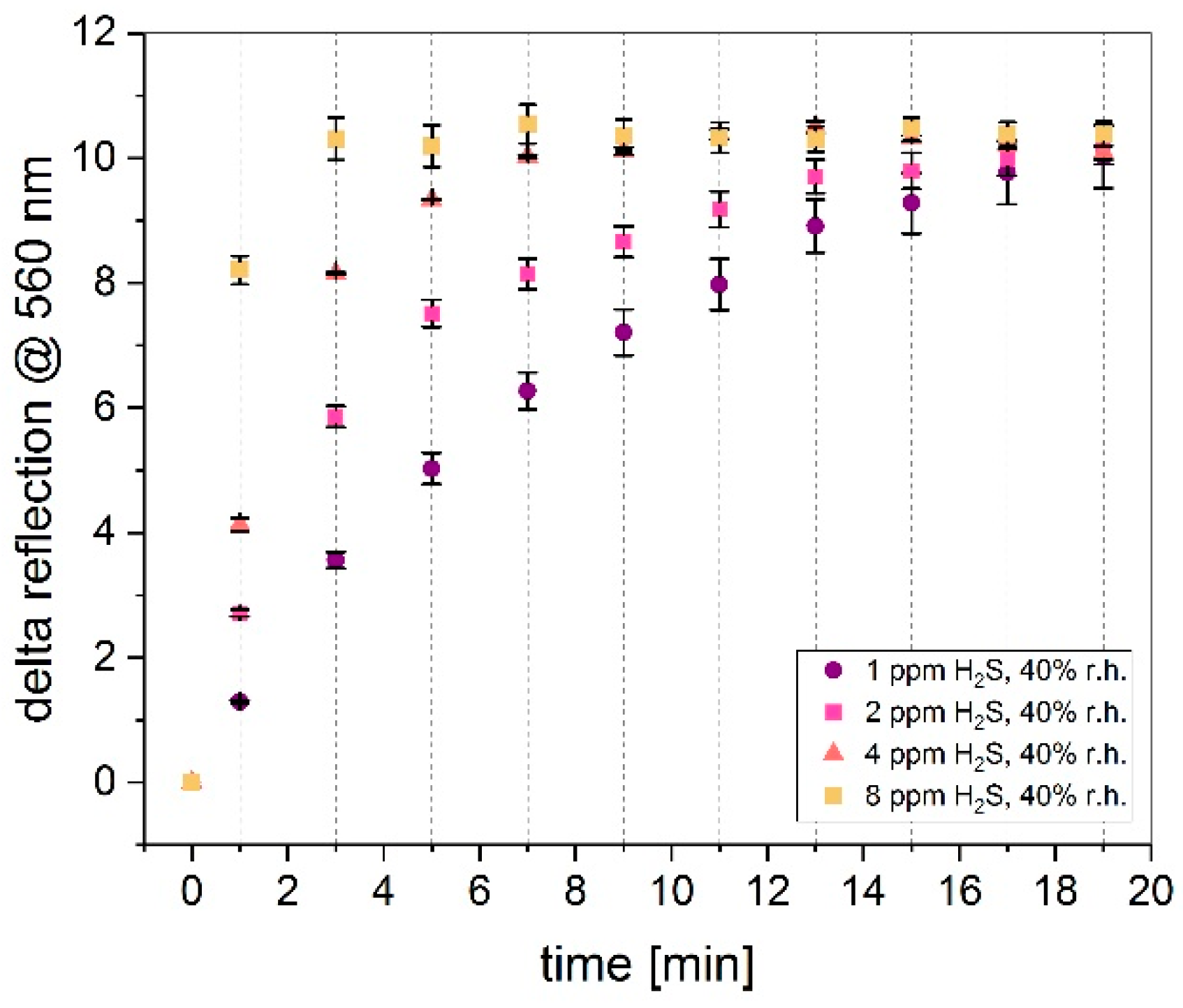
3.3. Influence on Gas Concentration
3.4. Cross-Sensitivities
3.5. Long-Term Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hydrogen Sulfide. The MAK-Collection for Occupational Health and Safety. American Cancer Society; pp. 1–27. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/3527600418.mb778306d0043 (accessed on 7 July 2018).
