Abstract
Toxic gases, such as NOx, SOx, H2S and other S-containing gases, cause numerous harmful effects on human health even at very low gas concentrations. Reliable detection of various gases in low concentration is mandatory in the fields such as industrial plants, environmental monitoring, air quality assurance, automotive technologies and so on. In this paper, the recent advances in electrochemical sensors for toxic gas detections were reviewed and summarized with a focus on NO2, SO2 and H2S gas sensors. The recent progress of the detection of each of these toxic gases was categorized by the highly explored sensing materials over the past few decades. The important sensing performance parameters like sensitivity/response, response and recovery times at certain gas concentration and operating temperature for different sensor materials and structures have been summarized and tabulated to provide a thorough performance comparison. A novel metric, sensitivity per ppm/response time ratio has been calculated for each sensor in order to compare the overall sensing performance on the same reference. It is found that hybrid materials-based sensors exhibit the highest average ratio for NO2 gas sensing, whereas GaN and metal-oxide based sensors possess the highest ratio for SO2 and H2S gas sensing, respectively. Recently, significant research efforts have been made exploring new sensor materials, such as graphene and its derivatives, transition metal dichalcogenides (TMDs), GaN, metal-metal oxide nanostructures, solid electrolytes and organic materials to detect the above-mentioned toxic gases. In addition, the contemporary progress in SO2 gas sensors based on zeolite and paper and H2S gas sensors based on colorimetric and metal-organic framework (MOF) structures have also been reviewed. Finally, this work reviewed the recent first principle studies on the interaction between gas molecules and novel promising materials like arsenene, borophene, blue phosphorene, GeSe monolayer and germanene. The goal is to understand the surface interaction mechanism.
Contents
- Introduction
- Recent Advances in NO2 Gas Detection
- 2.1.
- Graphene and Its Derivatives-Based NO2 Sensors
- 2.2.
- Transition Metal Dichalcogenide (TMD)-Based NO2 Sensors
- 2.3.
- Metal and Metal-Oxide Nanostructure-Based NO2 Sensors
- 2.4.
- GaN-Based NO2 Sensors
- 2.5.
- Organic Materials-Based NO2 Sensors
- 2.6.
- Hybrid Materials-Based NO2 Sensors
- Recent Advances in SO2 Gas Detection
- 3.1.
- Carbon Material-Based SO2 Sensors
- 3.2.
- Metal and Metal-Oxide Nanostructures-Based SO2 Sensors
- 3.3.
- GaN-Based SO2 Sensors
- 3.4.
- Solid Electrolyte-Based SO2 Sensors
- 3.5.
- Zeolite-Based SO2 Sensors
- 3.6.
- Paper-Based SO2 Sensors
- Recent Advances in H2S Gas Detection
- 4.1.
- Carbon Material-Based H2S Sensors
- 4.2.
- GaN-Based H2S Sensors
- 4.3.
- Metal and Metal Oxide-Based H2S Sensors
- 4.3.1.
- Nanostructured Metal Oxide-Based Sensors
- 4.3.2.
- Mesoporous Metal Oxide-Based Sensors
- 4.3.3.
- Metal Oxide Microsphere-Based Sensors
- 4.4.
- MOF-Based H2S Sensors
- 4.5.
- Organic Materials-Based H2S Sensors
- 4.6.
- Solid Electrolytes-Based H2S Sensors
- Recent Density-Functional Theory (DFT) Study of Gas Molecule-Sensor Interaction
- Calibration of Toxic Gas Sensors
- Toxic Gas Sensors in Internet of Things (IoT) Applications
- Future Perspectives and Conclusions
Acknowledgments
Conflicts of Interest
References
1. Introduction
Humans are exposed to various air toxins in the indoor and outdoor environment. Poor air quality is a well-known trigger for various health problems which can often result in life threatening and expensive emergency care. Therefore, precise toxic gas sensing will not only bring a major benefit to industries but also to day-to-day life for all people. Nitrogen dioxide (NO2) is one of the common toxic air pollutants, which is mostly found as a mixture of nitrogen oxides (NOx) with different ratios (x). NO2 is a reddish-brown, irritant, toxic gas having a characteristic sharp and biting odor. The LC50 (the lethal concentration for 50% of those exposed) for one hour of NO2 exposure for humans has been estimated as 174 ppm. The major sources of NO2 are from combustion of fuels such as certain coals and oil [1], biomass burning due to the extreme heat of lightning during thunderstorms [2], and nitrogen fixation by microorganisms due to agricultural fertilization [3]. The noteworthy impacts of NO2 include: respiratory inflammation of the airways, decreased lung function due to long term exposure, increased risk of respiratory conditions [4,5], increased responsiveness to allergens, contribution to the formation of fine particulate matter (PM) and ground level ozone which have adverse health effects, and contribution to acid rain causing damage to vegetation, buildings and acidification of lakes and streams [6,7].
Sulphur dioxide (SO2) is the most common air pollutant, mostly found as a mixture of sulfur oxides (SOx). It is an invisible gas with a nasty, sharp smell. The maximum concentration for SO2 exposures of 30 min to 1 h has been estimated as 50 to 100 ppm. The main sources of SO2 include burning of fossil fuels (fuel oil, coal) in power stations, oil refineries, other large industrial plants, motor vehicles and domestic boilers [8,9]. It is also produced from natural sources like active volcanoes and forest fires. When mineral ores containing sulfur are processed, SO2 is released to atmosphere as well. Excessive exposure of SO2 causes harms on the eye, lung and throat [10,11]. It is toxic to some plants, inducing visible signs of injury and reducing yields. SO2 gas combined with air moisture causes gradual damage to some building materials (e.g., limestone). SO2 can readily dissolve in the water droplets in clouds, causing acid rain that affects natural balance of rivers, lakes and soils, resulting in damage to wildlife and vegetation.
Hydrogen sulfide (H2S) is a highly toxic, malodorous, intensely irritating gas. The maximum concentration for H2S exposure for one hour without grave after-effects has been estimated as 170 to 300 ppm. The key sources of H2S gas are from decaying organic materials, natural gas, volcanic gas, petroleum, sewage plants and sulfur deposits [12,13]. Minimal exposure to H2S gas causes nose/eye irritation, olfactory nerve paralysis. Moderate amount may cause sore throat, cough, keratoconjunctivitis, chest tightness and pulmonary edema. Excessive exposure causes headaches, disorientation, loss of reasoning, coma, convulsions and even death [14,15].
In comparison to gas detection techniques like optical [16], acoustic [17] and gas chromatographic methods [18], electrochemical sensing is the most popular technique for ambient toxic gas monitoring. The key advantages of electrochemical detection are having low energy linear output with high resolution, good selectivity and repeatability, ppm level detection with high accuracy, and being more inexpensive than other techniques [19]. However, electrochemical sensors are highly sensitive to temperature fluctuations and have minimal shell life. The operating temperature should be kept as steady as possible to get the best sensor performances. Sensors with high operating temperature are generally employed in industrial and space applications. Over the last decades, research on toxic gas sensing was mostly focused on using electrochemical sensors which were built from various functional materials, such as carbon nanomaterials [20,21,22,23,24,25,26,27,28,29,30,31], metal oxide/metallic nanostructures [32,33,34,35,36,37,38], transition metal dichalcogenides (TMDs) [39,40,41], gallium nitride (GaN) [42,43,44,45], organic materials [46,47,48,49,50], solid electrolytes [51,52,53,54], zeolites [55,56,57,58] and others [59,60,61,62,63]. Recently numerous research efforts have been made on suitable gas sensing materials to detect nitrogen oxides, sulfur oxides and hydrogen sulfide gases. In this work, we have reviewed the recent advances in electrochemical sensors for toxic gas detection focusing mainly on NO2, SO2 and H2S gas sensors. The recent progress of each of these toxic gas detections has been categorized based on various sensing materials of high interest. The goal is to shine a light on the future development trend of toxic gas detection, a vital technology for the emerging Internet of Things era.
2. Recent Advances in NO2 Gas Detection
2.1. Graphene and Its Derivatives-Based NO2 Sensors
Graphene provides a large surface area, atom-thick 2D conjugated structures, low electrical noise, high conductivity, and excellent electronic properties [64]. Having high surface-to volume ratio, reduced graphene oxide (RGO) provides large surface areas, defects and low electrical noise as well [65]. All these characteristics make both graphene and RGO suitable candidates for gas adsorption and detection.
Recently epitaxial graphene has been utilized to detect ppb levels of NO2 gas and it was found that single-layer graphene is superior to bilayer graphene in terms of carrier concentration response [66]. Wang et al. [67] incorporated Pd nanoparticles (NPs) and SnO2 NPs on reduced graphene oxide to form Pd-SnO2-RGO hybrids as NO2 gas sensing materials. A high resolution transmission electron microscopy (HR-TEM) image of Pd-SnO2-RGO reveals that 3–5 nm sized nanoparticles (NPs) are deposited on RGO nanosheets (Figure 1A). Lattice distances of 0.33 nm and 0.23 nm indicated the presence of SnO2 NPs and Pd NPs, respectively. When a Pd-SnO2-RGO nanosheet was exposed to 1 ppm of NO2 gas at room temperature, a response of 3.92 was obtained with a response time of 13 s which are better compared to RGO as well as SnO2-RGO hybrids. However, the recovery time (105 s) was slower due to addition of Pd NPs. To perform a concentration response test, the fabricated sensor was exposed to 50 ppb to 2 ppm of NO2 gas. The sensor showed an increasing response trend with NO2 concentration (Figure 1B). The selectivity response of the Pd-SnO2-RGO sensor was examined towards Cl2, NO and some common volatile organic compounds (VOCs). Results indicated that the Pd-SnO2-RGO hybrid is highly selective to NO2 gas (Figure 1C). Preferred adsorption sites providing for NO2, high conductivity and the catalytic properties of Pd NPs are mainly responsible for the sensing performance improvement. However, no sulfur-containing gas was included in the test interference gases. Since Pd is known to interact strongly with S [68], the fabricated sensor should have been tested with S-containing gases to get the complete selectivity test picture. The same Wang et al. group [69] experimented with the introduction of oxygen vacancies (OV) into reduced graphene oxide nanosheets decorated with SnO2 nanoparticles (NPs). OVs enhance the adsorption of O2 molecules which in turn enhances the adsorption of NO2 molecules onto SnO2 NPs. Upon exposure to 1 ppm of NO2 gas, the SnO2-RGO-OVs-based sensor showed a response of 3.80 with reasonable response and recovery time. These NO2 sensing performances are better than those of other previously reported RGO-based sensors.
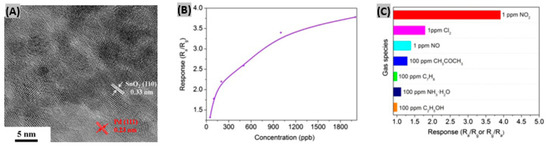
Figure 1.
(A) HR-TEM image of fabricated Pd-SnO2-RGO hybrid. (B) Sensor response toward different concentrations of NO2 gas at room temperature. (C) The response of the sensor to Cl2, NO, NO2, acetone, toluene, ammonia and ethanol in a selectivity test. Figures adapted with permission from [67], Copyright 2018 Elsevier.
In another study, Akbari et al. [70] decomposed methane in an arc discharge experiment to get carbonaceous materials (C-strands) between graphite electrodes. Upon NO2 exposure, the conductivity of the fabricated C strands was altered due to charge transfer between the carbon film and NO2 molecules. Previously, Zhang et al. [71] reported a rGO/Au nanocomposite-based NO2 sensor using a hydrothermal treatment. It provided good sensitivity with a quick response–recovery process at 50 °C.
2.2. Transition Metal Dichalcogenide (TMD)-Based NO2 Sensors
Two-dimensional (2D) transition metal dichalcogenides (TMDs) possess semiconducting nature, high surface-to-volume ratio and atomically thin-layered structures which are useful properties required to be a convincing sensing material [72]. MoS2, WS2, ReS2, MoSe2, MoTe2, WSe2 and ReSe2 are very promising 2D TMDs for gas sensing purposes [73,74,75]. Agrawal et al. prepared in-plane and edge-enriched p-MoS2 flakes (mixed MoS2) to detect NO2 gas at room temperature [76]. A FE-SEM image of the mixed MoS2 flakes is shown in Figure 2A. The blackish region represents the in-plane MoS2 flakes and the white region represents the edge-enriched MoS2 flakes. Most likely, the edge-enriched MoS2 flakes are white due to their height from the substrate surface. Figure 2B displays a sensitivity vs. NO2 concentration bar graph at RT and 125 °C. NO2 is an electron acceptor and it withdraws electrons from the MoS2 flakes, thus causing the resistance decrease of the mixed MoS2 flake-based sensor. The response and recovery time of the sensor were better at 125 °C than at RT. This happened because the adsorption energy of the NO2 gas molecule with the MoS2 flakes is very high at RT. The sensitivity of the sensor had been enhanced under UV light illumination as shown in Figure 2C. This improvement is attributed to the photoactivated desorption of adsorbed oxygen and creation of fresh active sites on the edges of MoS2 flakes. In another study, Kumar et al. [77] prepared a 1D MoS2 nanowire network which showed a detection limit of 4.6 ppb NO2 with good sensitivity. At the estimated optimum operating temperature (60 °C), response and recovery times were found as 16 s and 172 s, respectively, at 5 ppm NO2 exposure. Previously, Choi et al. [78] introduced Nb atoms into 2D MoSe2 host films. Figure 2D displays the low magnification planar annular dark-field scanning transmission electron microscopy (ADF-STEM) images and FFT patterns (inset) of MoSe2: Nb 1C, where 1C indicates one deposition cycle in the plasma-enhanced atomic layer deposition (PEALD) process. The polycrystal ring patterns in the image represent the presence of a few grains. Variably Nb-doped MoSe2 sensor films were exposed to different NO2 concentrations as shown in Figure 2E. The highest gas response was found for a MoSe2:Nb 1C device among the three tested devices because at low Nb dopant concentrations, MoSe2 showed an improved NO2 gas response due to its small grains and stabilized grain boundaries. At high Nb dopant concentrations, the NO2 gas response was degraded due to the increase of gas-unresponsive metallic NbSe2 regions, so an optimum Nb concentration is required for achieving a better gas response. The resistance of the MoSe2-based sensor gradually increased due to oxidation, whereas the Nb-doped MoSe2 sensor showed very stable response (Figure 2F). This means, introduction of Nb atoms onto 2D layered MoSe2 promotes a stable gas response and the long-term stability of the sensor. Also, a significant enhancement in sensing response with quick response-recovery toward NO2 was observed on WS2 nanosheet functionalized with Ag NWs [79].
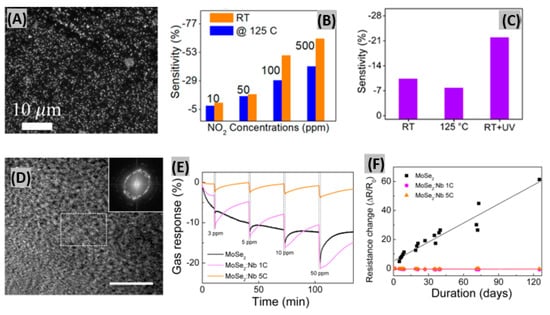
Figure 2.
(A) FE-SEM image of the mixed MoS2 flakes. (B) Sensitivity vs. NO2 concentration bar graph at RT and 125 °C. (C) Sensitivity bar graph of the mixed MoS2 flakes-based sensor for a NO2 gas concentration of 10 ppm. Figures adapted with permission from [76], Copyright 2018 American Chemical Society. (D) Planar ADF-STEM image of the MoSe2:Nb 1C film (white scale bar = 10 nm). Inset shows the corresponding FFT patterns. (E) Percent gas response for MoSe2, MoSe2:Nb 1C, and MoSe2:Nb 5C sensors at 3 to 50 ppm of NO2 gas. (F) Response of the MoSe2, MoSe2:Nb 1C, and MoSe2:Nb 5C sensors over 120 days. Figures adapted with permission from [78], Copyright 2017 American Chemical Society.
