An All-Solid-State Silicate Ion-Selective Electrode Using PbSiO3 as a Sensitive Membrane
Abstract
1. Introduction
2. Preparation of the Silicate Electrode
3. Results and Discussion
3.1. Linear Range, Response Time, and Reproductivity of the Silicate ISE
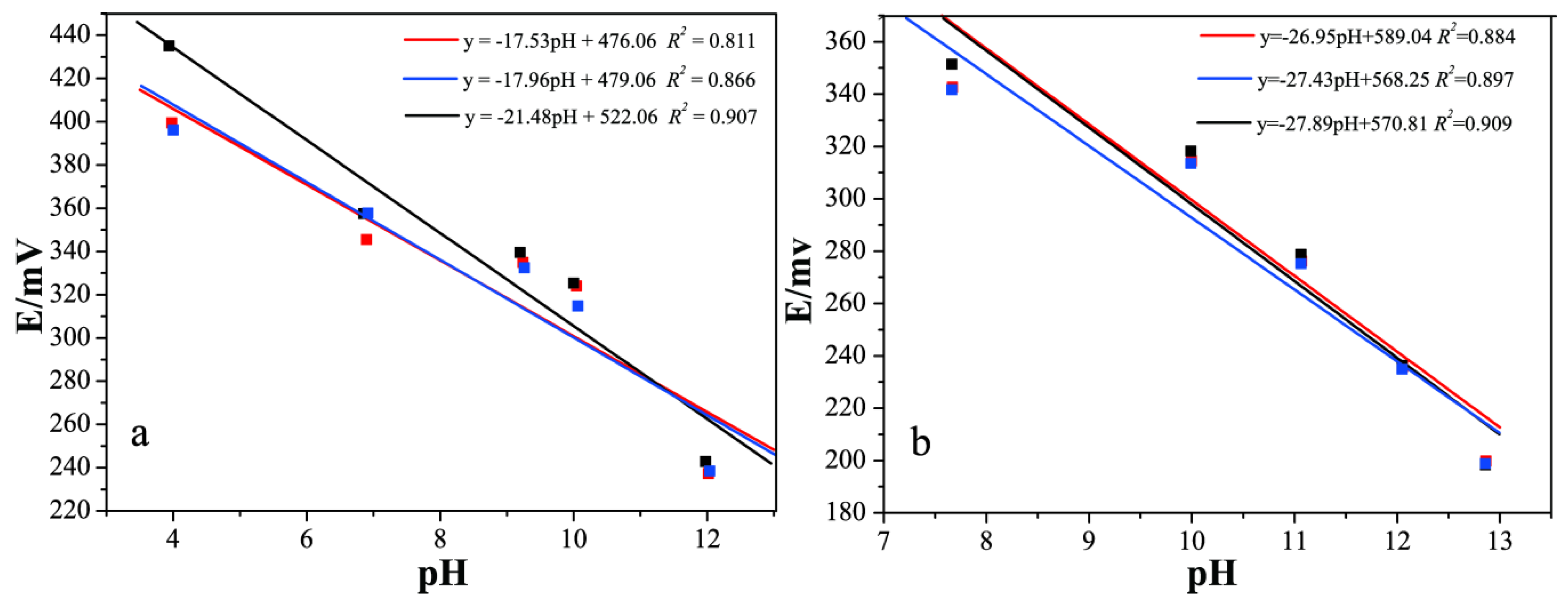
3.2. pH Response of the Prepared Silicate ISE
3.3. Selectivity
- ai = lower detection limit of primary ions when interfering ions existed
- aj = activity of the interfering ions
- zi = charge of the primary ions
- zj = charge of the interfering ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Burguera, M.; Burguera, J.L.; Carrero, P.; Rondon, C. A flow injection-ETAAS system for the on-line determination of total and dissolved silica in waters. Talanta 2002, 58, 1157–1166. [Google Scholar] [CrossRef]
- Svensen, C. Eutrophication and vertical flux: A critical evaluation of silicate addition. Mar. Ecol. Prog. Ser. 2002, 240, 21–26. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Danno, T.; Haginoya, M.; Yaso, Y. Simultaneous determination of silicate and phosphate in environmental waters using pre-column derivatization ion-pair liquid chromatography. Talanta 2009, 79, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Qi, L.; Tao, X.D.; Liu, J. Determination of Mn, Cr, Ni, Si, P., Cu, Mo in Chromium Nickel Stainless Steel by-ICP-AES. Chem. Anal. Meterage 2016, 25, 84–87. [Google Scholar]
- Proost, J.; Santoro, R.; Jeriban, S.A.; Guiot, I. Spectrophotometric determination of silicon in ultrapure, dilute hydrofluoric acid solutions. Microchem. J. 2008, 89, 48–51. [Google Scholar] [CrossRef]
- Pytlakowska, K. Energy-dispersive X-ray spectrometry combined with directly suspended droplet microextraction for determination of dissolved silicate in surface water via silicomolybdenum blue complex. Talanta 2014, 128, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Cheong, C.; Nonose, N.; Miura, T.; Hioki, A. Improved accuracy of determination of dissolved silicate in seawater using absorption spectrometry. Accredit. Qual. Assur. 2014, 19, 31–40. [Google Scholar] [CrossRef]
- Ma, J.; Byrne, R.H. Flow injection analysis of nanomolar silicate using long pathlength absorbance spectroscopy. Talanta 2012, 88, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Kozak, J.; Latocha, K.; Kochana, J.; Wiecaorek, M. Simultaneous spectrophotometric flow injection determination of phosphate and silicate. Talanta 2014, 133, 150–154. [Google Scholar] [CrossRef]
- Sabarudin, A.; Oshima, M.; Ishii, N.; Motomizu, S. Novel flow injection-fluorometric method for the determination of trace silicate and its application to ultrapurified water analysis. Talanta 2003, 60, 1277–1285. [Google Scholar] [CrossRef]
- Grudpan, K.; Ampan, P.; Udnan, Y.; Jayasvat, S. Stopped-flow injection simultaneous determination of phosphate and silicate using molybdenum blue. Talanta 2002, 58, 1319–1326. [Google Scholar] [CrossRef]