- OSHA Standards, Occupational Safety and Health Administration. Health Hazards of Hydrogen Sulfide. Available online: https://www.osha.gov/SLTC/hydrogensulfide/hazards.html (accessed on 1 January 2019).
- Liptàk, B.G. Instrument Engineer’s Handbook, Volume One: Process Measurement and Analysis; CRC Press: Boca Raton, FL, USA, 2003; pp. 1366–1376. [Google Scholar]
- Safety Data Sheet, Lead(II) Acetate, Carl Roth. Available online: https://www.carlroth.com/downloads/sdb/en/2/SDB_2559_MT_EN.pdf (accessed on 1 January 2019).
- Sanderson, H.P.; Thomas, R.; Katz, M. Limitations of the Lead Acetate Impregnated Paper Tape Method for Hydrogen Sulfide. J. Air Pollut. Control Assoc. 2012, 16, 328–330. [Google Scholar] [CrossRef]
- Pla-Tolós, J.; Moliner-Martínez, Y.; Verdú-Andés, J.; Casanova-Chafer, J.; Molins-Legua, C.; Campíns-Falcó, P. New optical paper sensor for in situ measurement of hydrogen sulphide in waters and atmospheres. Talanta 2016, 156–157, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Imamura, T.; Cheng, K.L. Handbook of Organic Analytical Reagents, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1992; pp. 209–217. [Google Scholar]
- Carpenter, T.S.; Rosolina, S.M.; Xue, Z.-L. Quantitative, colorimetric paper probe for hydrogen sulfide gas. Sens. Actuators B Chem. 2017, 253, 843–851. [Google Scholar] [CrossRef]
- Ariza-Avidad, M.; Agudo-Acemel, M.; Salinas-Castillo, A.; Capitán-Vallvey, L.F. Inkjet-printed disposable metal complexing indicator-displacement assay for sulphide determination in water. Spectrochimica 2014, 872, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Köhler, M.; Fritzsche, W. Nanotechnology: An Introduction to Nanostructuring Techniques, 2nd ed.; WILEY-VCH Verlag GmbH & Co., KGaA: Berlin, German, 2007; pp. 19–20. [Google Scholar]
- Kneer, J.; Eberhardt, A.; Walden, P.; Ortiz Pérez, A.; Wöllenstein, J.; Palzer, S. Apparatus to characterize gas sensor response under real-world conditions in the lab. Rev. Sci. Instrum. 2014, 85. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engel, L.; Tarantik, K.R.; Pannek, C.; Wöllenstein, J. Screen-Printed Sensors for Colorimetric Detection of Hydrogen Sulfide in Ambient Air. Sensors 2019, 19, 1182. https://doi.org/10.3390/s19051182
Engel L, Tarantik KR, Pannek C, Wöllenstein J. Screen-Printed Sensors for Colorimetric Detection of Hydrogen Sulfide in Ambient Air. Sensors. 2019; 19(5):1182. https://doi.org/10.3390/s19051182
Chicago/Turabian StyleEngel, Laura, Karina R. Tarantik, Carolin Pannek, and Jürgen Wöllenstein. 2019. "Screen-Printed Sensors for Colorimetric Detection of Hydrogen Sulfide in Ambient Air" Sensors 19, no. 5: 1182. https://doi.org/10.3390/s19051182
APA StyleEngel, L., Tarantik, K. R., Pannek, C., & Wöllenstein, J. (2019). Screen-Printed Sensors for Colorimetric Detection of Hydrogen Sulfide in Ambient Air. Sensors, 19(5), 1182. https://doi.org/10.3390/s19051182