2.3. Metal and Metal-Oxide Nanostructure-Based NO2 Sensors
Metal oxides can be synthesized in various nanostructure morphologies like nanowires, nanoparticles, nanotubes, nanoflowers, nanocomposites and nanosheets for the enhancement of sensing performance [80,81,82]. Besides, porosity and permeable shell layers contribute to absolute electron depletion and gas diffusion that allow sensor devices to achieve high sensitivity toward gases [83]. Qiang et al. reported a NO2 gas sensor based on porous silicon (PS)/WO3 nanorods (NRs) functionalized with Pd NPs [84]. PS WO3 NRs were synthesized by electrochemical methods and thermal oxidation of W film, respectively. Pd NPs were deposited onto WO3 NRs, by the reduction of a Pd complex solution. Three different samples of PS/WO3 NRs–Pd NPs were prepared by varying the amount of Pd NPs on the substrate. These are PS/WO3–Pd20, PS/WO3–Pd40 and PS/WO3–Pd60, where the order of the amount of Pd NPs is Pd60 > Pd40 > Pd20. A TEM image of PS/WO3–Pd60 displays the agglomeration of Pd NPs on WO3 NRs (Figure 3A). Gas concentration tests on the PS/WO3–Pd60 sensor revealed a ppb level detection capacity at RT with a faster response time (Figure 3B). The catalytic activity of Pd NPs enhanced the NO2 molecule adsorption and thereby enhanced the sensor response, so a PS/WO3–Pd60 sensor having the highest amount of Pd NPs showed the highest sensor response at room temperature (Figure 3C). With a facile fabrication process and being compatible with the planar processes of the microelectronics industry, ultra-thin PdO films provided good sensing performances toward NO2 [85], but they require a long recovery period (600–700 s) because of the lack of immediate interaction between NO2 molecules and oxygen molecules adsorbed on sensor material surface. Also, ZnO nanostructured films obtained by a thermal evaporation method offered significantly enhanced response (622 at 100 ppm NO2) with good response-recovery at 200 °C [86]. The microwave-synthesized NiO film has been found to operate using ultra-low power of 0.2 μW at room temperature. It achieved a response of 4991% to 3 ppm NO2 along with fast response-recovery [87]. Moreover, a reasonable sensor response toward low concentration of NO2 was exhibited by the multicomponent oxide CuBi2O4 at 400°C [88]. Recently, Hung et al. synthesized three sensors of ZnO (Z2, Z4 and Z6) and Zn2SnO4 (ZS2, ZS4 and ZS6) NWs on microelectrode chips at 2, 4 and 6 cm from the thermal evaporation source, respectively [89]. It was found that the distance between the source and substrate strongly affected the gas response of the Zn2SnO4 NW sensors. Figure 3D,E show FESEM images of the on-chip grown ZnO (Z2) NW and Zn2SnO4 (ZS2) NW respectively.
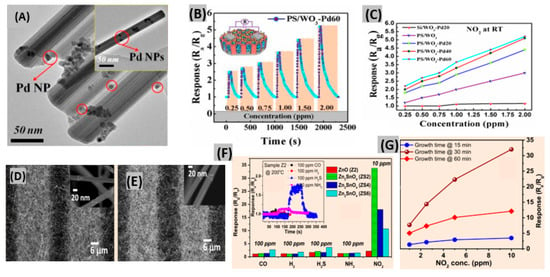
Figure 3.
(A) TEM images of PS/WO3–Pd60 (inset shows a single PS/WO3–Pd60 NR). (B) Sensor response of PS/WO3–Pd60 to different concentrations of NO2 at RT. (C) Response of Si/WO3–Pd20, PS/WO3, PS/WO3–Pd20, PS/WO3–Pd40 and PS/WO3–Pd60 sensors at different NO2 concentrations. Figures adapted with permission from [84], Copyright 2018 MDPI. (D,E) FESEM images of on-chip grown ZnO and Zn2SnO4 NWs. (F) Response comparison of Z2, ZS2, ZS4, ZS6 to 100 ppm CO, H2, H2S, NH3 and 10 ppm NO2. (G) Sensor responses of the devices with growth times of 15 min, 30 min and 60 min as a function of NO2 concentrations. Figures adapted with permission from [89], Copyright 2018 Elsevier.
The sensing performances of ZnO and Zn2SnO4 NW sensors to NO2 and other reducing gases are displayed in Figure 3F. Zn2SnO4 NW exhibited significantly better response towards NO2 gas in comparison to ZnO NW. Also, ZS2 showed higher response than ZS4 and ZS6, because placing the sensors far from the source resulted in several surface defects due to the lack of a Sn source. Responses for Zn2SnO4 NW sensors with growth times of 15, 30 and 60 min are shown in Figure 3G. It is revealed that comparatively high or low density of NWs decreases the gas response.
2.4. GaN-Based NO2 Sensors
Having a wide bandgap energy (3.4 eV), gallium nitride (GaN) is found to support higher peak internal electric fields than silicon or gallium arsenide (GaAs). This wide bandgap causes lower thermal electron-hole pair generation, hence allowing high working temperatures. GaN is less vulnerable to attack in caustic environments, and resistant to radiation because of the larger cohesion energies among its constituent atoms [90,91,92,93]. Bishop et al. proposed a double Schottky junction gas sensor based on BGaN/GaN [94]. Two devices were developed; first, 10 periods of 20 nm thick undoped GaN, and 20 nm thick BGaN formed the BGaN/GaN superlattice structure. Then a circular diode having 300 µm as diameter was made with a 200 µm spacing between two Pt contacts on the n-type GaN sample (Figure 4A). When the sensors were exposed to 450 ppm NO2 gas at different temperatures, BGaN/GaN SL sensor exhibited higher current change and sensitivity than GaN monolayer sensors (Figure 4B). This enhancement is caused by two main reasons: firstly, BGaN has more interface traps than GaN, which creates more adsorption sites at the interface for gas molecules resulting a greater SBH change. Secondly, BGaN shows columnar growth thus a decrease in the volume-to-area ratio at the interface that provides more interface traps within a given area. It was found that at higher temperatures and concentrations, saturation of the signal change leads to a nonlinear response for the BGaN/GaN SL resulting into a decrease in the responsivity of the device (Figure 4C). In another study, an AlGaN/GaN high electron mobility transistor (HEMT) with Pt functionalized gate demonstrated a high sensitivity of 38.5% toward 900 ppm NO2 at high operating temperature of 600 °C [95]. The fabricated heterostructure sensor exhibited robustness under severe environmental conditions with a very quick response time of 1 s. When sensors are integrated in chips, low power sensor operation is required. Lim et al. [96] made SnO2 sensitized AlGaN/GaN sensor operating at ultra-low power without using any heater. The fabricated sensor exhibited ppb level detection as well as fast response times.
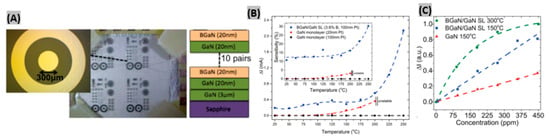
Figure 4.
(A) Fabricated BGaN/GaN SL and GaN devices. (B) Current change and Sensitivity (Inset) of BGaN/GaN SL and GaN sensors to 450 ppm NO2 at 5 V bias at different temperatures. (C) Sensor current changes vs. NO2 concentrations for various temperatures. Figures adapted with permission from [94], Copyright 2015 AIP Publishing LLC.
2.5. Organic Materials-Based NO2 Sensors
Conducting and semiconducting organic films are promising gas sensing materials due to their excellent ability of tuning the chemical and physical properties on exposure to gas molecules. Also, recognition groups can be integrated covalently on organic sensing materials in order to get high selectivity and response [97]. Organic field effect transistors (OFETs) and thin film transistors (TFTs) are two major forms of organic material-based sensors. Kumar et al. [98] synthesized an OFET to detect NO2 gas using gate bias as control unit. The active layer of the OFET was the polymer poly [N-90-heptadecanyl-2,7-carbazole-alt-5,5-(40,70-di-2-thienyl-20,10,30-benzothiadiazole] (PCDTBT). The electron removal of NO2 molecule from the p–type conducting polymer PCDTBT led to an increase of conductivity. The typical transfer and output characteristics of OFET sensor are shown in Figure 5A,B, respectively. From the attained transfer and output characteristics, the mobility (µsat) and threshold voltage (Vth) were obtained as 1.13 × 10−4 cm2 V−1 s−1 and −9 V. From the gas concentration test, as shown in Figure 5C, the response increases linearly up to 10 ppm of exposure and then the increasing trend drops at higher concentrations. This happens because most of the active adsorption sites of the active PCDTBT layer get populated by NO2 molecules. The response and recovery time of the sensor at 1 ppm of NO2 exposure were obtained as shown in the inset of Figure 5C. The selectivity of the sensor upon exposure 10 ppm of different toxic gases was studied. Figure 5D displays that the OFET sensor exhibits the highest selectivity towards NO2 gas. Although the H2S gas response was moderate, the recovery was incomplete. In another study, a 6,13-bis(triisopropylsilylethynyl)-pentacene (TIPS-pentacene) film-based NO2 sensor attained a sensitivity above 1000%/ppm along with quick response-recovery [99]. It was predicted that the high sensing performance is attributable to the effective charge transport on the top of low original carrier concentration. Huang et al. [100] fabricated TFTs using copper phthalocyanine (CuPc) for NO2 gas detection. The gate dielectric used here is a UV–ozone (UVO)-treated polymer. Figure 5E shows sensitivities of the TFT biased at VD = VG = −40 V toward different NO2 concentrations and UVO treatment times (tUVO). It is seen that the sensitivity enhances significantly for sensors with longer tUVO at all NO2 gas concentrations because of UVO-derived hydroxylated species on the dielectric surface. Gas selectivity tests revealed that without UVO treatment of the dielectric, the sensors are not at all selective to NO2 gas. However, at tUVO = 360 s, the sensitivity increased from 10% to almost 600% at a concentration of 20 ppm NO2 which is six times more sensitive than all other test gases (Figure 5F).
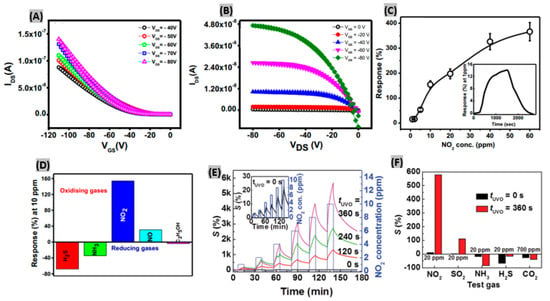
Figure 5.
(A) Transfer (B) Output characteristics of the PCDTBT based OFET. (C) Sensor response at different NO2 concentrations. Response-recovery of the sensor at 1 ppm of NO2 (inset). (D) Selectivity graph of the sensor towards various analytes. Figures adapted with permission from [98], Copyright 2018 Elsevier. (E) Sensitivity profile at different NO2 concentrations and with UVO treatment times (tUVO). Inset shows the sensitivity at tUVO = 0 s. (F) Sensitivities obtained for TFT sensors with tUVO = 0 and 360 s at 20 ppm NO2, SO2, NH3, H2S and 700 ppm CO2. Figures adapted with permission from [100], Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
2.6. Hybrid Materials-Based NO2 Sensors
In most cases hybrid materials combine and exhibit the useful characteristics of their constituent materials to promote high sensing performances. For instances, both MoS2 and RGO show good conductivity changes upon adsorption of NO2 molecules, and thus a combination of MoS2 and RGO results in high performance NO2 gas sensors [101]. Recently, Wang et al. synthesized a MoS2 nanoparticles-incorporated RGO hybrid material for NO2 detection by a two-step wet-chemical method [102]. In the first step, from powdered MoS2 NPs were prepared by a modified liquid exfoliation method. Next, self-assembly of MoS2 NPs and GO nanosheets, and hydrothermal treatment provided MoS2-RGO hybrid nanosheets. A high magnification SEM image of MoS2-RGO hybrids, shown in Figure 6A, reveals the presence of NPs on the RGO surface. It was found that the response time and recovery time decrease with increasing operating temperature while the sensor responses to NO2 remain almost unchanged. The optimum operating temperature was obtained as 160 °C. A response-recovery curve to 3 ppm NO2 gas at 160 °C is illustrated in Figure 6B. When the fabricated MoS2-RGO based sensor was exposed to NO2 gas concentrations ranging from 100 ppb to 3 ppm, the response followed an increasing trend due to the increased amount of NO2 molecules absorbed (Figure 6C). Wang et al. [103] synthesized a hybrid sensing material made of ZnO and poly(3-hexylthiophene) for the detection of NO2 at room temperature. The fabricated nanosheet-nanorod structured bilayer film sensor showed a sensitivity of 180% at 50 ppm of gas exposure.
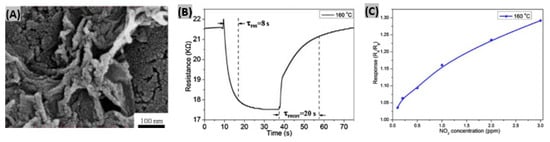
Figure 6.
(A) High magnification SEM images of the fabricated MoS2-RGO hybrids. (B) Response- recovery curve of MoS2-RGO sensor to 3 ppm NO2 at 160 °C. (C) Sensor responses to different NO2 concentrations at 160 °C. Figures adapted with permission from [102], Copyright 2017 Elsevier.
The sensing performance metrics like sensitivity/response, response and recovery times at certain gas concentration and operating temperatures, and sensitivity per ppm/response time ratio for different NO2 sensor materials and structures are summarized in Table 1. It provides a brief comparative performances outline among different NO2 sensor reported in recent years.

Table 1.
Gas sensing properties of recently developed NO2 gas sensors.
3. Recent Advances in SO2 Gas Detection
3.1. Carbon Material-Based SO2 Sensors
Aligned carbon nanotubes possess high surface-to-volume ratios which promote efficient physical and chemical adsorption of target gases [114]. Recently Zouaghi et al. have initiated research on vertically aligned carbon nanotube (VACNT)-based gas sensors interrogated by THz radiation [115]. They synthesized VACNT on SiO2 coated, boron-doped Si substrate by a water-assisted chemical vapor deposition method. Figure 7A shows a SEM image of vertically aligned CNT indicating a layer thickness of 95μm. The transmission spectrum upon SO2 gas exposure is illustrated in Figure 7B. The denser rotational spectrum of SO2 is attributed to the bent structure of SO2 molecule. The highest relative transmittance was obtained around 0.2 THz. When SO2 gas was flowed abruptly into a Si/SiO2/VACNT sensor, the maximum of transmitted electric field amplitude decreased to a steady value with fast response time of 2–3 min (Figure 7C). However, the recovery time was too long (>70 min). It has been predicted that the slow recovery was caused from the high sticking coefficient of SO2 gas to steel walls in the system. In a previous research, cholesteric-nematic mixture intercalated with CNT walls had been prepared and physical adsorption between the CNT and SO2 molecules was observed [116]. This adsorption phenomenon altered the CNT conductivity that in turn resulted into sensing signal for SO2. Zhang et al. synthesized TiO2/graphene film using layer-by-layer self-assembly technique for room temperature SO2 detection [117]. Excellent contacts between TiO2 and rGO are achieved from the wrapping of rGO flakes on TiO2 nanosphere surface or bridge-connection between TiO2 balls as shown in SEM image (Figure 7D). The sensor was exposed to 1, 50, 250, 1000 ppb SO2 gas to study the response-recovery behavior plotted in Figure 7E. It was observed that with increasing gas concentration, the sensor response kept increasing but the response-recovery time became longer. It has been predicted that the large interspace is responsible for the increase of response and recovery time. The TiO2/rGO film sensor showed much higher sensitivity to 1 ppm SO2 gas at room temperature than other target gases such as-—CH4, C2H2, H2, CO, NO2 (Figure 7F). So, the synthesized sensor was selective enough to SO2 gas.
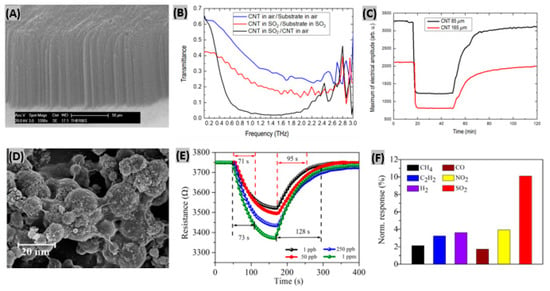
Figure 7.
(A) SEM image of a VACNT layer on Si/SiO2 substrate. (B) Normalized transmittance spectra of Si/SiO2/VACNT in air and SO2. (C) Maximum of electrical amplitude vs. time response in transmission measurements with 85 um and 165 um thick layers of VACNT. Figures adapted with permission from [114], Copyright 2018 Lietuvos mokslų akademija. (D) SEM image of self-assembled TiO2/rGO film. (E) Response/recovery curves of TiO2/rGO sensor exposed to four different concentrations of SO2 gas. (F) Normalized response of TiO2/rGO sensor toward 1 ppm of various gases at room temperature. Figures adapted with permission from [116], Copyright 2017 Elsevier.
3.2. Metal and Metal-Oxide Nanostructures-Based SO2 Sensors
Many attempts had been made for SO2 gas detection using various semi-conducting metal oxides, such as- CeO2, WO3, V2O5-TiO2, MoO3-SnO2 and NiO [118]. However, due to instability in the highly reducing atmospheres, these sensors can only operate at low temperature (<500 °C) [119]. Liu et al. fabricated ZnO nanosheets decorated with Ru/Al2O3 catalyst and integrated them with a microsensor to detect SO2 gas [120]. Inkjet printing technology was used to load the sensor. AFM image in Figure 8A reveals the uniformity of the prepared ZnO 2D nanosheet and the thickness is indicated as about 1.5 nm. Different concentrations of SO2 gas had been exposed to Ru/Al2O3/ZnO sensor and the corresponding resistance responses are shown in Figure 8B. It is seen that resistance notably decreased at SO2 exposure and percent sensor response increased linearly with SO2 concentration. At 25 ppm of SO2, the obtained response and recovery times were about 1 min and 6 min, respectively. The SO2 selectivity test is displayed in Figure 8C, where the fabricated sensor responded negligibly to the test gases CO, CH3OH, C2H5OH, acetone, CO2, NO and HCHO in comparison to SO2 gas. From on-line mass spectrometry experiments, it was found that the catalyst Ru/Al2O3 dissociates SO2 molecules into easily detectable SO• species. Being captured by ZnO nanosheet, these species contribute to the sensor output signal. In another study, Ciftyürek et al. prepared and then evaluated molybdenum and tungsten binary and ternary oxide thick films for gas sulfur species sensing [121]. It was found that hydrothermally synthesized nano-SrMoO4 exhibited the highest sensor response among those fabricated oxide films. The SrMoO4-based sensors were able to operate at very high temperature (>600 °C) while maintaining their sensing performances, and thus can be useful in gas monitoring at industries. SnO2 thin film had been prepared by Tyagi et al. [122] using sputtering technique. Then, the film was functionalized with various metal oxide catalyst such as- PdO, CuO, NiO, MgO, V2O5 to make SO2 gas sensor. The uniform distribution of NiO nanoclusters on the surface of SnO2 film is noticed in the SEM image (Figure 8D). 500 ppm of SO2 gas was exposed to different metal-oxides deposited on SnO2 sensors to study the response at various operating temperatures (Figure 8E). NiO/SnO2 structure showed the highest response (∼56) at 180 °C due to two main reasons. Firstly, the spill-over effect from NiO nanoclusters toward SO2 molecules. Secondly, increase of adsorbed oxygen species sites at the porous and rough surface of SnO2 film [123]. The response and recovery time of NiO/SnO2 sensor were estimated as 80 s and 70 s respectively towards 500 ppm of SO2 gas at 180 °C as shown in Figure 8F. Also, the sensor exhibited good reproducibility and selectivity under SO2 exposure. In another study, it had been reported that BiFeO3 is highly selective to SO2 against carbon monoxide and butane [124]. Also, it was found that BiFeO3 synthesized by a sonochemical method provides better sensing performances than when prepared by conventional methods.
