Discrimination of Two Cultivars of Alpinia Officinarum Hance Using an Electronic Nose and Gas Chromatography-Mass Spectrometry Coupled with Chemometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. A. officinarum Material
2.2. E-Nose Equipment and Measurements
2.3. GC-MS Analysis
2.3.1. Preparation of Volatile Oil
2.3.2. The GC-MS Parameters and Conditions
2.4. Statistical Processing
3. Results
3.1. Application of the E-Nose to the Odor of Two A. officinarum Cultivars
3.1.1. Repeatability of E-Nose Experiment
3.1.2. E-nose Response of the Two A. officinarum Cultivars
3.1.3. Discrimination between the Two A. officinarum Cultivars by PCA
3.2. Investigation of the GC-MS Data from Two A. officinarum Cultivars
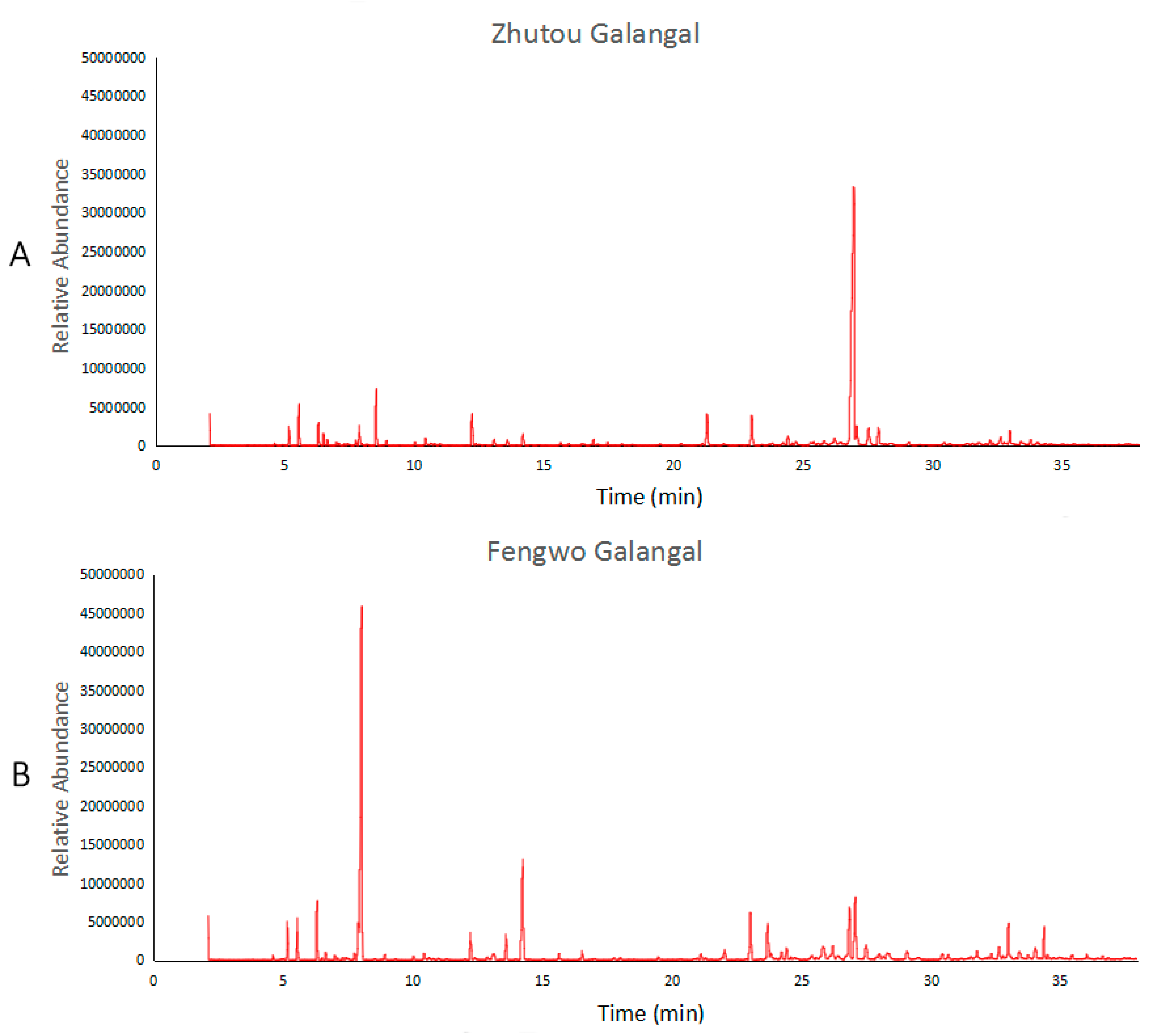
3.2.1. Identification and Comparison of Volatile Compounds between Zhutou Galangal and Fengwo Galangal
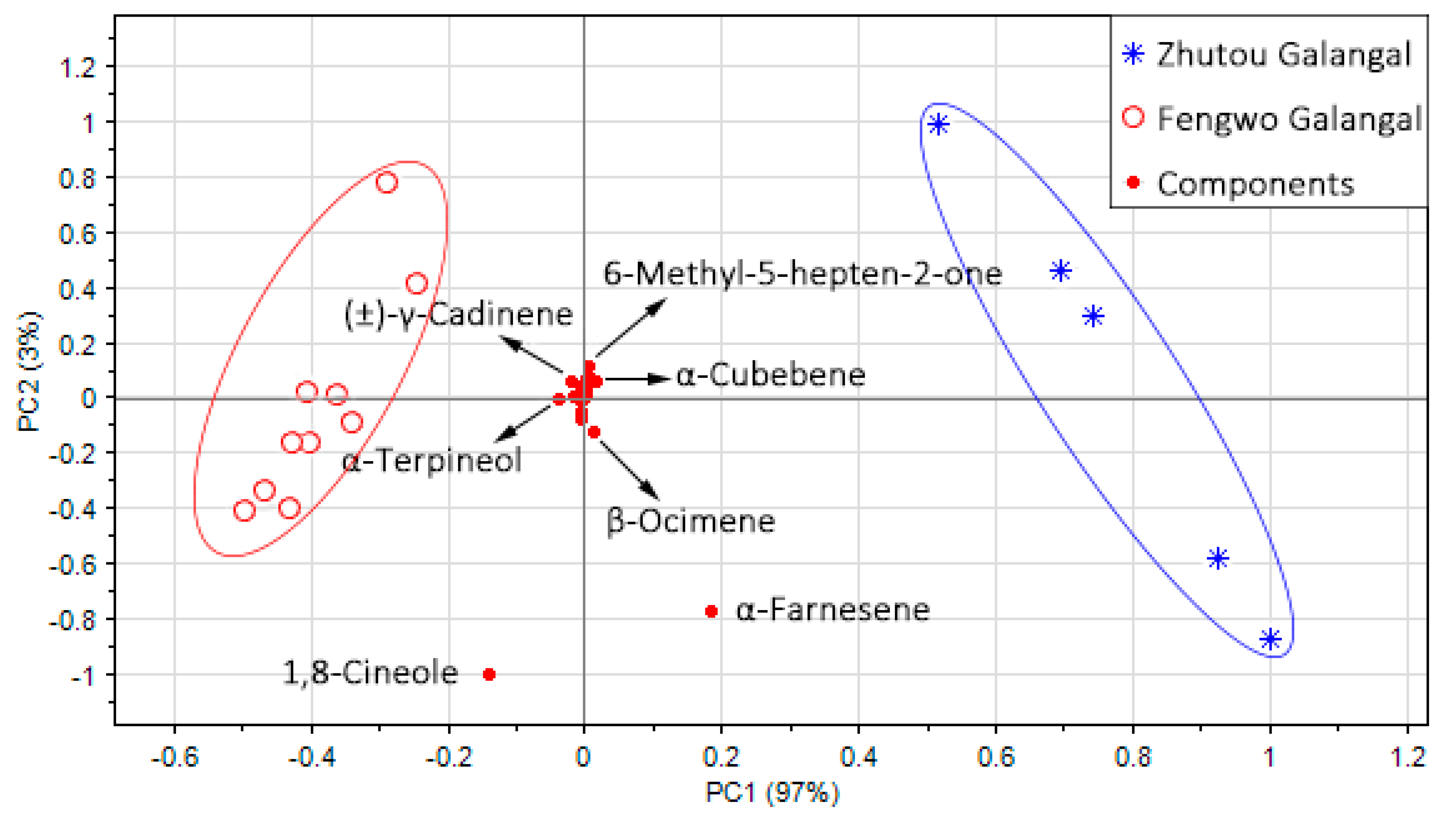
3.2.2. Analysis of Volatile Compounds of Two A. officinarum Cultivars by PCA
3.3. Correlation between E-Nose and GC-MS Data
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hadjzadeh, M.-A.-R.; Ghanbari, H.; Keshavarzi, Z.; Tavakol-Afshari, J. The Effects of Aqueous Extract of Alpinia Galangal on Gastric Cancer Cells (AGS) and L929 Cells in Vitro. Iran. J. Cancer Prev. 2014, 7, 142–146. [Google Scholar] [PubMed]
- Basri, A.M.; Taha, H.; Ahmad, N. A Review on the Pharmacological Activities and Phytochemicals of Alpinia officinarum (Galangal) Extracts Derived from Bioassay-Guided Fractionation and Isolation. Pharmacogn. Rev. 2017, 11, 43–56. [Google Scholar] [PubMed]
- Zhan, R.T.; Huang, H.B.; Pan, C.M.; Xu, H.H.; Chen, W.W. Morphological Comparison of Different Cultured Varieties of Rhizoma Alpiniae officinarum in GAP Base. Res. Pract. Chin. Med. 2007, 22, 3–6. [Google Scholar]