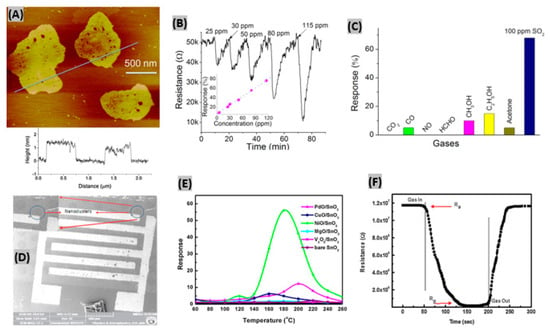
Figure 8.
(A) Two-dimensional AFM image of ZnO nanosheets, height profile of two ZnO nanosheets as marked is shown below. (B) Resistance changes to SO2 gas at different concentrations, inset shows plotting of sensing response vs. SO2 concentration (C) Selectivity test of Ru/Al2O3/ZnO sensor to various gases under same concentration. Figures adapted with permission from [119], Copyright 2018 Elsevier. (D) SEM image of NiO/SnO2 sensor structure, inset displays the presence of NiO nanoclusters. (E) Sensor response of NiO/SnO2, PdO/SnO2, CuO/SnO2, MgO/SnO2, V2O5/SnO2 and bare SnO2 structures with operating temperatures towards 500 ppm of SO2 gas. (F) SO2 gas response-recovery illustration for NiO/SnO2 sensor. Figures adapted with permission from [121], Copyright 2015 Elsevier.
3.3. GaN-Based SO2 Sensors
AlGaN/GaN heterostructure semiconductors facilitate low power consumption, miniaturization and excellent sensing performances [125]. Also, AlGaN/GaN-based sensors can operate in chemically harsh environments, at high temperatures and under radiation fluxes due to having thermally and chemically stable structures [126]. Triet et al. synthesized Al0.27Ga0.73N/GaN-based Schottky diode sensors for SO2 gas detection [127]. Vertical zinc oxide nanorods (ZnO NRs) and a RGO nanosheet hybrid was formed on a AlGaN/GaN/sapphire heterostructure where the RGO and AlGaN surface made a Schottky contact with each other. From the FE-SEM image in Figure 9A, it is observed that neighboring ZnO NRs are attached to each other by RGO. During the gas exposure, the Schottky barrier between RGO and AlGaN layers changes. As a result, thermionic emission carrier transport is altered which in turn modifies the reverse saturation current. In the case of detecting SO2 (Figure 9B), the resistance response increased with increasing gas concentration because SO2 molecules are electron withdrawers. The non-linearity of the response with gas concentration is attributed to incomplete recovery of the sensing material RGO-ZnO NRs (Figure 9C). Here, SO2 gas molecules react with interaction sites resulting into slow diffusion of gas molecules within the RGO multilayer structure [128].
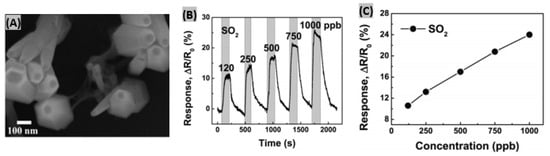
Figure 9.
(A) Top-view of FE-SEM high magnification image of the RGO nanosheets connecting to ZnO NRs on AlGaN/GaN heterostructure. (B) Sensor resistance variations at the exposure to SO2 gas of different concentrations. (C) Response vs. gas concentration relationship under SO2 gas exposure. Figures adapted with permission from [126], Copyright 2017 American Chemical Society.
3.4. Solid Electrolyte-Based SO2 Sensors
Different solid electrolytes such as- NASICON [129], YSZ [130] and alkali metal sulfates [131] have been exploited during the past decades to fabricate high performance SO2 sensors. Among all the solid electrolytes, NASICON is widely used in the mixed-potential sensors due to its high ionic conductivity. Ma et al. [132] reported a mixed-potential gas sensor using NASICON and orthoferrite (La0.5Sm0.5FeO3) as sensing electrode. The SEM image of powdered La0.5Sm0.5FeO3 having a perovskite crystal structure reveals the uniformity of size and porosity (Figure 10A). La3+ doping level had been varied to study the variation of sensing performances. The highest response (−86.5 mV) was obtained for sensor with La0.5Sm0.5FeO3 as sensing electrode to 1 ppm SO2 (Figure 10B). The response order was found as ΔV(La0.5) > ΔV(La0.4) > ΔV(La0.6) > ΔV(La0.8) > ΔV (La0.2). The porous structure and electrocatalytic property are possibly responsible for the variation of responses. Responses were recorded at different operating temperatures. The equity between the amount of adhering gas and the activation energy demand indicated 275 °C as the optimum operating temperature with the highest response. The prepared mixed-potential sensor was exposed to other test gases such as- NO2, Cl2, NH3, CO, NO, acetone, H2, CH4, ethanol and methanol for a gas selectivity test. The sensor remained selective enough to detect SO2 gas even in very low amounts as illustrated in Figure 10C. In another study, a zirconia-based solid state electrochemical SO2 sensor had been demonstrated with MnNb2O6 as sensing electrode [133]. Under very high operating temperature (700 °C), the sensor attained good sensitivity along with rapid and stable response-recovery of gas molecules.
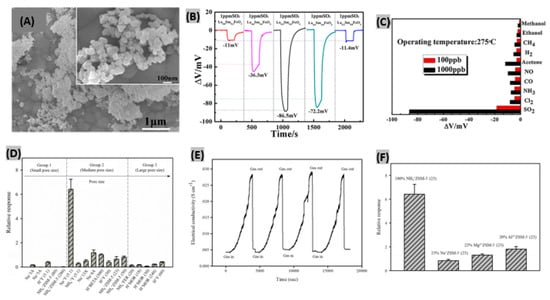
Figure 10.
(A) SEM image of powdered La0.5Sm0.5FeO3, inset shows higher magnified image. (B) The voltage change response of the gas sensor made with LaxSm1−xFeO3 sensing electrodes (x = 0.2, 0.4, 0.5, 0.6, 0.8). (C) The selectivity test for the sensor using La0.5Sm0.5FeO3 as sensing electrode to various gases at 275 °C. Figures adapted with permission from [131], Copyright 2017 Elsevier. (D) Comparative responses of different pristine zeolites. (E) Sensor response of NH4+ZSM-5 (23) exposed to 4200 ppm SO2 for four cycles. (F) Comparative responses of different ion-exchanged ZSM-5 (23) zeolite sensors. Figures adapted with permission from [134], Copyright 2018 Springer-Verlag GmbH Germany, part of Springer Nature.
3.5. Zeolite-Based SO2 Sensors
Zeolites are aluminosilicates possessing immensely porous crystal structure, high specific surface area, high chemical and thermal stability, good adsorption properties, alterable chemical composition, presence of mobile ions, ability to undergo ion-exchange process and variable hydrophobic or hydrophilic features [134,135]. These characteristics make zeolites very attractive for gas detection. Choeichom et al. studied the effects of zeolite type, cation type and Si/Al ratio within various zeolites when exposed to SO2 gas [136]. During the exposure to 4200 ppm SO2, pristine zeolites exhibited the different sensor responses plotted in Figure 10D. It was found that the relative response of each pristine zeolite type showed the following decreasing order: ZSM-5 > beta > 13X > Y > 4A > ferrierite > mordenite > 5A > 3A. The three key factors contributing to the variation of these zeolite responses are pore size, cation type and Si/Al ratio. It was observed that the relative response increases with increasing zeolite pore size, however, decreases with a too large pore size. Among the monovalent cation zeolites focused here, the NH4+ zeolite response was the highest because of formation of hydrogen bonds with more than one SO2 molecule. With decreasing of Si/Al ratio, the responses kept increasing. The combined effect of the above discussed factors contributed to NH4+ZSM-5 (23) achieving the highest relative response toward SO2 with 23 as Si/Al ratio and medium pore size. Recovery and repeatability assessments were performed by flowing 4200 ppm SO2 for four cycles as illustrated in Figure 10E. The sensor conductivity returned to its initial value after SO2 was removed and again produced the same response to SO2 in the subsequent cycles. These results indicate the complete recovery and strong repeatability of the zeolite sensor. The sensor responses of various ion-exchanged ZSM-5 (23) towards 4200 ppm SO2 had been investigated as well (Figure 10F). It was found that Al3+ZSM-5 (23) provides the highest relative response due to two key factors: firstly, the magnitude of the ion-dipole attraction increases with the increasing ionic charge. Al3+ having higher ionic charge than Mg2+ and Na+, promotes a higher degree of interaction with SO2 molecules which in turn results in a higher sensor response. Secondly, the higher electronegativity of Al3+ZSM-5 (23) governs the stronger cation-dipole interaction with SO2 and thus facilitates a higher sensor response. Recently, a zinc-doped zeolitic imidazolate framework (ZIF-67) attached with CNT has been reported as SO2 sensing material [137]. It provided notable sensing performances at room temperature due to its porous polyhedral structure of metal particles with numerous interlinked CNTs. Previously, conductive polymer/zeolite composite based SO2 detection had been studied [138]. It was observed that PEDOT-PSS/KY zeolite composite achieved the highest sensor response having gas adsorption–desorption dependence on the cation types of Y zeolite.
3.6. Paper-Based SO2 Sensors
Sensing materials incorporated onto paper offer color transition sensing with the eyes, whereby measurement systems and electric circuits are not needed [139]. Paper-based analytical devices (PADs) provide the advantages of ease of production, low cost, flexibility, efficient sample collection, and easy disposability [140]. Li et al. coupled headspace sampling (HS) with PAD in order to detect SO2 through surface-enhanced Raman scattering (SERS) [141]. Hybrids consisting of 4-mercapto-pyridine (Mpy)-modified gold nanorods (GNRs) and reduced graphene oxide (rGO) were prepared. Then along with anhydrous methanol and starch iodine complex, the rGO/MPy-GNRs hybrids were immobilized upon cellulose-based filter papers using a vacuum filtration method. This process promotes the formation of a dense blue colored film on the filter paper as shown in the SEM images (Figure 11A–C). Uniform cellulose fibers of 12.5 μm width adopt wrinkle-like structures because of the attachment with rGO (Figure 11B). On exposing the fabricated rGO/MPy-GNRs/SIC paper to SO2, the blue color faded within minutes as illustrated in Figure 11C. It was found that the intermolecular charge-transfer complex between starch and iodine produces a broad band at 600 nm as indicated by curve d of Figure 11D.
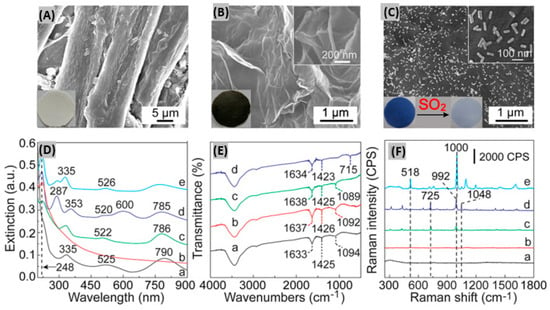
Figure 11.
SEM images of (A) pure cellulose paper, (B) after assembling with rGO and (C) rGO/MPy-GNRs/SIC paper. Insets of figures (A–C) show pictures of the corresponding paper substrate under light. (D) UV-vis-NIR extinction spectra of (a) MPy-GNRs paper, (b) rGO paper, (c) rGO/MPy-GNRs paper, rGO/MPy-GNRs/SIC paper (d) before and (e) after the adsorption of SO2, respectively. (E) FT-IR spectra of (a) MPy-GNRs, (b) rGO/MPy-GNRs, rGO/MPy-GNRs/SIC (c) before and (d) after the adsorption of SO2, respectively. (F) SERS spectra of (a) the pure cellulose paper, (b) the rGO paper, (c) the MPy-GNRs paper, rGO/MPy-GNRs/SIC paper (d) before and (e) after adsorption of SO2, respectively. Figures adapted with permission from [140], Copyright 2018 American Chemical Society.
The IR spectrum of rGO/MPy-GNRs/SIC is displayed in Figure 11E indicating the modification in the response after SO2 exposure. Along with the distinct and typical peaks for MPy, SO2 adsorption introduces a new peak having increased intensity in the SERS spectra of rGO/MPy-GNRs/SIC as shown by curve e in Figure 11F. This additional peak occurs because SO2 possibly affects the bending vibration of pyridine, and the characteristic peaks of SO2-pyridine complex are reflected in the bands. Recently, an amino-functionalized luminescent MOF material (MOF-5-NH2) was incorporated onto test paper for portable SO2 sensing [142]. It was seen that the prepared luminescent paper got lightened upon SO2 gas exposure with high selectivity. Also, it detected as low as 0.05 ppm SO2 having reusability advantages. In another research work, a microfluidic paper-based integrated detection system had been reported to monitor SO2 concentrations using RGB color analysis software [143]. The sensing performance metrics like sensitivity/response, response and recovery times at certain gas concentration and operating temperatures, and sensitivity per ppm/response time ratio for different SO2 sensor materials and structures have been summarized in Table 2. It provides a brief comparative performances outline among different SO2 sensor reported in recent years.

Table 2.
Gas sensing properties of recently developed SO2 gas sensors.
4. Recent Advances in H2S Gas Detection
4.1. Carbon Material-Based H2S Sensors
In recent years, many research efforts have been made for H2S detection using graphene, reduced graphene oxide and carbon nanofibers. Ovsianytskyi et al. [152] proposed a graphene-based H2S gas sensor functionalized with Ag nanoparticles (Ag NPs) and charged impurities. Graphene was grown by the CVD technique, and then Ag NPs and impurities were incorporated on the graphene by a wet chemical method. The SEM image of graphene after immersing into AgNO3/Fe(NO3)3 solution reveals the presence of large number of nanoparticles (10–100 nm) uniformly distributed on its surface (Figure 12A). The comparative responses obtained on exposing 500 ppb of H2S gas for 400 s to pristine graphene, graphene doped with Fe(NO3)3 solution, graphene doped with AgNO3 solution, and graphene doped with a mixed AgNO3/Fe(NO3)3 solution are displayed in Figure 12B. Graphene doped with the mixed solution exhibited the highest response. Since Ag is less electronegative than graphene, adsorption of H2S occurs because of its interaction with the adsorbed oxygen species on Ag mostly. Then, electrons released from dissociation of H2S are accumulated in graphene. This phenomenon causes a decrease in graphene hole concentration, and thus resistance of Ag-doped graphene increases.
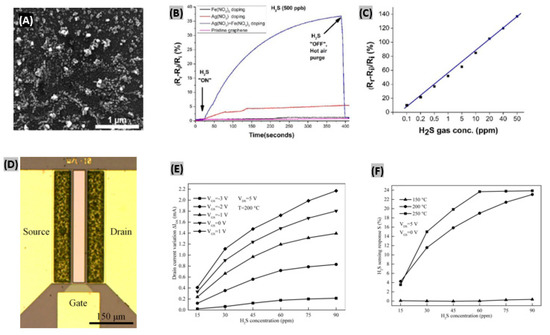
Figure 12.
(A) SEM image of graphene after immersion into AgNO3/Fe(NO3)3 solution. (B) Comparative sensor responses of pristine graphene, graphene doped with Fe(NO3)3 solution, graphene doped with AgNO3 solution, and graphene doped with mixed Fe(NO3)3 and AgNO3 solution. (C) Relationship between H2S gas concentrations and corresponding relative responses of the fabricated sensor. Figures adapted with permission from [152], Copyright 2017 Elsevier. (D) Top view of the optical micrograph of the fabricated Pt-AlGaN/GaN HEMT sensor. (E) Changes in drain current with different concentrations of H2S gas and gate biases at T = 200 °C, VDS = 5 V. (F) Sensitivity of the fabricated sensor toward H2S at different temperatures. Figures adapted with permission from [155], Copyright 2018 Elsevier.
The relationship between gas concentrations and corresponding relative responses of the synthesized sensor is quite linear, as plotted in Figure 12C. Also, the sensor was strongly selective to H2S gas against CH4, CO2, N2, and O2 gases. Similarly, Chu et al. [153] obtained a sensitivity of 34.31% toward 100 ppm H2S with tin oxide-modified reduced graphene oxide (SnO2-rGO) at 125 °C. In another study, Zhang et al. [154] developed a stable sensor using ZnO-carbon nanofibers (30.34 wt% carbon) that exhibited good H2S sensing performances. It was found that the protection of carbon provides high stability of ZnO and oxygen vacancies to allow improved sensor responses.
4.2. GaN-Based H2S Sensors
Several AlGaN/GaN-based gas sensors have been reported, including NO, NO2, NH3, Cl2, CO, CO2 and CH4 [156,157,158]. However, H2S sensing using wide bandgap semiconductors like GaN have not been explored yet that much. In order to sense H2S gas even at very low amounts, Sokolovskij et al. [155] synthesized AlGaN/GaN HEMT-based sensor with platinum as gate. The top view optical micrograph of the synthesized device shown in Figure 12D reveals the gate dimensions, gate-source and gate-drain spacing. For high temperature operations, each device was wire bonded to ceramic substrates. The variation of drain current was observed under different H2S concentrations and gate bias voltages (Figure 12E).
Because of the increasing baseline current with increasing gate bias, variation of the drain current was highly influenced. Also, the fabricated HEMT sensor operates in a wide range of biasing conditions without degrading the sensing performances and thus shows an excellent stability. It was found that when gate bias approaches pinch-off state, it minimizes power consumption and thus enables the sensor to operate at high response mode. The sensitivity of AlGaN/GaN HEMT sensor clearly increases with higher temperatures as plotted in Figure 12F. The rise and fall time were estimated 219 s and 507 s, respectively, at 250 °C. At lower temperatures, rise and fall times have gone higher. Further, Zhang et al. [159] pre-treated the Pt-gated AlGaN/GaN HEMT sensor with H2 pulses in dry air ambient at 250 °C. This treatment facilitated the enlargement of the H2S detection range up to 90 ppm.
4.3. Metal and Metal Oxide-Based H2S Sensors
4.3.1. Nanostructured Metal Oxide-Based Sensors
It was found that metal oxides such as- SnO2, WO3, ZnO, and α-Fe2O3 based sensors exhibited superior sensing performances toward H2S due to their stable nanostructures [160,161,162]. Zhang et al. [163] reported a α-Fe2O3 nanosheet-based H2S gas sensor using a solvothermal method. Figure 13A shows the SEM image of a sample obtained at reaction temperature of 160 °C denoted as S160. It was observed that at low temperatures, the morphology of the samples is not uniform. Since both α-Fe2O3 and Fe3O4 exist in the nanostructure, uniform morphology can’t be obtained under the reaction temperature of 160 °C. It was seen that sensor response of α-Fe2O3 to H2S decreases with the increasing working temperature. However, the recovery time is too long at low temperature, so taking sensor response, response time and recovery time into account, 135 °C was estimated as the optimum working temperature. Figure 13B displays the response of the prepared sensor to different concentrations of H2S ranging from 1 to 50 ppm at 135 °C. The response and recovery time were estimated to be less than 10 s and 45 s, respectively, indicating very a rapid response in comparison to other H2S gas sensors. The changes in the electric resistances were found negligible for the sensor to 50 ppm acetone, ethanol, methanol and H2 gases at 135 °C. On the contrary, the sensor response was very large to H2S under same conditions, thus reflecting excellent selectivity of the α-Fe2O3 nanosheet-based H2S sensor (Figure 13C).
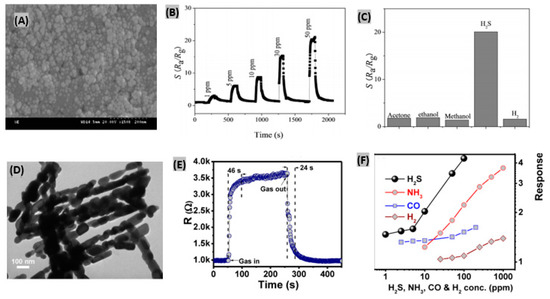
Figure 13.
(A) SEM image of α-Fe2O3 nanosheets sample obtained at reaction temperature of 160 °C denoted as S160. (B) Sensor responses of α-Fe2O3 nanosheets to various H2S concentrations at 135 °C. (C) Sensor responses of the synthesized sensor to 50 ppm of various gases at 135 °C. Figures adapted with permission from [163], Copyright 2018 Elsevier. (D) TEM image of the fabricated mesoporous Co3O4 nanochains. (E) Sensor response–recovery curve at 100 ppm of H2S gas at 300 °C. (F) Sensing response of the prepared Co3O4 sensor to various gases at 300 °C. Figures adapted with permission from [169], Copyright 2018 Elsevier.
In another study, Li et al. [164] developed ZnO/CuO nanotube arrays to sense H2S at low-working temperatures. It was observed that the nanotube structures promoted the diffusion and adsorption of gas with many active sites between H2S molecules and adsorbed oxygen molecules. Thus, they contributed to achieve good sensitivity along with fast response time. It was found that porous In2O3 nanoparticles provide large surface areas and pore volumes which create numerous active sites to produce active oxygen species [165]. These sites facilitate a significant improvement in H2S gas sensing with 1 ppb of detection limit. Also, a dense array of intrinsic ZnO NWs has been reported for H2S detection by exploiting a sulfuration–desulfuration reaction mechanism [166]. In another work, Eom et al. [167] fabricated Cux(x=1,2)O:SnO2 thin films to detect H2S gas at room temperature. Enhanced sensitivity with rapid response-recovery was obtained due to enhanced adsorption sites arising from abounding domains of p-n heterojunctions on the CuxO:SnO2 film surfaces. Besides, a Cu-doped BaSrTiO3-based H2S sensor was reported [168]. Herein along with gas-surface interaction, the role of pre-adsorbed oxygen species and surface dipolar hydroxyl groups has been investigated as well.
4.3.2. Mesoporous Metal Oxide-Based Sensors
Generally mesoporous materials contain pores with diameters of 2–50 nm. Mesoporous metal-oxides offer efficient gas detection because they have large surface areas, open porosity, small pore sizes, and the ability to coat the surface of the mesoporous structure with one or more compounds. Quang et al. [169] reported a mesoporous Co3O4 nanochains-based H2S sensor. At first cobalt carbonate hydroxide (Co (CO3)0.5(OH)·11H2O) nanowires were synthesized using a hydrothermal route. Then heat treatment was applied in air at 600 °C for 5 h to form rough-surfaced mesoporous Co3O4 nanochains as shown by the TEM image in Figure 13D. From the gas responses vs. working temperatures analysis, the optimum working temperature was obtained as 300 °C. At lower operating temperatures than the optimum, Co3O4 nanochains displayed sluggish chemical activity causing weak responses. Moreover, at higher working temperatures, adsorbed H2S molecules start escaping from the Co3O4 nanochain surface because of increased activation. As a result, the sensor response starts decreasing. The fabricated sensor exhibited quick responses and recovery to 1–100 ppm H2S at 300 °C. At 100 ppm H2S, response and recovery times were estimated as 46 s and 24 s, respectively, as illustrated in Figure 13E. The nanochain structure provides a high specific surface area, narrow pore size and rich mesopores which make the fabricated sensor more suitable for H2S sensing than other toxic gases. The comparative responses of Co3O4 nanochains toward H2S and other target gases are plotted in Figure 13F indicate strong selectivity for H2S. Previously, Stanoiu et al. [170] prepared a mesoporous SnO2-CuWO4-based cost-effective H2S sensor having high sensitivity at low working temperature. In addition, a short temperature trigger of 500 °C was applied to enhance the recovery operation of the fabricated sensor.
4.3.3. Metal Oxide Microsphere-Based Sensors
Typically, microspheres are small spherical particles having diameters in the micrometer range. Hu et al. [171] reported CuFe2O4 nanoparticles-decorated CuO microspheres-based H2S gas sensors. The synthesized CuO/CuFe2O4 heterostructures provided a porous and rough surface due to the arbitrary deposition of nanoparticles as displayed by the FE-SEM image in Figure 14A. When the temperature is low, the response becomes low because of the weak chemical interaction between the gas molecules and adsorbed oxygen species. At higher working temperatures, the mentioned chemical interaction is strong and the responses keep increasing, but gas molecule diffusion becomes slower than the surface interaction causing a decrease of the response again, as illustrated in Figure 14B. The optimal operating temperature was estimated as 240 °C. The dependence of responses of the sensor on H2S concentrations is plotted in Figure 14C which exhibits a gradual increasing trend. The response and recovery time of the fabricated CuO/CuFe2O4 sensor were obtained as 31 s and 40 s, respectively, at the optimal operating temperature (240 °C) with good reproducibility and selectivity toward H2S gas. In another study, Li et al. [172] prepared SiO2@TiO2 microspheres and then formed Cd2+-doped TiO2 shell-modified ITO electrodes for H2S detection. Exploiting the mismatch of energy band levels between TiO2 shells and induced CdS nanoparticles, this device provided good sensing performances.
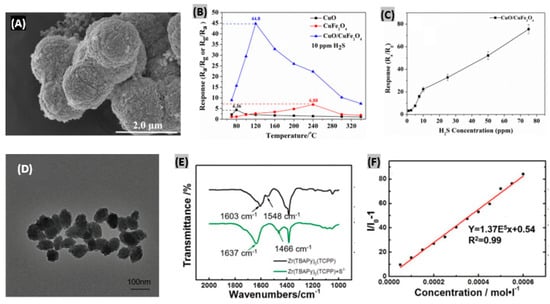
Figure 14.
(A) FE-SEM image of synthesized CuO/CuFe2O4 heterostructure. (B) Variation of gas sensing responses of CuO microspheres, CuFe2O4 nanoparticles and CuO/CuFe2O4 heterostructure with working temperatures. (C) H2S concentrations dependent sensing responses of CuO/CuFe2O4 sensor at 240 °C. Figures adapted with permission from [171], Copyright 2018 Elsevier. (D) TEM image of the synthesized Zr(TBAPy)5(TCPP). (E) FTIR spectra of Zr(TBAPy)5(TCPP) before and after H2S exposure. (F) Fluorescent enhancement response of the fabricated sensor under increasing S2− concentration. Figures adapted with permission from [174], Copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
4.4. MOF-Based H2S Sensors
Metal organic frameworks (MOFs) offer highly selective and sensitive detection of H2S because of possessing chemical stability, custom tuning of porosity and functionalities, and various pre- or post-synthetic modifications to the structural framework [173]. Guo et al. [174] synthesized a MOF material named as Zr(TBAPy)5(TCPP) using a solvothermal method, where Zr is the metal center, and 1,3,6,8-tetra(4-carboxylphenyl) pyrene (TBAPy) and tetrakis(4-carboxyphenyl) porphyrin (TCPP) act as double linkers. The prepared Zr(TBAPy)5(TCPP) exhibited well-shaped shuttle structures with a particle size of about 100 nm as seen from the transmission electron microscope (TEM) image (Figure 14D). However, Zr-MOF NU-1000 (synthesized for comparison) exhibited an irregular structure indicating the structural effect of TCPP on the synthesized materials. The FTIR spectrum of Zr(TBAPy)5(TCPP) is displayed in Figure 14E. There is a clear shift in the N-H and C=N peak on the addition of S2− due to the attachment between S and N in the materials. Fluorescence enhancement of the fabricated Zr(TBAPy)5(TCPP) sensor provides a linear trend with the increase of S2− concentration as plotted in Figure 14F. The interference effects of other anions such as- SO42−, CNS−, COOH−, Br−, I−, IO3−, F−, HSO3−, Cl− and NO3− was investigated and the results confirm that Zr(TBAPy)5(TCPP) is highly selective for S2− sensing. In another study, Dong et al. [175] developed a ZIF-67-derived porous dodecahedra Co3O4 sensor showing enhanced linear trend with H2S concentration. The significant improvement in the overall sensing performances is attributed to a high specific surface and the exposed {110} lattice planes of the fabricated structure.
4.5. Organic Materials-Based H2S Sensors
In recent years, several attempts have been made for H2S sensing using organic semiconducting films and polymers. Different types of interactions like crosslinking, doping, grafting and scissioning between electrons and organic materials take place in subject to energy as well as the dose of incident electron beam [176] which help to attain high selectivity and sensitivity to target gas. Chaudhary et al. reported a polyaniline-silver (PANI-Ag) nanocomposite film-based H2S sensor [177]. After protonation of aniline monomers, photopolymerization of aniline on a bi-axially oriented polyethylene terephthalate (BOPET) sheet was performed. The prepared PANI-Ag films were irradiated by a 10 MeV electron beam. As the dose was increased, the nanofiber diameter increased and a 30 kGy dose promoted an interconnected microstructure with larger sized Ag particles. At a very high dose of 100 kGy, Ag clusters submerged inside the polymer matrix with denser structure. The bright spots observed in SEM image as shown in Figure 15A reveal that Ag nanoparticles are incorporated in the PANI matrix. After EB irradiation, Ohmic nature was retained as seen from the linear I-V relationship displayed in Figure 15B. The electrical conductivity kept increasing with the dose to achieve the highest value at 30 kGy and then started going down. The percent sensor responses under different H2S concentrations and irradiation doses are plotted in Figure 15C. Since lower irradiation doses cause higher conductivity changes, the corresponding sensing responses become lower upon H2S exposure. On the contrary, higher doses cause lower electrical conductivity due to crosslinking-induced structural defects, so, the corresponding sensing responses to H2S become larger. Abu-Hani et al. [178] engineered the conductivity of chitosan (CS) film to obtain a highly sensitive and selective sensor toward H2S gas. Glycerol ionic liquid (IL) had been incorporated to tune the conductivity of the CS film. It was able to operate at lower temperature and provided rapid response-recovery with low-power consumption. In another study, Cu2+-doped SnO2 nanograin/polypyrrole nanospheres-based H2S gas detection was reported [179]. The enhanced sensing performance of the fabricated organic-inorganic nanohybrids is mainly attributed to improved surface potential barrier by surface defects tailoring, and numerous reaction sites to accelerate gas diffusion and adsorption.
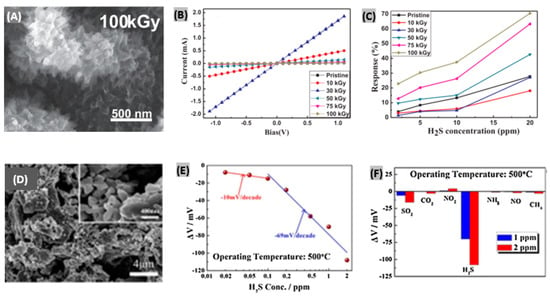
Figure 15.
(A) FE-SEM image of PANI-Ag film irradiated with EB at 100kGy dose. (B) Current-Voltage relationship of PANI-Ag film at pristine and EB irradiated condition. (C) Percent sensor responses under different H2S concentrations and irradiation doses. Figures adapted with permission from [177], Copyright 2018 Elsevier. (D) SEM image of LNO-1 sensing electrode (La2NiO4). (E) Electrode potential difference as a function of the logarithm of H2S concentrations (0.02–2 ppm) at 500 °C. (F) Selectivity evaluation of the fabricated sensor at 1 and 2 ppm of every test gas. Figures adapted with permission from [180], Copyright 2017 Elsevier.
4.6. Solid Electrolytes-Based H2S Sensors
The mixed potential type sensor requires a solid electrolyte having the ability to transfer oxygen ions between reference electrode and sensing electrode. Hao et al. [180] prepared a mixed-potential H2S gas sensor using YSZ as solid electrolyte and La2NiO4 as sensing electrode. The microstructure of the sensing material La2NiO4 was varied with three equivalents of citric acid and total metal irons which were 0.5:1 (LNO-0.5), 1:1 (LNO-1) and 1:2 (LNO-2), respectively. The SEM image of LNO-1 sensing material (La2NiO4 powders) reveals the porous structure and it has the largest pore size among the three samples as displayed in Figure 15D. Also, it was found that LNO-1 possesses the highest BET surface area and pore volume. On increasing the H2S exposure concentration, the electrode potential difference exhibited linear changes with the logarithm of H2S concentration at 500 °C as plotted in Figure 15E. The sensitivity of the sensor to H2S was changed to −10 mV/decade from −69 mV/decade. The recovery time was improved by applying a temperature pulse of 700 °C. For 500 ppb of H2S exposure, it decreased from 20 min to 150 s. The fabricated sensor was proved to be highly selective toward H2S compared to other target gases as observed from the responses toward 1 and 2 ppm of every test gas (Figure 15F). Moreover, it was found quite stable in long term performance with lower detection limit. In another study, Yang et al. [181] used Nafion as a proton exchange membrane to demonstrate H2S sensing with the help of a sensing electrode. The electrode was made of Pt-Rh nanoparticles loaded on carbon fibers. The sensitivity was obtained 0.191 μA/ppm from the linear plot between sensor current changes and corresponding H2S concentrations. The fabricated sensor was highly selective toward H2S at room temperature with a fast recovery time (16 s) under 50 ppm of gas exposure. Recently, a promising TMD material, WS2 has been utilized to detect H2S with high sensitivity and selectivity [182]. It was observed that oxygen doping in the sulfur sites of the WS2 lattice promotes enhanced sensing performances towards H2S. Earlier, the adsorption properties of WS2 had been analyzed toward various target gas molecules along with its Fermi level pinning [183]. The sensing performance metrics like sensitivity/response, response and recovery times at certain gas concentration and operating temperatures, and sensitivity per ppm/response time ratio for different H2S sensor materials and structures have been summarized in Table 3. It provides a brief comparative performances outline among different H2S sensors reported in recent years.