- Long, Q.; Lin, D.G.; Hu, J.L.; Li, Z.; Wang, S.M. Study on HPLC Fingerprint and Content Determination of Index Componentsof Different Cultivars of Alpinia officinarum Hance. J. Guangzhou Univ. Tradit. Chin. Med. 2019, 36, 109–114. [Google Scholar]
- Fang, D.Y.; Xiong, Z.M.; Xu, J.M.; Yin, J.; Luo, R.L. Chemopreventive mechanisms of galangin against hepatocellular carcinoma: A review. Biomed. Pharmacother. 2019, 109, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, Q.; Bi, J.; Ding, J.; Ge, S.; Chen, F. Galangin inhibits growth of human head and neck squamous carcinoma cells in vitro and in vivo. Chem. Biol. Interact. 2014, 224, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhu, L.; Ding, J.; Zhuang, X.; Xu, L.; Chen, F. Protective effect of galangin in Concanavalin A-induced hepatitis in mice. Drug Des. Dev. Ther. 2015, 9, 2983–2992. [Google Scholar]
- Jung, Y.C.; Kim, M.E.; Yoon, J.H.; Park, P.R.; Youn, H.-Y.; Lee, H.-W.; Lee, J.S. Anti-inflammatory effects of galangin on lipopolysaccharide-activated macrophages via ERK and NF-kappa B pathway regulation. Immunopharmacol. Immunotoxicol. 2014, 36, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Hamilton, V.E.S.; Chapman, D.G.; Taylor, P.W.; Lamb, A.J. Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin. J. Appl. Microbiol. 2007, 103, 1562–1567. [Google Scholar] [CrossRef]
- Honmore, V.S.; Kandhare, A.D.; Kadam, P.P.; Khedkar, V.M.; Sarkar, D.; Bodhankar, S.L.; Zanwar, A.A.; Rojatkar, S.R.; Natu, A.D. Isolates of Alpinia officinarum Hance as COX-2 inhibitors: Evidence from anti-inflammatory, antioxidant and molecular docking studies. Int. Immunopharmacol. 