Table 3.
Gas sensing properties of recently developed H2S gas sensors.
A novel metric, sensitivity per ppm/response time ratio has been calculated for each sensor in order to compare the overall sensing performance on the same reference. The higher value of the calculated ratio indicates the better overall sensor performance. Average ratios have been obtained by taking the recently reported gas sensors into account for the highly focused sensing materials as illustrated in Figure 16. It is found that hybrid materials-based sensors exhibit the highest average ratio for NO2 gas sensing, whereas GaN and Metal-oxide based sensors possess the highest ratio for SO2 and H2S gas sensing respectively.
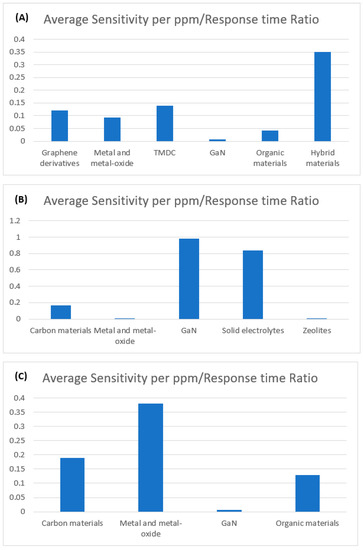
Figure 16.
Average sensitivity per ppm/response time ratio comparison among various sensing materials reported in recent years for (A) NO2 (B) SO2 and (C) H2S gas sensors.
5. Recent Density-Functional Theory (DFT) Study of Gas Molecule-Sensor Interaction
Numerous efforts have been made to investigate the adsorption properties of various sensing materials toward different toxic gases including NO2, SO2 and H2S by first-principle method calculations using density functional theory (DFT) in recent years as shown in Table 4.

Table 4.
The adsorption energy (eV), Shortest adsorption distance (Å) and charge transfer (e) between NO2, SO2, H2S and various sensing materials at the most stable adsorption configuration based on DFT.
For instance, Chen et al. [202] reported that NO2 and SO2 adsorptions show chemisorption character on boron-doped arsenene whereas physisorption character on pristine and nitrogen-doped arsenene. Mao et al. [203] explored the effect of Ge/Se vacancy, anti-site defect, and P atom substituted defect on GeSe monolayer toward toxic gas adsorption. It was found that, the point defect engineering alters electronic structure and work function of GeSe monolayer and thus influence on the adsorption properties of target gas molecules. Besides, adsorption properties such as- adsorption energy, shortest adsorption distance, charge transfer estimation, stability etc. toward NO2, SO2 and H2S have been studied based on various sensing materials like borophene [204], monolayer C3N [205], blue phosphorene [206], organolithium (C2H4Li) complex [207], Ni-MoS2 monolayer [208], germanene nanosheet (Ge-NS) [209], Fe-atom-functionalized CNTs [210], 2D tetragonal GaN [211], graphitic GaN sheet [212], etc.
6. Calibration of Toxic Gas Sensors
In order to check sensor precision, toxic gas sensors must be calibrated at regular intervals. The sensor producers typically suggest a time interval between calibrations. Single toxic gas detectors are normally calibrated with a defined toxic gas depending on the gas type whereas multi-gas detectors are calibrated with their own specific calibration gas mixtures. There are mainly two steps in the gas sensor calibration. Firstly, a reference zero reading must be established using pure nitrogen or pure synthetic air. Secondly, the sensor operating range must be calibrated using a standard gas mixture. The ideal practice is to apply a mixture of the target gas balanced in the natural air as the calibration gas. Premixed calibration gas, permeation devices, cross calibration, gas mixing, Gaussian processes are some of the practical methods of calibrating the gas sensors [213,214,215].
7. Toxic Gas Sensors in Internet of Things (IoT) Applications
The Internet of Things is a network of physical objects that utilizes sensors and application programming interfaces (APIs) to collect and exchange data over the internet. IoT network requires ultra-low power, low cost, long lifetime, integrable into electronic circuits, and mini-sized gas sensors for remote air quality monitoring and enhanced automated system [216]. Electrochemical gas sensors can provide these characteristics required by IoT platforms, thus become suitable candidate for the IoT applications such as creating smart environment, smart home, smart parking system and so on [217,218]. Toxic gas sensors were incorporated into a multi-purpose field surveillance robot which uses multiple IoT cloud servers [219]. High performance gas sensors are utilized in IoT-based vehicle emission monitoring systems [220]. Besides, wireless sensor networks have been employed for toxic gas boundary area detection in large-scale petrochemical plants [221]. However, the gas sensing performances are strongly affected by miniaturization of sensor in terms of length and width between the electrodes, number of electrodes, sensing area etc. [222]. Extensive studies on sensing properties of miniaturized gas sensors can further facilitate the implementation of toxic gas sensors in IoT platforms.
8. Future Perspectives and Conclusions
Toxic gas sensors play an important role in many aspects of technology, industry, or daily life. In recent years, researchers have exploited the fundamental properties of various gas sensing materials to achieve high performance toxic gas sensors. Particularly, excellent improvements have been attained in terms of sensitivity, selectivity, limit of detection, miniaturization and portability for NO2, SO2 and H2S gas sensors using novel combination of nanomaterials exhibiting various morphologies. However, the toxic gas sensors reported so far have limitations in some of the important performance metrics, such as- response and recovery times, stability, operating temperature, reproducibility, fabrication cost, reliability etc. These limitations can be overcome by further exploiting the hybrid and heterostructure, exploring more in surface functionalization, and adopting novel, efficient and cost-effective fabrication technique. This work reviews and categorizes the recent progress in electrochemical detection of NO2, SO2 and H2S gases based on various highly explored sensing materials over the past few decades. Moreover, the sensing performance parameters like sensitivity/ response, response and recovery times at certain gas exposure concentration and operating temperature for various sensor materials and structures have been tabulated which provide a brief comparative performances outline to the reader. This study will give an overview on the research trend of the above-mentioned toxic gas sensors to the current and future researchers.
Acknowledgments
The authors M.A.H.K. and M.V.R. would like to acknowledge the support of NSF grant: 204541. Q.L. would like to acknowledge the support of a Virginia Microelectronics Consortium research grant.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Anenberg, S.C.; Miller, J.; Minjares, R.; Du, L.; Henze, D.K.; Lacey, F.; Malley, C.S.; Emberson, L.; Franco, V.; Klimont, Z.; et al. Impacts and mitigation of excess diesel-related NOx emissions in 11 major vehicle markets. Nature 2017, 545, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Boleij, J.S.M.; Ruigewaard, P.; Hoek, F.; Thairu, H.; Wafula, E.; Onyango, F.; de Koning, H. Domestic air pollution from biomass burning in Kenya. Atmos. Environ. 1989, 23, 1677–1681. [Google Scholar] [CrossRef]
- Robinson, E.; Robbins, R.C. Gaseous Nitrogen Compound Pollutants from Urban and Natural Sources. J. Air Pollut. Control Assoc. 1970, 20, 303–306. [Google Scholar] [CrossRef]
- Bauer, M.A.; Utell, M.J.; Morrow, P.E.; Speers, D.M.; Gibb, F.R. Inhalation of 0.30 ppm nitrogen dioxide potentiates exercise-induced bronchospasm in asthmatics. Am. Rev. Respir. Dis. 1986, 134, 1203–1208. [Google Scholar] [PubMed]
- Ehrlich, R. Effect of nitrogen dioxide on resistance to respiratory infection. Bacteriol. Rev. 1966, 30, 604–614. [Google Scholar] [PubMed]
- Genc, S.; Zadeoglulari, Z.; Fuss, S.H.; Genc, K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012, 2012. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, W.; Hong, Y.; Lee, G.; Yoon, D.S. Recent advances in carbon material-based NO2 gas sensors. Sens. Actuators B Chem. 2018, 255, 1788–1804. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Li, Y.R.; Zhu, T.Y.; Ye, M. Investigation of SO2 and NO adsorption species on activated carbon and the mechanism of NO promotion effect on SO2. Fuel 2015, 143, 536–542. [Google Scholar] [CrossRef]
- Chatterjee, C.; Sen, A. Sensitive colorimetric sensors for visual detection of carbon dioxide and sulfur dioxide. J. Mater. Chem. A 2015, 3, 5642–5647. [Google Scholar] [CrossRef]
- Khan, R.R.; Siddiqui, M.J.A. Review on effects of Particulates; Sulfur Dioxide and Nitrogen Dioxide on Human Health. Int. Res. J. Environment Sci. 2014, 3, 70–73. [Google Scholar]
- Nisar, J.; Topalian, Z.; Sarkar, A.D.; Osterlund, L.; Ahuja, R. TiO2-based gas sensor: A possible application to SO2. ACS Appl. Mater. Interfaces 2013, 5, 8516–8522. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, K.; Wang, X.; Sui, Y.; Zou, B.; Zheng, W.; Zou, G. Rapid and selective H2S detection of hierarchical ZnSnO3 nanocages. Sens. Actuators B Chem. 2011, 159, 245–250. [Google Scholar] [CrossRef]
- Bari, H.R.; Patil, P.P.; Patil, S.B.; Bari, A.R. Detection of H2S gas at lower operating temperature using sprayed nanostructured In2O3 thin films. Bull. Mater. Sci. 2013, 36, 967–972. [Google Scholar] [CrossRef]
- Chou, C. Hydrogen Sulfide: Human Health Aspects: Concise International Chemical Assessment Document 53; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Wiheeb, A.D.; Shamsudin, I.K.; Ahmad, M.A.; Murat, M.N.; Kim, J.; Othman, M.R. Present Technologies for Hydrogen Sulfide Removal from Gaseous Mixtures. Rev. Chem. Eng. 2013, 29, 449–470. [Google Scholar] [CrossRef]
- Levitsky, I.A. Porous Silicon Structures as Optical Gas Sensors. Sensors 2015, 15, 19968–19991. [Google Scholar] [CrossRef] [PubMed]
- Hök, B.; Blückert, A.; Löfving, J. Acoustic gas sensor with ppm resolution. Sens. Rev. 2000, 20, 139–142. [Google Scholar] [CrossRef]
- Li, M.; Myers, E.B.; Tang, H.X.; Aldridge, S.J.; McCaig, H.C.; Whiting, J.J.; Simonson, R.J.; Lewis, N.S.; Roukes, M.L. Nanoelectromechanical Resonator Arrays for Ultrafast, Gas-Phase Chromatographic Chemical Analysis. Nano Lett. 2010, 10, 3899–3903. [Google Scholar] [CrossRef]
- Yunusa, Z.; Hamidon, M.N.; Kaiser, A.; Awang, Z. Gas Sensors: A Review. Sens. Transducers 2014, 168, 61–75. [Google Scholar]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Ko, G.; Kim, H.Y.; Ahn, J.; Park, Y.M.; Lee, K.Y.; Kim, J. Graphene-based nitrogen dioxide gas sensors. Curr. Appl. Phys. 2010, 10, 1002–1004. [Google Scholar] [CrossRef]
- Iqbal, N.; Afzal, A.; Cioffi, N.; Sabbatini, L.; Torsi, L. NOx sensing one- and two-dimensional carbon nanostructures and nanohybrids: Progress and perspectives. Sens. Actuators B Chem. 2013, 181, 9–21. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, C.; Cai, W.; Li, H.; Ji, H.; Kholmanov, I.; Wu, Y.; Piner, R.D. Ruoff RS Detection of sulfur dioxide gas with graphene field effect transistor. Appl. Phys. Lett. 2012, 100, 163114. [Google Scholar] [CrossRef]
- Rana, M.M.; Ibrahim, D.S.; Asyraf, M.R.M.; Jarin, S.; Tomal, A. A review on recent advances of CNTs as gas sensors. Sens. Rev. 2017, 37, 127–136. [Google Scholar] [CrossRef]
- Kumar, D.; Chaturvedi, P.; Saho, P.; Jha, P.; Chouksey, A.; Lal, M.; Rawat, J.S.B.S.; Tandon, R.P.; Chaudhury, P.K. Effect of single wall carbon nanotube networks on gas sensor response and detection limit. Sens. Actuators B Chem. 2017, 240, 1134–1140. [Google Scholar] [CrossRef]
- Kang, I.-S.; So, H.-M.; Bang, G.-S.; Kwak, J.-H.; Lee, J.-O.; Won Ahn, C. Recovery improvement of graphene-based gas sensors functionalized with nanoscale heterojunctions. Appl. Phys. Lett. 2012, 101, 123504. [Google Scholar] [CrossRef]
- Chen, G.; Paronyan, T.M.; Pigos, E.M.; Harutyunyan, A.R. Enhanced gas sensing in pristine carbon nanotubes under continuous ultraviolet light illumination. Sci. Rep. 2012, 2, 343. [Google Scholar] [CrossRef]
- Ye, H.; Nallon, E.C.; Schnee, V.P.; Shi, C.; Jiang, K.; Xu, J.; Feng, S.; Wang, H.; Li, Q. Enhance the Discrimination Precision of Graphene Gas Sensors with a Hidden Markov Model. Anal. Chem. 2018, 90, 13790–13795. [Google Scholar] [CrossRef]
- Pearce, R.; Iakimov, T.; Andersson, M.; Hultman, L.; Spetz, A.L.; Yakimova, R. Epitaxially grown graphene based gas sensors for ultra-sensitive NO2 detection. Sens. Actuators B Chem. 2011, 155, 451–455. [Google Scholar] [CrossRef]
- Nallon, E.C.; Schnee, V.P.; Bright, C.; Polcha, M.P.; Li, Q. Chemical Discrimination with an Unmodified Graphene Chemical Sensor. ACS Sens. 2016, 1, 26–31. [Google Scholar] [CrossRef]
- Esmaeilzadeh, J.; Marzbanrad, E.; Zamani, C.; Raissi, B. Fabrication of undoped-TiO2 nanostructure-based NO2 high temperature gas sensor using low frequency AC electrophoretic deposition method. Sens. Actuators B Chem. 2012, 161, 401–405. [Google Scholar] [CrossRef]
- Shubhda, S.; Kiran, J.; Singh, V.N.; Sukhvir, S.; Vijayan, N.; Nita, D.; Gupta, G.; Senguttuvan, T.D. Faster response of NO2 sensing in graphene–WO3 nanocomposites. Nanotechnology 2012, 23, 205501. [Google Scholar]
- Wang, Y.; Jiang, X.; Xia, Y. Precursor Route to Polycrystalline SnO2 Nanowires That Can Be Used for Gas Sensing under Ambient Conditions. J. Am. Chem. Soc. 2003, 125, 16176–16177. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Xing, L.; Chen, Y.; Shi, S.; Wang, Y.; Wang, T. Synthesis and H2S Sensing Properties of CuO-SnO2 Core/Shell PN-Junction Nanorods. J. Phys. Chem. C 2008, 112, 12157–12160. [Google Scholar] [CrossRef]
- Domènech-Gil, G.; Barth, S.; Samà, J.; Pellegrino, P.; Gràcia, I.; Cané, C.; Romano-Rodriguez, A. Gas sensors based on individual indium oxide nanowire. Sens. Actuators B Chem. 2017, 238, 447–454. [Google Scholar] [CrossRef]
- Young-Jin, C.; In-Sung, H.; Jae-Gwan, P.; Jin, C.K.; Jae-Hwan, P.; Jong-Heun, L. Novel fabrication of an SnO2 nanowire gas sensor with high sensitivity. Nanotechnology 2008, 19, 095508. [Google Scholar]
- Shi, C.; Ye, H.; Wang, H.; Ioannou, D.E.; Li, Q. Precise gas discrimination with cross-reactive graphene and metal oxide sensor arrays. Appl. Phys. Lett. 2018, 113, 222102. [Google Scholar] [CrossRef]
- Lee, K.; Gatensby, R.; McEvoy, N.; Hallam, T.; Duesberg, G.S. High-Performance Sensors Based on Molybdenum Disulfide Thin Films. Adv. Mater. 2013, 25, 6699–6702. [Google Scholar] [CrossRef]
- Shokri, A.; Salami, N. Gas sensor based on MoS2 monolayer. Sens. Actuators B Chem. 2016, 236, 378–385. [Google Scholar] [CrossRef]
- Liu, B.; Chen, L.; Liu, G.; Abbas, A.N.; Fathi, M.; Zhou, C. High Performance Chemical Sensing Using Schottky-Contacted Chemical Vapor Deposition Grown Monolayer MoS2 Transistors. ACS Nano 2014, 8, 5304–5314. [Google Scholar] [CrossRef]
- Schalwig, J.; Muller, G.; Eickhoff, M.; Ambacher, O.; Stutzmann, M. Gas sensitive GaN/AlGaN-heterostructures. Sens. Actuators B Chem. 2002, 87, 425–430. [Google Scholar] [CrossRef]
- Makoto, M.; Shu, F.; Takashi, E. Demonstration of NOx gas sensing for Pd/ZnO/GaN heterojunction diodes. J. Vac. Sci. Technol. 2015, 33, 013001. [Google Scholar]
- Song, J.; Lu, W.; Flynn, J.; Brandes, G. Pt-AlGaN/GaN Schottky diodes operated at 800 °C for hydrogen sensing. Appl. Phys. Lett. 2005, 87, 133501. [Google Scholar] [CrossRef]
- Tilak, V.; Matocha, K.; Sandvik, P. Pt/GaN Schottky diodes for harsh environment NO sensing applications. Phys. Status Solidi 2005, 7, 2555–2558. [Google Scholar] [CrossRef]
- Das, A.; Dost, R.; Richardson, T.; Grell, M.; Morrison, J.J.; Turner, M.L. A nitrogen dioxide sensor based on an organic transistor constructed from amorphous semiconducting polymers. Adv. Mater. 2007, 19, 4018–4023. [Google Scholar] [CrossRef]
- Agbor, N.E.; Petty, M.C.; Monkman, A.P. Polyaniline thin films for gas sensing. Sens. Actuators B Chem. 1995, 28, 173–179. [Google Scholar] [CrossRef]
- Yuan, W.; Huang, L.; Zhou, Q.; Shi, G. Ultrasensitive and selective nitrogen dioxide sensor based on self-Assembled Graphene/Polymer composite nanofibers. ACS Appl. Mater. Interfaces 2014, 6, 17003–17008. [Google Scholar] [CrossRef]
- Mekki, A.; Joshi, N.; Singh, A.; Salmi, Z.; Jha PDecorse, P.; Lau-Truong, S.; Mahmoud, R.; Chehimi, M.M.; Aswal, D.K.; Gupta, S.K. H2S sensing using in situ photopolymerized polyaniline-silver nanocomposite films on flexible substrates. Org. Electron. 2014, 15, 71–81. [Google Scholar] [CrossRef]
- Hyodo, T.; Sasahara, K.; Shimizu, Y.; Egashira, M. Preparation of microporous SnO2 films using PMMA microspheres and their sensing properties to NOx and H2. Sens. Actuators B Chem. 2005, 106, 580–590. [Google Scholar]
- Liang, X.; Zhong, T.; Quan, B.; Wang, B.; Guan, H. Solid-state potentiometric SO2 sensor combining NASICON with V2O5-doped TiO2 electrode. Sens. Actuators B Chem. 2008, 134, 25–30. [Google Scholar] [CrossRef]
- Giang, H.T.; Duy, H.T.; Ngan, P.Q.; Thai, G.H.; Thu, D.T.A.; Thu, D.T.; Toan, N.N. High sensitivity and selectivity of mixed potential sensor based on Pt/YSZ/SmFeO3 to NO2 gas. Sens. Actuators B Chem. 2013, 183, 550–555. [Google Scholar] [CrossRef]
- Guan, Y.; Yin, C.; Cheng, X.; Liang, X.; Diao, Q.; Zhang, H.; Lu, G. Sub-ppm H2S sensor based on YSZ and hollow balls NiMn2O4 sensing electrode. Sens. Actuators B Chem. 2014, 193, 501–508. [Google Scholar] [CrossRef]
- Min, B.; Choi, S. SO2-sensing characteristics of NASICON sensors with Na2SO4–BaSO4 auxiliary electrolytes. Sens. Actuators B Chem. 2003, 93, 209–213. [Google Scholar] [CrossRef]
- Marcu, I.C.; Sandulescu, I. Study of sulfur dioxide adsorption on Y zeolite. J. Serb. Chem. Soc. 2004, 69, 563–569. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Long, Y. Zeolite-based materials for gas sensors. Sensors 2006, 6, 1751–1764. [Google Scholar] [CrossRef]
- Mohan, K.J.; Jagadeesan, K.; Yadav, A. A novel approach for zeolite-based materials for gas sensors. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013, 6, 1–5. [Google Scholar] [CrossRef]
- Dragan, G. The simultaneous adsorption of sulphur dioxide and carbon dioxide by Y zeolites. Rev. Chim. 2010, 61, 1071–1075. [Google Scholar]
- Ma, J.M.; Mei, L.; Chen, Y.J.; Li, Q.H.; Wang, T.H.; Xu, Z.; Duan, X.C.; Zheng, W.J. Ammonium acetate-based ionothermal synthesis and ultrasensitive sensors for low-ppm-level H2S gas. Nanoscale 2013, 5, 895–898. [Google Scholar] [CrossRef]
- Swain, S.K.; Barik, S.; Das, R. Nanomaterials as Sensor for Hazardous Gas Detection. In Handbook of Ecomaterials; Martínez, L., Kharissova, O., Kharisov, B., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Hoffmann, M.W.G.; Casals, O.; Gad, A.E.; Mayrhofer, L.; Caccamo, C.F.L.; Hernández-Ramírez, F.; Lilienkamp, G.; Daum, W.; Moseler, M.; Shen, H.; et al. Novel Approaches towards Highly Selective Self-Powered Gas Sensors. Procedia Eng. 2015, 120, 623–627. [Google Scholar] [CrossRef]
- Lahade, S.V.; Pardhi, P.D. Gas Sensing Technologies: Review, Scope and Challenges. Int. J. Recent Trends Eng. Res. 2018, 4, 108–115. [Google Scholar]
- Sharma, S.; Madou, M. Review: A new approach to gas sensing with nanotechnology. Phil. Trans. R. Soc. A 2012, 370, 2448–2473. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.J.; Liu, A.R.; Huang, L.; Li, C.; Shi, G.Q. High Performance NO2 Sensors Based on Chemically Modified Graphene. Adv. Mater. 2013, 25, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Duy, L.T.; Kim, D.-J.; Trung, T.Q.; Dang, V.Q.; Kim, B.-Y.; Moon, H.K.; Lee, N.-E. High Performance Three-Dimensional Chemical Sensor Platform Using Reduced Graphene Oxide Formed on High Aspect-Ratio Micro-Pillars. Adv. Funct. Mater. 2015, 25, 883–890. [Google Scholar] [CrossRef]
- Melios, C.; Panchal, V.; Edmonds, K.; Lartsev, A.; Yakimova, R.; Kazakova, O. Detection of ultra-low concentration NO2 in complex environment using epitaxial graphene sensors. ACS Sens. 2018, 3, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, T.; Zhao, C.; Han, T.; Fei, T.; Liu, S.; Lu, G. Anchoring ultrafine Pd nanoparticles and SnO2 nanoparticles on reduced graphene oxide for high-performance room temperature NO2 sensing. J. Colloid Interface Sci. 2018, 514, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Opalka, S.M.; Løvvik, O.M.; Emerson, S.C.; She, Y.; Vanderspurt, T.H. Electronic origins for sulfur interactions with palladium alloys for hydrogen-selective membranes. J. Membr. Sci. 2011, 375, 96–103. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Han, T.; Fei, T.; Liu, S.; Lu, G. Oxygen vacancy engineering for enhanced sensing performances: A case of SnO2 nanoparticles-reduced graphene oxide hybrids for ultrasensitive ppb-level room-temperature NO2 sensing. Sens. Actuators B Chem. 2018, 266, 812–822. [Google Scholar] [CrossRef]
- Akbari, E.; Buntat, Z.; Iqbal, S.M.Z.; Azman, Z.; Sahari, N.; Nawawi, Z.; Jambak, M.I.; Sidik, M.A.B. NO2 Gas Sensing Properties of Carbon Films Fabricated by Arc Discharge Methane Decomposition Technique. TELKOMNIKA 2018, 16, 69–76. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Huang, J.; Du, Y.; Ruan, S.C. Reduced Graphene Oxide/Au Nanocomposite for NO2 Sensing at Low Operating Temperature. Sensors 2016, 16, 1152. [Google Scholar] [CrossRef] [PubMed]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Perkins, F.K.; Friedman, A.L.; Cobas, E.; Campbell, P.M.; Jernigan, G.G.; Jonker, B.T. Chemical Vapor Sensing with Monolayer MoS2. Nano Lett. 2013, 13, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jiang, C.; Wei, S.H. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar] [CrossRef]
- Cho, B.; Hahm, M.G.; Choi, M.; Yoon, J.; Kim, A.R.; Lee, Y.-J.; Park, S.-G.; Kwon, J.-D.; Kim, C.S.; Song, M.; et al. Charge transfer-based Gas Sensing Using Atomic-layer MoS2. Sci. Rep. 2015, 5, 8052. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.V.; Kumar, R.; Venkatesan, S.; Zakhidov, A.; Yang, G.; Bao, J.; Kumar, M.; Kumar, M. Photoactivated Mixed In-Plane and Edge-Enriched p-Type MoS2 Flake-Based NO2 Sensor Working at Room Temperature. ACS Sens. 2018, 3, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Goel, N.; Kumar, M. High performance NO2 sensor using MoS2 nanowires network. Appl. Phys. Lett. 2018, 112, 053502. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, Y.; Chung, H.-S.; Kim, A.R.; Kwon, J.-D.; Park, J.; Kim, Y.L.; Kwon, S.; Hahm, M.G.; Cho, B. Effect of Nb Doping on Chemical Sensing Performance of Two-Dimensional Layered MoSe2. ACS Appl. Mater. Interfaces 2017, 9, 3817–3823. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.Y.; Song, J.-G.; Kim, Y.; Choi, T.; Shin, S.; Lee, C.W.; Lee, K.; Koo, J.; Lee, H.; Kim, J.; et al. Improvement of Gas-Sensing Performance of Large-Area Tungsten Disulfide Nanosheets by Surface Functionalization. ACS Nano 2016, 10, 9287–9296. [Google Scholar] [CrossRef]
- Gönüllü, Y.; Rodríguez, G.C.M.; Saruhan, B.; Ürgen, M. Improvement of gas sensing performance of TiO2 towards NO2 by nano-tubular structuring. Sens. Actuators B Chem. 2012, 169, 151–160. [Google Scholar] [CrossRef]
- Vyas, R.; Sharma, S.; Gupta, P.; Vijay, Y.K.; Prasad, A.K.; Tyagi, A.K.; Sachdev, K.; Sharma, S.K. Enhanced NO2 sensing using ZnO–TiO2 nanocomposite thin films. J. Alloy. Compd. 2013, 554, 59–63. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, D.; Zhu, Q.; Wu, J.; Huang, Q.; Xie, C. Effect of Nickel Vacancies on the Room-Temperature NO2 Sensing Properties of Mesoporous NiO Nanosheets. J. Phys. Chem. C 2016, 120, 3936–3945. [Google Scholar] [CrossRef]
- Postica, V.; Gröttrup, J.; Adelung, R.; Lupan, O.; Mishra, A.K.; de Leeuw, N.H.; Ababii, N.; Carreira, J.F.C.; Rodrigues, J.; Sedrine, N.B.; et al. Nanosensors: Multifunctional Materials: A Case Study of the Effects of Metal Doping on ZnO Tetrapods with Bismuth and Tin Oxides. Adv Funct. Mater. 2017, 27, 1604676. [Google Scholar] [CrossRef]
- Qiang, X.; Hu, M.; Zhao, B.; Qin, Y.; Yang, R.; Zhou, L.; Qin, Y. Effect of the Functionalization of Porous Silicon/WO3 Nanorods with Pd Nanoparticles and Their Enhanced NO2-Sensing Performance at Room Temperature. Materials 2018, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Ievlev, V.M.; Ryabtsev, S.V.; Samoylov, A.M.; Shaposhnik, A.V.; Kuschev, S.B.; Sinelnikov, A.A. Thin and ultrathin films of palladium oxide for oxidizing gases detection. Sens. Actuators B Chem. 2018, 255, 1335–1342. [Google Scholar] [CrossRef]
- Navale, Y.H.; Navale, S.T.; Ramgir, N.S.; Stadler, F.J.; Gupta, S.K.; Aswal, D.K.; Patil, V.B. Zinc oxide hierarchical nanostructures as potential NO2 sensors. Sens. Actuators B Chem. 2017, 251, 551–563. [Google Scholar] [CrossRef]
- Benedict, S.; Singh, M.; Naik, T.R.R.; Shivashankar, S.A.; Bhat, N. Microwave-Synthesized NiO as a Highly Sensitive and Selective Room-Temperature NO2 Sensor. ECS J. Solid State Sci. Technol. 2018, 7, Q3143–Q3147. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Kim, D.-H.; Hong, S.-H. CuBi2O4 Prepared by the Polymerized Complex Method for Gas Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 14901–14913. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.M.; Phuong, H.V.; Duy, N.V.; Hoa, N.D.; Hieu, N.V. Comparative effects of synthesis parameters on the NO2 gas-sensing performance of on-chip grown ZnO and Zn2SnO4 nanowire sensors. J. Alloy. Compd. 2018, 765, 1237–1242. [Google Scholar] [CrossRef]
- Aluri, G.S.; Motayed, A.; Davydov, A.V.; Oleshko, V.P.; Bertness, K.A.; Rao, M.V. Nitro-Aromatic Explosive Sensing Using GaN Nanowire-Titania Nanocluster Hybrids. IEEE Sens. J. 2013, 13, 1883–1888. [Google Scholar] [CrossRef]
- Aluri, G.S.; Motayed, A.; Bertness, K.; Sanford, N.; Oleshko, V.; Davydov, A.; Rao, M.V. Highly selective GaN-nanowire/TiO2 nanocluster hybrid sensors for detection of benzene and related environmental pollutants. Nanotechnology 2011, 22, 295503. [Google Scholar] [CrossRef] [PubMed]
- Aluri, G.S.; Motayed, A.; Davydov, A.V.; Oleshko, V.; Bertness, K.; Sanford, N.; Rao, M.V. GaN-nanowire/TiO2-nanocluster hybrid sensors for detection of Benzene and related aromatic compounds. Proc. SPIE 2011, 8024, 80240M. [Google Scholar]
- Aluri, G.S.; Motayed, A.; Bertness, K.; Sanford, N.; Oleshko, V.; Davydov, A.; Rao, M.V. Methanol, Ethanol, and Hydrogen Sensing using Metal-Oxide and Metal (TiO2-Pt) Composite Nanoclusters on GaN Nanowires: A New Route towards Tailoring the Selectivity of Nanowire/Nanocluster Chemical Sensors. Nanotechnology 2012, 23, 17550. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.; Salvestrini, J.P.; Halfaya, Y.; Sundaram, S.; el Gmili, Y.; Pradere, L.; Marteau, J.Y.; Assouar, M.B.; Voss, P.L.; Ougazzaden, A. Highly sensitive detection of NO2 gas using BGaN/GaN superlattice-based double Schottky junction sensors. Appl. Phys. Lett. 2015, 106, 243504. [Google Scholar] [CrossRef]
- Halfaya, Y.; Bishop, C.; Soltani, A.; Sundaram, S.; Aubry, V.; Voss, P.L.; Salvestrini, J.P.; Ougazzaden, A. Investigation of the Performance of HEMT-Based NO, NO2 and NH3 Exhaust Gas Sensors for Automotive Antipollution Systems. Sensors 2016, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Mills, S.; Lee, B.; Misra, V. Application of AlGaN/GaN Heterostructures for Ultra-Low Power Nitrogen Dioxide Sensing. ECS J. Solid State Sci. Technol. 2015, 4, S3034–S3037. [Google Scholar] [CrossRef]
- Lv, A.; Pan, Y.; Chi, L. Gas Sensors Based on Polymer Field-Effect Transistors. Sensors 2017, 17, 213. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jha, P.; Singh, A.; Chauhan, A.K.; Gupta, S.K.; Aswal, D.K.; Muthe, K.P.; Gadkari, S.C. Modeling of gate bias controlled NO2 response of the PCDTBT based organic field effect transistor. Chem. Phys. Lett. 2018, 698, 7–10. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Zhu, X.; Zhou, X.; Chi, L. An Ultrasensitive Organic Semiconductor NO2 Sensor Based on Crystalline TIPS-Pentacene Films. Adv. Mater. 2017, 29, 1703192. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhuang, X.; Melkonyan, F.S.; Wang, B.; Zeng, L.; Wang, G.; Han, S.; Bedzyk, M.J.; Yu, J.; Marks, T.J.; et al. UV–Ozone Interfacial Modification in Organic Transistors for High-Sensitivity NO2 Detection. Adv. Mater. 2017, 29, 1701706. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, R.; Jiao, W.; Ding, G.; Hao, L.; Yang, F.; He, X. MoS2 graphene fiber-based gas sensing devices. Carbon 2015, 95, 34–41. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Zhao, C.; Han, T.; Fei, T.; Liu, S.; Lu, G. Rational synthesis of molybdenum disulfide nanoparticles decorated reduced graphene oxide hybrids and their application for high-performance NO2 sensing. Sens. Actuators B Chem. 2018, 260, 508–518. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Xia, Y.; Komarneni, S.; Chen, H.; Xu, J.; Xiang, L.; Xie, D. Hierarchical ZnO Nanosheet-Nanorod Architectures for Fabrication of Poly(3-hexylthiophene)/ZnO Hybrid NO2 Sensor. ACS Appl. Mater. Interfaces 2016, 8, 8600–8607. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, C.; Han, T.; Zhang, Y.; Liu, S.; Fei, T.; Lu, G.; Zhang, T. High-performance reduced graphene oxide-based room-temperature NO2 sensors: A combined surface modification of SnO2 nanoparticles and nitrogen doping approach. Sens. Actuators B Chem. 2017, 242, 269–279. [Google Scholar] [CrossRef]