2016, 33, 8–17. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, D.; GholamHosseini, H.; Li, Z.; He, J. Identification of Chinese Herbal Medicines with Electronic Nose Technology: Applications and Challenges. Sensors 2017, 17, 1073. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, X.; Liu, L.; Zhang, R. A novel method for qualitative analysis of edible oil oxidation using an electronic nose. Food Chem. 2016, 202, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, S.; Sun, D.W. Application of the electronic nose to the identification of different milk flavorings. Food Res. Int. 2010, 43, 255–262. [Google Scholar] [CrossRef]
- Westenbrink, E.; Arasaradnam, R.P.; O’Connell, N.; Bailey, C.; Nwokolo, C.; Bardhan, K.D.; Covington, J.A. Development and application of a new electronic nose instrument for the detection of colorectal cancer. Biosens. Bioelectron. 2015, 67, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Kolk, A.; Hoelscher, M.; Maboko, L.; Jung, J.; Kuijper, S.; Cauchi, M.; Bessant, C.; van Beers, S.; Dutta, R.; Gibson, T.; et al. Electronic-Nose Technology Using Sputum Samples in Diagnosis of Patients with Tuberculosis. J. Clin. Microbiol. 2010, 48, 4235–4238. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.; Bandyopadhyay, R.; Bhattacharyya, N.; Pandey, R.A.; Jana, A. Application of electronic nose for industrial odors and gaseous emissions measurement and monitoring—An overview. Talanta 2015, 144, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J. Application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. Trac-Trends Anal. Chem. 2016, 77, 1–13. [Google Scholar] [CrossRef]