- Kim, H.W.; Na, H.G.; Kwon, Y.J.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Wu, P.; Kim, S.S. Microwave-Assisted Synthesis of Graphene-SnO2 Nanocomposites and Their Applications in Gas Sensors. ACS Appl. Mater. Interfaces 2017, 9, 31667–31682. [Google Scholar] [CrossRef] [PubMed]
- Tammanoon, N.; Wisitsoraat, A.; Sriprachuabwong, C.; Phokharatkul, D.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Ultrasensitive NO2 Sensor Based on Ohmic Metal-Semiconductor Interfaces of Electrolytically Exfoliated Graphene/Flame-Spray-Made SnO2 Nanoparticles Composite Operating at Low Temperatures. ACS Appl. Mater. Interfaces 2015, 7, 24338–24352. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Guo, J.; Sun, J.; Tang, P.; Chen, A.; Luo, R.; Li, D. Enhancement of NO2-Sensing Performance at Room Temperature by Graphene-Modified Polythiophene. Ind. Eng. Chem. Res. 2016, 55, 5788–5794. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kang, S.Y.; Wu, P.; Peng, Y.; Kim, S.S.; Kim, H.W. Selective Improvement of NO2 Gas Sensing Behavior in SnO2 Nanowires by Ion-Beam Irradiation. ACS Appl. Mater. Interfaces 2016, 8, 13646–13658. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Goel, N.; Kumar, M. UV-Activated MoS2 Based Fast and Reversible NO2 Sensor at Room Temperature. ACS Sens. 2017, 2, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Zhang, B.; Xiao, Y.; Gao, Y.; Yang, Q.; Wang, Y.; Lu, G. Ultrasensitive and low detection limit of nitrogen dioxide gas sensor based on flower-like ZnO hierarchical nanostructure modified by reduced graphene oxide. Sens. Actuators B Chem. 2017, 249, 715–724. [Google Scholar] [CrossRef]
- Long, H.; Trochimczyk, A.H.; Pham, T.; Zettl, A.; Carraro, C.; Worsley, M.A.; Maboudian, R. High Surface Area 3D MoS2/Graphene Hybrid Aerogel for Ultrasensitive NO2 Detection. Adv. Funct. Mater. 2016, 26, 5158–5165. [Google Scholar] [CrossRef]
- Betty, C.A.; Choudhury, S.; Arora, S. Tin oxide–polyaniline heterostructure sensors for highly sensitive and selective detection of toxic gases at room temperature. Sens. Actuators B Chem. 2015, 220, 288–294. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Yang, W.; Yuan, W.; Xu, J.; Jiang, Y. In Situ Polymerization Deposition of Porous Conducting Polymer on Reduced Graphene Oxide for Gas Sensor. ACS Appl. Mater. Interfaces 2014, 6, 13807–13814. [Google Scholar] [CrossRef] [PubMed]
- Popp, A.; Yilmazoglu, O.; Kaldirim, O.; Schneider, J.J.; Pavlidis, D. A self-supporting monolith of highly aligned carbon nanotubes as device structure for sensor applications. Chem. Comm. 2009, 22, 3205–3207. [Google Scholar] [CrossRef] [PubMed]
- Zouaghi, W.; Hussein, L.; Tomson, M.D.; Islam, Q.; Nicoloso, N.; Heinlein, T.; Engstler, J.; Schneider, J.J.; Roskos, H.G. Towards gas sensing with vertically aligned carbon nanotubes interrogated by thz radiation pulses. Lith. J. Phys. 2018, 58, 38–48. [Google Scholar] [CrossRef]
- Petryshak, V.; Mikityuk, Z.; Vistak, M.; Gotra, Z.; Akhmetova, A.; Wójcik, W.; Assembay, A. Highly sensitive active medium of primary converter SO2 sensors based on cholesteric-nematic mixtures, doped by carbon nanotubes. Przeglad Elektrotech. 2017, 1, 119–122. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Jiang, C.; Li, P.; Sun, Y. High-performance sulfur dioxide sensing properties of layer-by-layer self-assembled titania-modified graphene hybrid nanocomposite. Sens. Actuators B Chem. 2017, 245, 560–567. [Google Scholar] [CrossRef]
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide Materials for Development of Integrated Gas Sensors—A Comprehensive Review. Crit. Rev. Solid State Mater. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- West, D.L.; Montgomery, F.C.; Armstrong, B.L. Compact, DC-electrical biased sulfur dioxide sensing elements for use at high temperatures. Sens. Actuators B Chem. 2012, 162, 409–417. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Chen, Y.; Zhang, Y.; Gao, X.; Xu, P.; Li, X.; Fang, J.; Wen, W. An integrated micro-chip with Ru/Al2O3/ZnO as sensing material for SO2 detection. Sens. Actuators B Chem. 2018, 262, 26–34. [Google Scholar] [CrossRef]
- Ciftyürek, E.; Sabolsky, K.; Sabolsky, E.M. Molybdenum and tungsten oxide-based gas sensors for high temperature detection of environmentally hazardous sulfur species. Sens. Actuators B Chem. 2016, 237, 262–274. [Google Scholar] [CrossRef]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. Metal oxide catalyst assisted SnO2 thin film based SO2 gas sensor. Sens. Actuators B Chem. 2016, 224, 282–289. [Google Scholar] [CrossRef]
- Haridas, D.; Chowdhuri, A.; Sreenivas, K.; Gupta, V. Effect of thickness of platinum catalyst clusters on response of SnO2 thin film sensor for LPG. Sens. Actuators B Chem. 2011, 153, 89–95. [Google Scholar] [CrossRef]
- Das, S.; Rana, S.; Mursalin, S.M.; Rana, P.; Sen, A. Sonochemically prepared nanosized BiFeO3 as novel SO2 sensor. Sens. Actuators B Chem. 2015, 218, 122–127. [Google Scholar] [CrossRef]
- Stephen, J.P.; Cammy, R.A.; Ren, F. Gallium Nitride Processing for Electronics, Sensors and Spintronics, 1st ed.; Springer: London, UK, 2006. [Google Scholar]
- Anderson, T.; Ren, F.; Pearton, S.; Kang, B.S.; Wang, H.-T.; Chang, C.-Y.; Lin, J. Advances in Hydrogen, Carbon Dioxide, and Hydrocarbon Gas Sensor Technology Using GaN and ZnO-Based Devices. Sensors 2009, 9, 4669–4694. [Google Scholar] [CrossRef] [PubMed]
- Triet, N.M.; Duy, L.T.; Hwang, B.-U.; Hanif, A.; Siddiqui, S.; Park, K.; Cho, C.; Lee, N. High-Performance Schottky Diode Gas Sensor Based on the Heterojunction of Three-Dimensional Nanohybrids of Reduced Graphene Oxide-Vertical ZnO Nanorods on an AlGaN/GaN Layer. ACS Appl. Mater. Interfaces 2017, 9, 30722–30732. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Lee, D.-S.; Choi, H.K.; Lee, D.H.; Kim, J.-E.; Lee, J.Y.; Lee, W.J.; Kim, S.O.; Choi, S.-Y. Flexible Room-Temperature NO2 Gas Sensors Based on Carbon Nanotubes/Reduced Graphene Hybrid Films. Appl. Phys. Lett. 2010, 96, 213105. [Google Scholar] [CrossRef]
- Wang, L.; Kumar, R.V. A SO2 gas sensor based upon composite NASICON/Sr--Al2O3 bielectrolyte. Mater. Res. Bull. 2005, 40, 1802–1815. [Google Scholar] [CrossRef]
- Suganuma, S.; Watanabe, M.; Kobayashi, T.; Wakabayashi, S. SO2 gas sensor utilizing stabilized zirconia and sulfate salts with a new working mechanism. Solid State Ionics 1999, 126, 175–179. [Google Scholar] [CrossRef]
- Wang, L.; Kumar, R.V. A new SO2 gas sensor based on an Mg2+ conducting solid electrolyte. J. Electroanal. Chem. 2003, 543, 109–114. [Google Scholar] [CrossRef]
- Ma, C.; Hao, X.; Yang, X.; Liang, X.; Liu, F.; Liu, T.; Yang, C.; Zhu, H.; Lu, G. Sub-ppb SO2 gas sensor based on NASICON and LaxSm1−xFeO3 sensing electrode. Sens. Actuators B Chem. 2018, 256, 648–655. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Wang, B.; Yang, X.; Wang, Q.; Liang, X.; Sun, P.; Chuai, X.; Wang, Y.; Lu, G. Stabilized zirconia-based mixed potential type sensors utilizing MnNb2O6 sensing electrode for detection of low-concentration SO2. Sens. Actuators B Chem. 2017, 238, 1024–1031. [Google Scholar] [CrossRef]
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Zeolite Science and Technology; Marcel Dekker, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Yimlamai, I.; Niamlang, S.; Chanthaanont, P.; Kunanuraksapong, R.; Changkhamchom, S.; Sirivat, A. Electrical conductivity response and sensitivity of ZSM-5, Y, and mordenite zeolites towards ethanol vapor. Ionics 2011, 17, 607–615. [Google Scholar] [CrossRef]
- Choeichom, P.; Sirivat, A. Discriminative sensing performances of ZSM-5, Y, mordenite, ferrierite, beta, 3A, 4A, 5A, and 13X zeolites towards sulfur dioxide. Ionics 2018, 24, 2829–2841. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Huang, L.; Gao, J.; Zhou, H.; Shi, Y.; Pan, Q.; Zhang, G.; Du, Y.; Liang, W. Sulfur dioxide gas-sensitive materials based on zeolitic imidazolate framework-derived carbon nanotubes. J. Mater. Chem. A 2018, 6, 12115–12124. [Google Scholar] [CrossRef]
- Chanthaanont, P.; Permpool, T.; Sirivat, A. Effect of alkaline and alkaline earth ion exchanged Y zeolites on electrical conductivity and response of PEDOT-PSS/Y zeolite composites toward SO2. Mater. Technol. 2015, 30, 193–199. [Google Scholar] [CrossRef]
- Lim, S.H.; Feng, L.; Kemling, J.W.; Musto, C.J.; Suslick, K.S. An Optoelectronic Nose for Detection of Toxic Gases. Nat. Chem. 2009, 1, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Duan, H.; Ma, Y.; Deng, W. Headspace-Sampling Paper-Based Analytical Device for Colorimetric/Surface-Enhanced Raman Scattering Dual Sensing of Sulfur Dioxide in Wine. Anal. Chem. 2018, 90, 5719–5727. [Google Scholar] [CrossRef]
- Wang, M.; Guo, L.; Cao, D. Amino-Functionalized Luminescent Metal-Organic Framework Test Paper for Rapid and Selective Sensing of SO2 Gas and Its Derivatives by Luminescence Turn-On Effect. Anal. Chem. 2018, 90, 3608–3614. [Google Scholar] [CrossRef]
- Liu, C.-C.; Wang, Y.-N.; Fu, L.-M.; Yang, D.-Y. Rapid integrated microfluidic paper-based system for sulfur dioxide detection. Chem. Eng. J. 2017, 316, 790–796. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kaur, A. Solitary surfactant assisted morphology dependent chemiresistive polyaniline sensors for room temperature monitoring of low parts per million sulfur di oxide. Polym. Int. 2015, 64, 1475–1481. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kaur, A. Enhanced room temperature sulfur dioxide sensing behaviour of in situ polymerized polyaniline-tungsten oxide nanocomposite possessing honeycomb morphology. RSC Adv. 2015, 5, 73535–73544. [Google Scholar] [CrossRef]
- Betty, C.A.; Choudhury, S. Charge carrier transport in nanocrystalline SnO2 thin film sensor and temperature dependence of toxic gas sensitivity. Sens. Actuators B Chem. 2016, 237, 787–794. [Google Scholar] [CrossRef]
- Gaiardo, A.; Fabbri, B.; Giberti, A.; Guidi, V.; Bellutti, P.; Malagù, C.; Valt, M.; Pepponi, G.; Gherardi, S.; Zonta, G.; et al. ZnO and Au/ZnO thin films: Room-temperature chemoresistive properties for gas sensing applications. Sens. Actuators B Chem. 2016, 237, 1085–1094. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Chen, D.; Jiang, Z. A new potentiometric SO2 sensor based on Li3PO4 electrolyte film and its response characteristics. Rev. Sci. Instrum. 2015, 86, 075007. [Google Scholar] [CrossRef] [PubMed]
- Mulmi, S.; Thangadurai, V. Semiconducting SnO2-TiO2 (ST) composites for detection of SO2 gas. Ionics 2016, 22, 1927–1935. [Google Scholar] [CrossRef]
- Chen, A.M.; Liu, R.; Peng, X.; Chen, Q.; Wu, J. 2D Hybrid Nanomaterials for Selective Detection of NO2 and SO2 Using “Light On and Off” Strategy. ACS Appl. Mater. Interfaces 2017, 9, 37191. [Google Scholar] [CrossRef]
- Chaudhary, V.; Singh, H.K.; Kaur, A. Effect of charge carrier transport on sulfur dioxide monitoring performance of highly porous polyaniline nanofibers. Polym. Int. 2017, 66, 699. [Google Scholar] [CrossRef]
- Ovsianytskyi, O.; Nam, Y.-S.; Tsymbalenko, O.; Lan, P.; Moon, M.; Lee, K. Highly sensitive chemiresistive H2S gas sensor based on graphene decorated with Ag nanoparticles and charged impurities. Sens. Actuators B Chem. 2018, 257, 278–285. [Google Scholar] [CrossRef]
- Chu, J.; Wang, X.; Wang, D.; Yang, A.; Lv, P.; Wu, Y.; Rong, M.; Gao, L. 3 Highly selective detection of sulfur hexafluoride decomposition components H2S and SOF2 employing sensors based on tin oxide modified reduced graphene oxide. Carbon 2018, 135, 95–103. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Z.; Chen, C. ZnO-carbon nanofibers for stable, high response, and selective H2S sensors. Nanotechnology 2018, 29, 275501. [Google Scholar] [CrossRef]
- Sokolovskij, R.; Zhangb, J.; Iervolino, E.; Zhao, C.; Santagata, F.; Wang, F.; Yu, H.; Sarro, P.M.; Zhang, G.Q. Hydrogen sulfide detection properties of Pt-gated AlGaN/GaN HEMT-sensor. Sens. Actuators B Chem. 2018, 274, 636–644. [Google Scholar] [CrossRef]
- Lo, C.-F.; Xi, Y.; Liu, L.; Pearton, S.J.; Doré, S.; Hsu, C.-H.; Dabiran, A.M.; Chow, P.P.; Ren, F. Effect of temperature on CO sensing response in air ambient by using ZnO nanorod-gated AlGaN/GaN high electron mobility transistors. Sens. Actuators B Chem. 2013, 176, 708–712. [Google Scholar] [CrossRef]
- Chen, T.Y.; Chen, H.I.; Hsu, C.S.; Huang, C.C.; Chang, C.F.; Chou, P.C.; Liu, W.C. On an Ammonia gas sensor based on a Pt/AlGaN heterostructure field-effect transistor. IEEE Electron Device Lett. 2012, 33, 612–614. [Google Scholar]
- Son, K.A.; Yang, B.; Prokopuk, N.; Moon, J.S.; Liao, A.; Katona, T.M.; Khan, M.A. RF GaN HEMT sensors for detection of caustic chemicals. IEEE Sens. J. 2011, 11, 3476–3478. [Google Scholar] [CrossRef]
- Zhanga, J.; Sokolovskijb, R.; Chen, G.; Zhu, Y.; Qi, Y.; Lin, X.; Li, W.; Zhang, G.Q.; Jiang, Y.; Yu, H. Impact of high temperature H2 pre-treatment on Pt-AlGaN/GaN HEMT sensor for H2S detection. Sens. Actuators B Chem. 2018, 280, 138–143. [Google Scholar] [CrossRef]
- Shukla, G.P.; Bhatnagar, M.C. H2S gas sensor based on Cu doped SnO2 nanostructure. J. Mater. Sci. Eng. A 2014, 4. [Google Scholar] [CrossRef]
- Hosseini, Z.S.; zad, A.I.; Mortezaali, A. Room temperature (24.0 ± 1 °C) H2S gas sensor based on rather aligned ZnO nanorods with flower-like structures. Sens. Actuators B Chem. 2015, 207, 865–871. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.; Zhang, S.; Chen, W.; Kuang, Z.; Ao, D.; Liu, W.; Fu, Y. A fast response & recovery H2S gas sensor based on α-Fe2O3 nanoparticles with ppb level detection limit. J. Hazard. Mater. 2015, 300, 167–174. [Google Scholar] [PubMed]
- Zhang, H.-J.; Meng, F.-N.; Liu, L.-Z.; Chen, Y.-J. Convenient route for synthesis of alpha-Fe2O3 and sensors for H2S gas. J. Alloy. Compd. 2019, 774, 1181–1188. [Google Scholar] [CrossRef]
- Li, D.; Qin, L.; Zhao, P.; Zhang, Y.; Liu, D.; Liu, F.; Kang, B.; Wang, Y.; Song, H.; Zhang, T.; et al. Preparation and gas-sensing performances of ZnO/CuO rough nanotubular arrays for low-working temperature H2S detection. Sens. Actuators B Chem. 2018, 254, 834–841. [Google Scholar] [CrossRef]
- Li, Z.; Yan, S.; Zhang, S.; Wang, J.; Shen, W.; Wang, Z.; Fu, Y.Q. Ultra-sensitive UV and H2S dual functional sensors based on porous In2O3 nanoparticles operated at room temperature. J. Alloy. Compd. 2019, 770, 721–731. [Google Scholar] [CrossRef]
- Huang, H.; Xu, P.; Zheng, D.; Chen, C.; Li, X. Sulfuration-desulfuration reaction sensing-effect of intrinsic ZnO nanowires for high-performance H2S detection. J. Mater. Chem. A 2015, 3, 6330–6339. [Google Scholar] [CrossRef]
- Eoma, N.S.A.; Chob, H.-B.; Song, Y.; Go, G.M.; Lee, J.; Choa, Y. Room-temperature H2S gas sensing by selectively synthesized Cux(x=1,2)O:SnO2 thin film nanocomposites with oblique & vertically assembled SnO2 ceramic nanorods. Sens. Actuators B Chem. 2018, 273, 1054–1061. [Google Scholar]
- Stanoiu, A.; Piticescu, R.M.; Simion, C.E.; Rusti-Ciobota, C.; Florea, O.; Teodorescu, V.; Osiceanu, P.; Sobetkii, A.; Badilita, V. H2S selective sensitivity of Cu doped BaSrTiO3 under operando conditions and the associated sensing mechanism. Sens. Actuators B Chem. 2018, 264, 327–336. [Google Scholar] [CrossRef]
- Quang, P.L.; Cuong, N.D.; Hoa, T.T.; Long, H.T.; Hung, C.M.; Le, D.T.T.; Hieu, N.V. Simple post-synthesis of mesoporous p-type Co3O4 nanochains for enhanced H2S gas sensing performance. Sens. Actuators B Chem. 2018, 270, 158–166. [Google Scholar] [CrossRef]
- Stanoiu, A.; Simion, C.E.; Calderon-Moreno, J.M.; Osiceanu, P.; Florea, M.; Teodorescu, V.S.; Somacescu, S. Sensors based on mesoporous SnO2-CuWO4 with high selective sensitivity to H2S at low operating temperature. J. Hazard. Mater. 2017, 331, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhu, Z.; Li, Z.; Xie, L.; Wu, Y.; Zheng, L. Heterostructure of CuO microspheres modified with CuFe2O4 nanoparticles for highly sensitive H2S gas sensor. Sens. Actuators B Chem. 2018, 264, 139–149. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Zhu, Y.; Xie, W.; Shao, R.; Yao, X.; Gao, A.; Yin, Y. Cd2+-Doped Amorphous TiO2 Hollow Spheres for Robust and Ultrasensitive Photoelectrochemical Sensing of Hydrogen Sulfide. Anal. Chem. 2018, 90, 5496–5502. [Google Scholar] [CrossRef]
- Yassine, O.; Shekhah, O.; Assen, A.H.; Belmabkhout, Y.; Salama, K.N.; Eddaoudi, M. H2S sensors: Fumarate-based fcu-MOF thin film grown on a capacitive interdigitated electrode. Angew. Chem. Int. Ed. 2016, 55, 15879–15883. [Google Scholar] [CrossRef]
- Guo, L.; Wang, M.; Cao, D. A Novel Zr-MOF as Fluorescence Turn-On Probe for Real-Time Detecting H2S Gas and Fingerprint Identification. Small 2018, 14, 1703822. [Google Scholar] [CrossRef]
- Dong, X.; Su, Y.; Lu, T.; Zhang, L.; Wu, L.; Lv, Y. MOFs-derived dodecahedra porous Co3O4: An efficient cataluminescence sensing material for H2S. Sens. Actuators B Chem. 2018, 258, 349–357. [Google Scholar] [CrossRef]
- Chaudhary, N.; Singh, A.; Debnath, A.K.; Acharya, S.; Aswal, D.K. Electron beam modified organic materials and their applications. Solid State Phenom. 2015, 239, 72–97. [Google Scholar] [CrossRef]
- Chaudhary, N.; Singh, A.; Aswal, D.K.; Jha, P.; Samanta, S.; Chauhan, A.; Debnath, A.; Acharya, S.; Shah, K.; Muthe, K.; et al. Electron beam induced modifications of polyaniline silver nano-composite films: Electrical conductivity and H2S gas sensing studies. Radiat. Phys. Chem. 2018, 153, 131–139. [Google Scholar] [CrossRef]
- Abu-Hani, A.F.; Greish, Y.E.; Mahmoud, S.T.; Awwad, F.; Ayesh, A.I. Low-temperature and fast response H2S gas sensor using semiconducting chitosan film. Sens. Actuators B Chem. 2017, 253, 677–684. [Google Scholar] [CrossRef]
- Shu, J.; Qiu, Z.; Lv, S.; Zhang, K.; Tang, D. Cu2+-Doped SnO2 Nanograin/Poly pyrrole Nanospheres with Synergic Enhanced Properties for Ultrasensitive Room-Temperature H2S Gas Sensing. Anal. Chem. 2017, 89, 11135–11142. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Ma, C.; Yang, X.; Liu, T.; Wang, B.; Liu, F.; Liang, X.; Yang, C.; Zhu, H.; Lu, G. YSZ-based mixed potential H2S sensor using La2NiO4 sensing electrode. Sens. Actuators B Chem. 2018, 255, 3033–3039. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Hao, X.; Song, Y.; Liang, X.; Liu, F.; Sun, P.; Gao, Y.; Yan, X.; Lu, G. Nafion-based amperometric H2S sensor using Pt Rh/C sensing electrode. Sens. Actuators B Chem. 2018, 273, 635–641. [Google Scholar] [CrossRef]
- Asres, G.A.; Baldoví, J.J.; Dombovari, A.; Järvinen, T.; Lorite, G.S.; Mohl, M.; Shchukarev, A.; Paz, A.P.; Xian, L.; Mikkola, J.; et al. Ultrasensitive H2S gas sensors based on p-type WS2 hybrid materials. Nano Res. 2018, 11, 4215–4224. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, W.; Zhu, H. Mechanism of charge transfer and its impacts on Fermi-level pinning for gas molecules adsorbed on monolayer WS2. J. Chem. Phys. 2015, 142, 214704. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Jain, N.; Sharma, K.; Bhattacharya, S.; Roy, M.; Tyagi, A.K.; Gupta, S.K.; Yakhmi, J.V. Room temperature H2S gas sensing at ppb level by single crystal In2O3 whiskers. Sens. Actuator. B Chem. 2008, 133, 456–461. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Cheng, X.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. Hierarchical NiO Cube/Nitrogen-Doped Reduced Graphene Oxide Composite with Enhanced H2S Sensing Properties at Low Temperature. ACS Appl. Mater. Interfaces 2017, 9, 26293–26303. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, Z.; Gao, L.; Zhang, Y.; Xu, J.; Zhao, H. Facile synthesis of reduced graphene oxide/hexagonal WO3 nanosheets composites with enhanced H2S sensing properties. Sens. Actuators B Chem. 2016, 230, 736–745. [Google Scholar] [CrossRef]
- Balouria, V.; Ramgir, N.S.; Singh, A.; Debnath, A.K.; Mahajan, A.; Bedi, R.K.; Aswal, D.K.; Gupta, S.K. Enhanced H2S sensing characteristics of Au modified Fe2O3 thin films. Sens. Actuators B Chem. 2015, 219, 125–132. [Google Scholar] [CrossRef]
- Li, Z.J.; Lin, Z.J.; Wang, N.N.; Huang, Y.W.; Wang, J.Q.; Liu, W.; Fu, Y.Q.; Wang, Z.G. Facile synthesis of α-Fe2O3 micro-ellipsoids by surfactant-free hydrothermal method for sub-ppm level H2S detection. Mater. Des. 2016, 110, 532–539. [Google Scholar] [CrossRef]
- Kheel, H.; Sun, G.J.; Lee, J.K.; Lee, S.; Dwivedi, R.P.; Lee, C. Enhanced H2S sensing performance of TiO2-decorated α-Fe2O3 nanorod sensors. Ceram. Int. 2016, 42, 18597–18604. [Google Scholar] [CrossRef]
- Benedict, S.; Lumdee, C.; Dmitriev, A.; Anand, S.; Bhat, N. Colloidal lithography nanostructured Pd/PdOx core–shell sensor for ppb level H2S detection. Nanotechnology 2018, 29, 255502. [Google Scholar] [CrossRef]
- Gao, X.; Sun, Y.; Zhu, C.; Li, C.; Ouyang, Q.; Chen, Y. Highly sensitive and selective H2S sensor based on porous ZnFe2O4 nanosheets. Sens. Actuators B Chem. 2017, 246, 662–672. [Google Scholar] [CrossRef]
- Fu, D.; Zhu, C.; Zhang, X.; Li, C.; Chen, Y. Two-dimensional net-like SnO2/ZnO heteronanostructures for high-performance H2S gas sensor. J. Mater. Chem. A 2016, 4, 1390–1398. [Google Scholar] [CrossRef]
- Gao, C.; Lin, Z.-D.; Li, N.; Fu, P.; Wang, X.-H. Preparation and H2S Gas-Sensing Performances of Coral Like SnO2–CuO Nanocomposite. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 1190–1197. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, F.; Liu, J.; Wang, Y.; Zhou, J.; Ruan, S. Enhanced H2S sensing characteristics of CuO-NiO core-shell microspheres sensors. Sens. Actuators B Chem. 2015, 209, 515–523. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Xiao, S.; Wang, X.; Sun, L.; Li, H.; Xie, W.; Li, Q.; Zhang, Q.; Wang, T. Low-Temperature H2S Detection with Hierarchical Cr-Doped WO3 Microspheres. ACS Appl. Mater. Interfaces 2016, 8, 9674–9683. [Google Scholar] [CrossRef]
- Tian, J.; Pan, F.; Xue, R.; Zhang, W.; Fang, X.; Liu, Q.; Wang, Y.; Zhang, Z.; Zhang, D. A highly sensitive room temperature H2S gas sensor based on SnO2 multi-tube arrays bio-templated from insect bristles. Dalton Trans. 2015, 44, 7911–7916. [Google Scholar] [CrossRef]
- Yu, T.; Cheng, X.; Zhang, X.; Sui, L.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. Highly sensitive H2S detection sensors at low temperature based on hierarchically structured NiO porous nanowall arrays. J. Mater. Chem. A 2015, 3, 11991–11999. [Google Scholar] [CrossRef]
- Asad, M.; Sheikhi, M.H.; Pourfath, M.; Moradi, M. High sensitive and selective flexible H2S gas sensors based on Cu nanoparticle decorated SWCNTs. Sens. Actuators B Chem. 2015, 210, 1–8. [Google Scholar] [CrossRef]
- Li, Z.; Wang, N.; Lin, Z.; Wang, J.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z. Room-Temperature High-Performance H2S Sensor Based on Porous CuO Nanosheets Prepared by Hydrothermal Method. ACS Appl. Mater. Interfaces 2016, 8, 20962–20968. [Google Scholar] [CrossRef]
- Su, P.; Peng, Y. Fabrication of a room-temperature H2S gas sensor based on ppy/WO3 nanocomposite films by in-situ photopolymerization. Sens. Actuator. B Chem. 2014, 193, 637–643. [Google Scholar] [CrossRef]
- Choi, S.; Jang, B.; Lee, S.; Min, B.K.; Rothschild, A.; Kim, I. Selective detection of acetone and hydrogen sulfide for the diagnosis of diabetes and halitosis using SnO2 nanofibers functionalized with reduced graphene oxide nanosheets. ACS Appl. Mater. Interface 2014, 6, 2588–2597. [Google Scholar] [CrossRef]
- Chen, X.-P.; Wang, L.-M.; Sun, X.; Meng, R.-S.; Xiao, J.; Ye, H.-Y.; Zhang, G.-Q. Sulfur Dioxide and Nitrogen Dioxide Gas Sensor Based on Arsenene: A First-Principle Study. IEEE Electron. Device Lett. 2017, 38, 661–664. [Google Scholar] [CrossRef]
- Mao, Y.; Long, L.; Yuan, J.; Zhong, J.; Zhao, H. Toxic gases molecules (NH3, SO2 and NO2) adsorption on GeSe monolayer with point defects engineering. Chem. Phys. Lett. 2018, 706, 501–508. [Google Scholar] [CrossRef]
- Huang, C.-S.; Murat, A.; Babar, V.; Montes, E.; Schwingenschlögl, U. Adsorption of the Gas Molecules NH3, NO, NO2, and CO on Borophene. J. Phys. Chem. C 2018, 122, 14665–14670. [Google Scholar] [CrossRef]
- Cui, H.; Zheng, K.; Zhang, Y.; Ye, H.; Chen, X. Superior Selectivity and Sensitivity of C3N Sensor in Probing Toxic Gases NO2 and SO2. IEEE Electron. Device Lett. 2018, 39, 284–287. [Google Scholar] [CrossRef]
- Niu, F.; Yang, D.; Cai, M.; Li, X.; Liu, D. A First Principles Study of Blue Phosphorene as A Superior Media for Gas Sensor. ICEPT 2018, 1149–1152. [Google Scholar] [CrossRef]
- Ingale, N.; Konda, R.; Chaudhari, A. Organolithium complex as a gas sensing material for oxides from ab initio calculations and molecular dynamics simulations. Int. J. Quantum Chem. 2018, 118, e25623. [Google Scholar] [CrossRef]
- Wei, H.; Gui, Y.; Kang, J.; Wang, W.; Tang, C. A DFT Study on the Adsorption of H2S and SO2 on Ni Doped MoS2 Monolayer. Nanomaterials 2018, 8, 646. [Google Scholar] [CrossRef]
- Hussain, T.; Kaewmaraya, T.; Chakraborty, S.; Vovusha, H.; Amornkitbamrung, V.; Ahuja, R. Defected and Functionalized Germanene-based Nanosensors under Sulfur Comprising Gas Exposure. ACS Sens. 2018, 3, 867–874. [Google Scholar] [CrossRef]
- Liao, T.; Kou, L.; Du, A.; Chen, L.; Cao, C.; Sun, Z. H2S Sensing and Splitting on Atom-Functionalized Carbon Nanotubes: A Theoretical Study. Adv. Theory Simul. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Yong, Y.; Su, X.; Cui, H.; Zhou, Q.; Kuang, Y.; Li, X. Two-Dimensional Tetragonal GaN as Potential Molecule Sensors for NO and NO2 Detection: A First-Principle Study. ACS Omega 2017, 2, 8888–8895. [Google Scholar] [CrossRef]
- Yong, Y.; Cui, H.; Zhou, Q.; Su, X.; Kuang, Y.; Li, X. Adsorption of gas molecules on a graphitic GaN sheet and its implications for molecule sensors. RSC Adv. 2017, 7, 51027. [Google Scholar] [CrossRef]
- Spinelle, L.; Aleixandre, M.; Gerboles, M. Protocol of Evaluation and Calibration of Low-Cost Gas Sensors for the Monitoring of air Pollution; JRC Technical Reports (JRC83791); Publications Office of the European Union: Brussels, Belgium, 2013. [Google Scholar]
- Monroy, J.G.; Lilienthal, A.; Blanco, J.L.; González-Jimenez, J.; Trincavelli, M. Calibration of MOX gas sensors in open sampling systems based on Gaussian Processes. Sensors 2012, 2012, 1–4. [Google Scholar]
- Perma Pure, L.L.C. Gas Sensor Calibration, Chapter 11. In Hazardous Gas Monitors; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Alreshaid, A.T.; Hester, J.G.; Su, W.; Fang, Y.; Tentzeris, M.M. Review—Ink-Jet Printed Wireless Liquid and Gas Sensors for IoT, SmartAg and Smart City Applications. J. Electrochem. Soc. 2018, 165, B407–B413. [Google Scholar] [CrossRef]
- Ramaswamy, P. IoT smart parking system for reducing green-house gas emission. In Proceedings of the 2016 International Conference on Recent Trends in Information Technology (ICRTIT), Chennai, India, 8–9 April 2016; pp. 1–6. [Google Scholar]
- Suh, J.H.; Cho, I.; Kang, K.; Kweon, S.-J.; Lee, M.; Yoo, H.-J.; Park, I. Fully integrated and portable semiconductor-type multi-gas sensing module for IoT applications. Sens. Actuators B Chem. 2018, 265, 660–667. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Thirumurugan, T. Integrated IOT based design and Android operated Multi-purpose Field Surveillance Robot for Military Use. In Advances in Engineering Research, Proceedings of the International Conference for Phoenixes on Emerging Current Trends in Engineering and Management (PECTEAM 2018), 9–10 February 2018, Chennai, India; Atlantis Press: Paris, France, 2018; Volume 142, pp. 236–243. [Google Scholar]
- Rushikesh, R.; Sivappagari, C.M.R. Development of IoT based vehicular pollution monitoring system. In Proceedings of the 2015 International Conference on Green Computing and Internet of Things (ICGCIoT), Noida, India, 8–10 October 2015; pp. 779–783. [Google Scholar]
- Shu, L.; Mukherjee, M.; Wu, X. Toxic gas boundary area detection in large-scale petrochemical plants with industrial wireless sensor networks. IEEE Commun. Mag. 2016, 54, 22–28. [Google Scholar] [CrossRef]
- Alcantara, G.P. A short review of gas sensors based on interdigital electrode. IEEE ICEMI 2015, 12, 1616–1621. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).