- Cui, S.Q.; Wu, J.F.; Wang, J.; Wang, X.L. Discrimination of American ginseng and Asian ginseng using electronic nose and gas chromatography-mass spectrometry coupled with chemometrics. J. Ginseng Res. 2017, 41, 85–95. [Google Scholar] [CrossRef]
- Ye, T.; Jin, C.; Zhou, J.; Li, X.; Wang, H.; Deng, P.; Yang, Y.; Wu, Y.; Xiao, X. Can odors of TCM be captured by electronic nose? The novel quality control method for musk by electronic nose coupled with chemometrics. J. Pharm. Biomed. Anal. 2011, 55, 1239–1244. [Google Scholar] [CrossRef]
- Luo, D.; Chen, H.; Yu, H.; Sun, Y. A Novel Approach for Classification of Chinese Herbal Medicines Using Diffusion Maps. Int. J. Pattern Recognit. Artif. Intell. 2015, 29, 1–18. [Google Scholar] [CrossRef]
- Xu, G.; Liao, C.; Ren, X.; Zhang, X.; Zhang, X.; Liu, S.; Fu, X.; Lin, H.; Wu, H.; Huang, L.; et al. Rapid assessment of quality of deer antler slices by using an electronic nose coupled with chemometric analysis. Rev. Bras. De Farmacogn. Braz. J. Pharmacogn. 2014, 24, 716–721. [Google Scholar] [CrossRef]
- Wold, S.; Sjostrom, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Pechous, S.W.; Whitaker, B.D. Cloning and functional expression of an (E,E)-alpha-farnesene synthase cDNA from peel tissue of apple fruit. Planta 2004, 219, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, Y.; Zhang, P.; Ren, M.; Xu, H.; Wang, X. A novel quality evaluation index and strategies to identify scenting quality of jasmine tea based on headspace volatiles analysis. Food Sci. Biotechnol. 2013, 22, 331–340. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, K.; Ni, X.X.; Bai, G.L. Study on the factors affecting the quality of Chinese medicinal materials. J. Chin. Med. Mater. 2016, 36, 1251–1256. [Google Scholar]
- Wang, C.C.; Cai, H.; Zhao, H.; Yan, Y.; Shi, J.J.; Chen, S.Y.; Tan, M.X.; Chen, J.L.; Zou, L.S.; Chen, C.H.; et al. Distribution patterns for metabolites in medicinal parts of wild and cultivated licorice. J. Pharm. Biomed. Anal. 2018, 161, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Jiang, C.; Xu, N.; Li, J.X.; Meng, F.Y.; Zhai, H.Q. Sorting and identification of Rehmannia glutinosa germplasm resources based on EST-SSR, scanning electron microscopy micromorphology, and quantitative taxonomy. Ind. Crop. Prod. 2018, 123, 303–314. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Zeng, X.; Peng, W.; Wu, Z.; Su, W.W. Study on the Discrimination between Citri Reticulatae Pericarpium Varieties Based on HS-SPME-GC-MS Combined with Multivariate Statistical Analyses. Molecules 2018, 23, 10. [Google Scholar] [CrossRef]
- Li, S.S.; Wu, Q.; Yin, D.D.; Feng, C.Y.; Liu, Z.A.; Wang, L.S. Phytochemical variation among the traditional Chinese medicine Mu Dan Pi from Paeonia suffruticosa (tree peony). Phytochemistry 2018, 146, 16–24. [Google Scholar] [CrossRef]
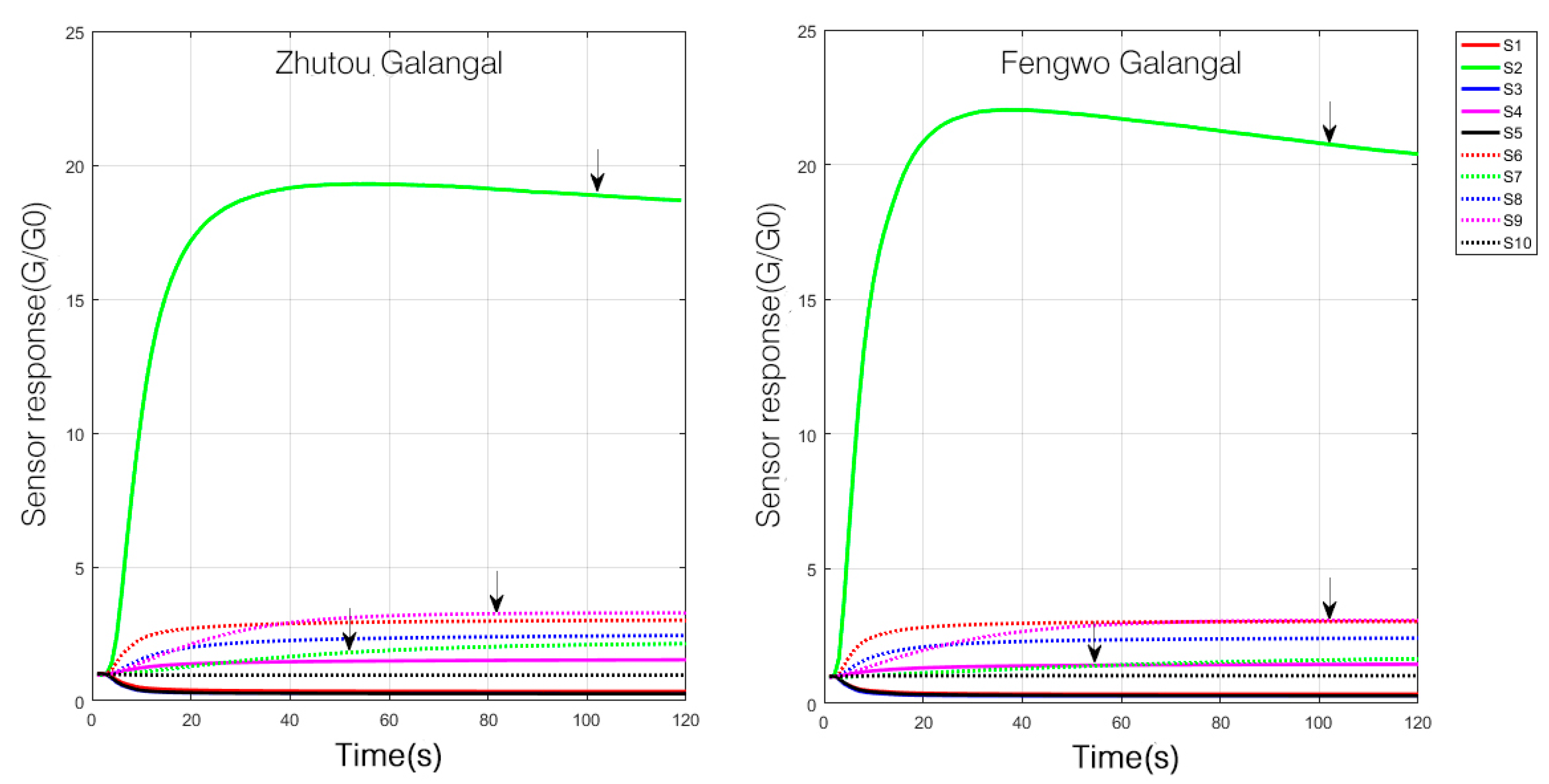
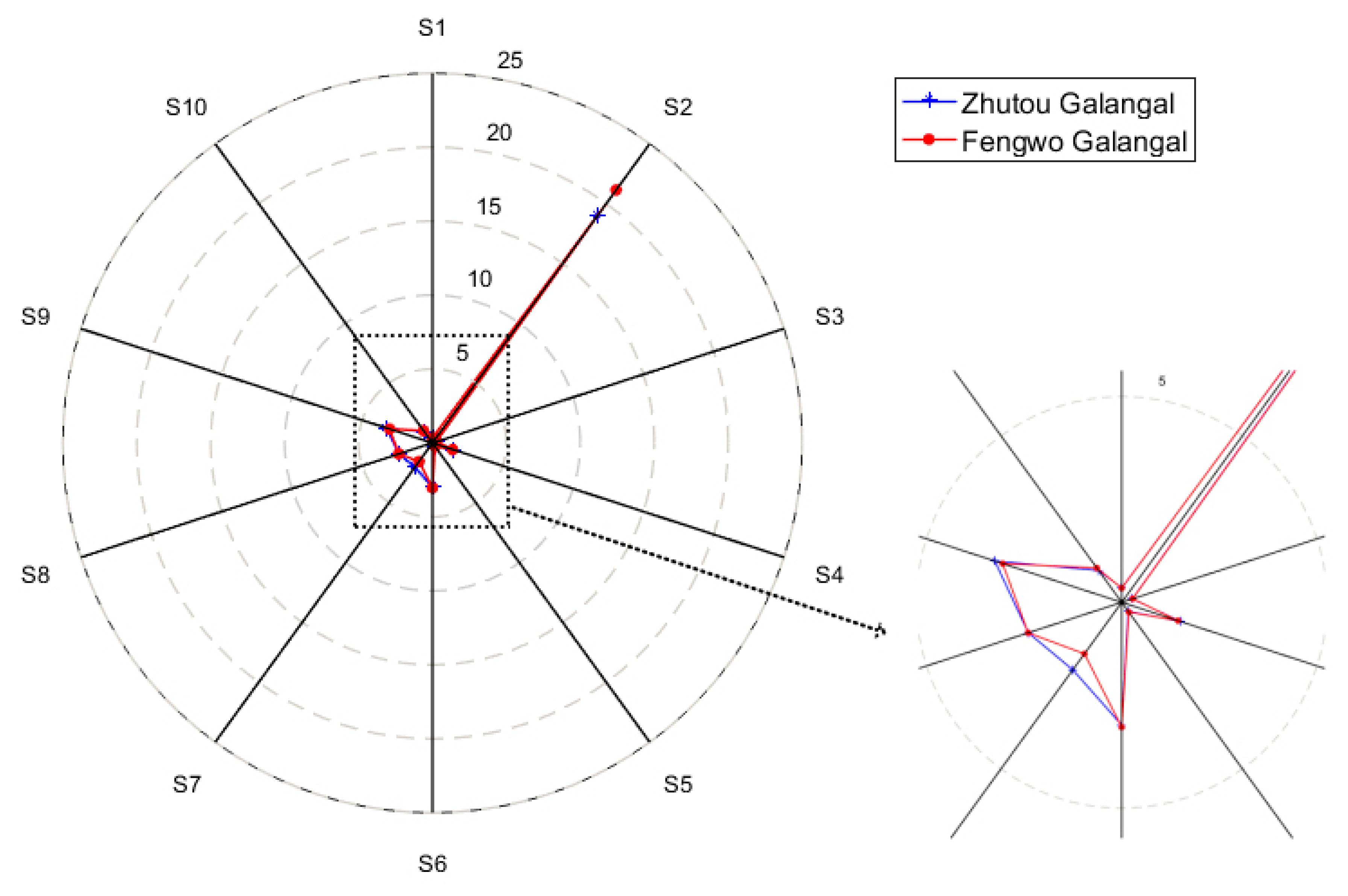
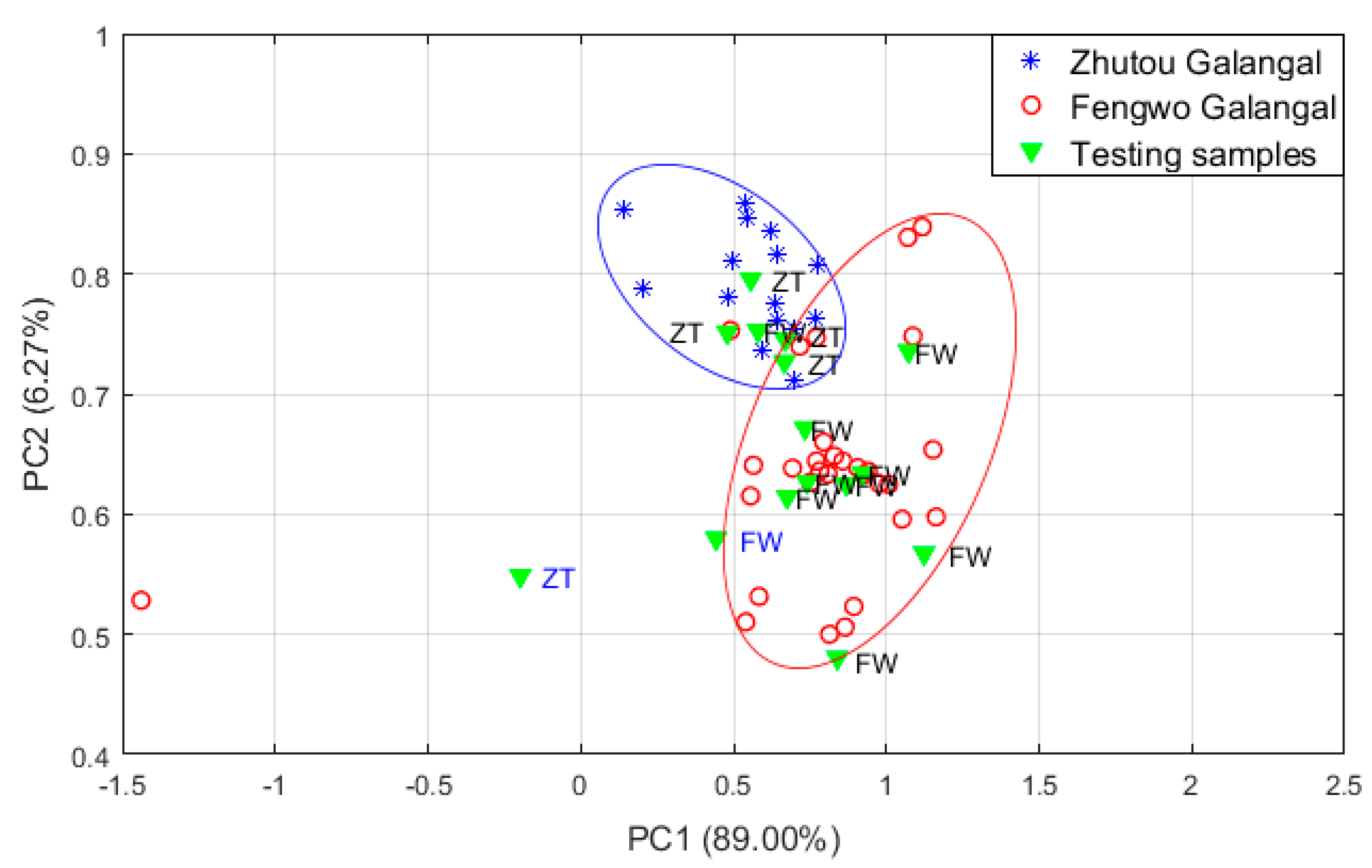
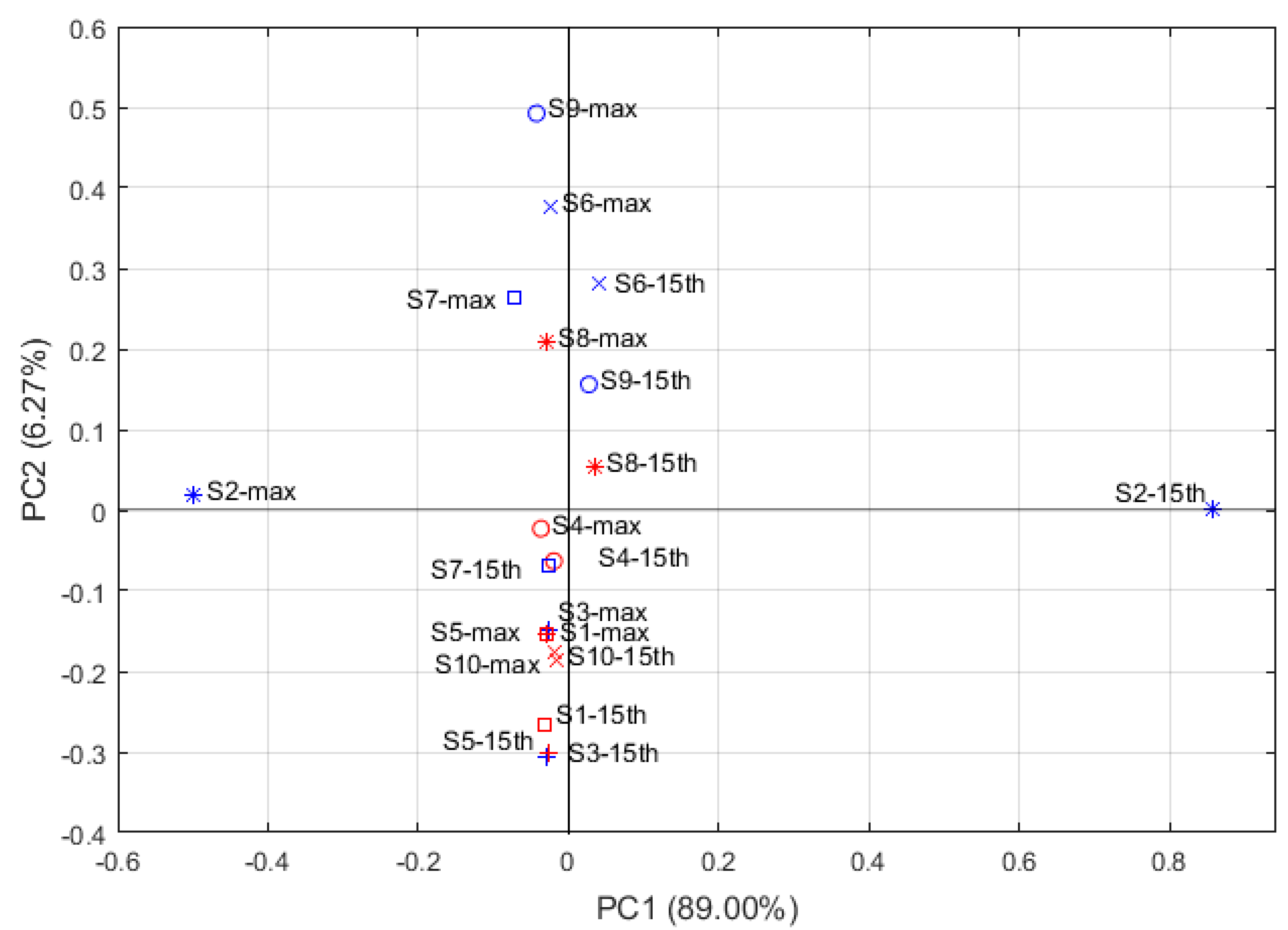

| No. | Detection of Chemical Components |
|---|---|
| S1 | Aromatic |
| S2 | Nitrogen Oxides |
| S3 | Ammonia, aromatic |
| S4 | hydrogen |
| S5 | Alkanes, aromatic ingredients |
| S6 | Methane |
| S7 | Sulfide |
| S8 | Ethanol |
| S9 | Aromatic ingredients, organic sulfur compounds |
| S10 | Alkanes |
| Sensor | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 |
|---|---|---|---|---|---|---|---|---|---|---|
| RSD/% | 0.93 | 3.40 | 0.80 | 0.65 | 0.79 | 4.20 | 3.15 | 3.98 | 4.13 | 0.74 |
| Peak No. | Category | Compound | CAS | Relative Content (%), Mean ± Standard | Selected 1 | |
|---|---|---|---|---|---|---|
| Zhutou Galangal | Fengwo Galangal | |||||
| 1 | Terpenes | Camphene | 79-92-5 | 1.83 ± 0.86 | 1.79 ± 0.67 | |
| 2 | β-Pinene | 127-91-3 | 1.36 ± 0.56 | 2.23 ± 0.86 | ||
| 3 | β-Myrcene | 123-35-3 | 0.326 ± 0.09 | 0.34 ± 0.11 | ||
| 4 | γ-Terpinene | 99-85-4 | 0.28 ± 0.11 | 0.32 ± 0.04 | ||
| 5 | Terpinolene | 586-62-9 | 0.23 ± 0.05 | 0.29 ± 0.08 | ||
| 6 | β-Caryophyllene | 87-44-5 | 3.48 ± 0.038 | 3.55 ± 1.14 | ||
| 7 | α-Caryophyllene | 6753-98-6 | 1.13 ± 0.08 | 1.02 ± 0.20 | ||
| 8 | γ-Muurolene | 30021-74-0 | 0.60 ± 0.11 | 0.79 ± 0.31 | ||
| 9 | α-Selinine | 473-13-2 | 1.87 ± 0.25 | 2.06 ± 0.26 | ||
| 10 | Germacrene B | 15423-57-1 | 0.50 ± 0.15 | 1.09 ± 0.67 | ||
| 11 | Calarene | 17334-55-3 | 0.41 ± 0.10 | 0.51 ± 0.04 | ||
| 12 | α-Elemene | 5951-67-7 | 0.42 ± 0.13 | 0.39 ± 0.03 | ||
| 13 | (-)-α-Pinene | 7785-26-4 | 0.78 ± 0.40 | 1.50 ± 0.65 * | 1 | |
| 14 | d-Limonene | 5989-27-5 | 1.27 ± 0.29 | 2.42 ± 0.59 * | 2 | |
| 15 | 1,8-Cineole | 470-82-6 | 0.37 ± 0.44 | 29.13 ± 4.16 * | 3 | |
| 16 | Camphor | 464-49-3 | 2.87 ± 0.41 | 1.78 ± 0.10 * | 4 | |
| 17 | Ylangene | 14912-44-8 | 0.24 ± 0.04 | 0.52 ± 0.05 * | 5 | |
| 18 | α-trans-Bergamotene | 13474-59-4 | 0.27 ± 0.05 | 3.40 ± 0.43 * | 6 | |
| 19 | α-Guaiene | 3691-12-1 | 0.29 ± 0.04 | 0.79 ± 0.09 * | 7 | |
| 20 | Isoledene | 95910-36-4 | 0.46 ± 0.04 | 0.77 ± 0.15 * | 8 | |
| 21 | β-Selinene | 17066-67-0 | 1.16 ± 0.22 | 2.33 ± 0.25 * | 9 | |
| 22 | α-Farnesene | 502-61-4 | 42.65 ± 9.83 | 6.00 ± 1.47 * | 10 | |
| 23 | ( ± )-γ-Cadinene | 39029-41-9 | 2.98 ± 0.46 | 7.15 ± 0.71 * | 11 | |
| 24 | (+)-δ-Cadinene | 483-76-1 | 3.22 ± 0.51 | 1.75 ± 0.18 * | 12 | |
| 25 | β-Ocimene | 13877-91-3 | 2.44 ± 1.35 | nd | 13 | |
| 26 | α-Cubebene | 17699-14-8 | 3.43 ± 0.42 | nd | 14 | |
| 27 | Alloaromadendrene | 25246-27-9 | 0.52 ± 0.09 | nd | 15 | |
| 28 | Cadina-1(6),4-diene | 16729-00-3 | 0.31 ± 0.04 | nd | 16 | |
| 29 | 1ξ,6ξ,7ξ-Cadina-4,9-diene | 31983-22-9 | 0.73 ± 0.15 | nd | 17 | |
| 30 | Epizonarene | 41702-63-0 | nd | 0.71 ± 0.14 | 18 | |
| 31 | γ-Selinene | 515-17-3 | nd | 0.72 ± 0.11 | 19 | |
| 32 | Selina-3,7(11)-diene | 6813-21-4 | nd | 1.18 ± 0.43 | 20 | |
| 33 | Alcohols | Borneol | 507-70-0 | 0.68 ± 0.18 | 0.69 ± 0.35 | |
| 34 | α-Cadinol | 481-34-5 | 2.30 ± 0.74 | 1.86 ± 0.55 | ||
| 35 | β-Bisabolol | 15352-77-9 | 0.67 ± 0.23 | 0.74 ± 0.13 | ||
| 36 | Linalool | 78-70-6 | 0.73 ± 0.13 | 0.45 ± 0.03 * | 21 | |
| 37 | Terpinen-4-ol | 562-74-3 | 0.62 ± 0.11 | 1.89 ± 0.8 * | 22 | |
| 38 | α-Terpineol | 98-55-5 | 1.45 ± 0.55 | 9.54 ± 0.82 * | 23 | |
| 39 | Epicubenol | 19912-67-5 | 0.45 ± 0.09 | 0.75 ± 0.05 * | 24 | |
| 40 | T-Cadinol | 5937-11-1 | 1.60 ± 0.38 | 0.86 ± 0.28 * | 25 | |
| 41 | α-Bisabolol | 515-69-5 | 0.90 ± 0.31 | 0.38 ± 0.07 * | 26 | |
| 42 | Juniper camphor | 473-04-1 | 0.39 ± 0.19 | 1.18 ± 0.14 * | 27 | |
| 43 | α-trans-Bergamotenol | 88034-74-6 | 0.32 ± 0.13 | 1.48 ± 0.18 * | 28 | |
| 44 | Geraniol | 106-24-1 | 0.44 ± 0.11 | nd | 29 | |
| 45 | Copaborneol | 21966-93-8 | 0.45 ± 0.12 | nd | 30 | |
| 46 | Epicubenol | 19912-67-5 | 0.73 ± 0.20 | nd | 31 | |
| 47 | Esters | Isobutyl 2-methylbutyrate | 2445-67-2 | 0.19 ± 0.05 | 0.22 ± 0.04 | |
| 48 | Fenchyl acetate | 13851-11-1 | 0.46 ± 0.29 | 0.48 ± 0.13 | ||
| 49 | 2-Methylbutyl-2-methyl-butyrate | 2445-78-5 | 0.30 ± 0.05 | nd | 32 | |
| 50 | 2-Methylbutyl-3-methyl-butanoate | 2445-77-4 | 0.18 ± 0.02 | nd | 33 | |
| 51 | Phenethyl butyrate | 103-52-6 | nd | 0.80 ± 0.19 | 34 | |
| 52 | Others | o-Cymene | 527-84-4 | 0.46 ± 0.15 | 0.47 ± 0.10 | |
| 53 | Benzylacetone | 2550-26-7 | 0.20 ± 0.07 | 0.39 ± 0.14 * | 35 | |
| 54 | 6-Methyl-5-hepten-2-one | 110-93-0 | 1.25 ± 0.70 | nd | 36 | |
| 55 | α-Citral | 141-27-5 | 0.22 ± 0.06 | nd | 37 | |
| 56 | Humulene oxide II | 19888-34-7 | 0.47 ± 0.20 | nd | 38 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Q.; Li, Z.; Han, B.; Gholam Hosseini, H.; Zhou, H.; Wang, S.; Luo, D. Discrimination of Two Cultivars of Alpinia Officinarum Hance Using an Electronic Nose and Gas Chromatography-Mass Spectrometry Coupled with Chemometrics. Sensors 2019, 19, 572. https://doi.org/10.3390/s19030572
Long Q, Li Z, Han B, Gholam Hosseini H, Zhou H, Wang S, Luo D. Discrimination of Two Cultivars of Alpinia Officinarum Hance Using an Electronic Nose and Gas Chromatography-Mass Spectrometry Coupled with Chemometrics. Sensors. 2019; 19(3):572. https://doi.org/10.3390/s19030572
Chicago/Turabian StyleLong, Qin, Zhong Li, Bin Han, Hamid Gholam Hosseini, Huaying Zhou, Shumei Wang, and Dehan Luo. 2019. "Discrimination of Two Cultivars of Alpinia Officinarum Hance Using an Electronic Nose and Gas Chromatography-Mass Spectrometry Coupled with Chemometrics" Sensors 19, no. 3: 572. https://doi.org/10.3390/s19030572
APA StyleLong, Q., Li, Z., Han, B., Gholam Hosseini, H., Zhou, H., Wang, S., & Luo, D. (2019). Discrimination of Two Cultivars of Alpinia Officinarum Hance Using an Electronic Nose and Gas Chromatography-Mass Spectrometry Coupled with Chemometrics. Sensors, 19(3), 572. https://doi.org/10.3390/s19030572