- Gallardogonzalez, J.; Baraket, A.; Bonhomme, A.; Zine, N. Sensitive potentiometric determination of amphetamine with an all-solid-state micro ion-selective-electrode. Anal. Lett. 2018, 3, 348–358. [Google Scholar] [CrossRef]
- Radu, A.; Anastasova, S.; Fay, C.; Diamond, D. Low cost, calibration-free sensors for in situ determination of natural water pollution. Sensors 2010, 143, 1487–1490. [Google Scholar]
- Zhao, Y.; Han, C.H.; Huang, Y.F.; Qin, W.L. New All-solid-state carbonate ion-selective electrode with Ag2CO3−BaCO3 as sensitive films. Chem. Res. Chin. Univ. 2016, 32, 655–660. [Google Scholar] [CrossRef]
- Huang, Y.F.; Ye, Y.; Zhao, C.G.; Wu, X.M. An all-solid-state phosphate electrode with H3PO4 doped polyaniline as the sensitive layer. Int. J. Electrochem. Sci. 2017, 12, 4677–4691. [Google Scholar] [CrossRef]
- Tang, W.; Ping, J.; Fan, K.; Wang, Y. All-solid-state nitrate-selective electrode and its application in drinking water. Electrochim. Acta 2012, 81, 186–190. [Google Scholar] [CrossRef]
- Kan, Y.T.; Han, C.H.; Ye, Y.; Zhang, X. An all-solid-state ammonium ion-selective electrode based on polyaniline as transducer and poly (o-phenylenediamine) as sensitive membrane. Int. J. Electrochem. Sci. 2016, 11, 9928–9940. [Google Scholar] [CrossRef]
- Ping, J.F.; Wang, Y.X.; Wu, J.; Ying, Y.B. Development of an all-solid-state potassium ion-selective electrode using graphene as the solid-contact transducer. Electrochem. Commun. 2011, 13, 1529–1532. [Google Scholar] [CrossRef]
- Yu, S.Y.; Li, F.H.; Qin, W. An all-solid-state Cd2+-selective electrode with a low detection limit. Sens. Actuators B Chem. 2011, 155, 919–922. [Google Scholar] [CrossRef]
- Lindfors, T.; Ivaska, A. All-solid-state calcium-selective electrode prepared of soluble electrically conducting polyaniline and di (2-ethylhexyl) phosphate with tetraoctylammonium chloride as cationic additive. Anal. Chim. Acta 2000, 404, 111–119. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Luo, Z.Y.; Pan, Y.W. A miniature all-solid-state calcium electrode applied to in situ seawater measurement. Meas. Sci. Technol. 2013, 24, 5105–5110. [Google Scholar] [CrossRef]
- Mikhelson, K.N. Ion-Selective Electrodes; Springer: Berlin, Germany, 2013. [Google Scholar]
- Xing, L.; Kan, Y.T.; Zhou, Y.F.; Ye, Y. Determination of sulfate in seawater by a novel all-solid- state sulfate sensor with H2SO4 doped polyaniline as sensitive membrane. Int. J. Electrochem. Sci. 2017, 12, 1506–1520. [Google Scholar] [CrossRef]
- Assirey, E.A. Development of a highly selective and sensitive sulfate-polymeric membrane sensor based on Nickel (II)-dioxime complex as neutral carrier. Desalin. Water Treat. 2016, 57, 3160–3167. [Google Scholar] [CrossRef]
- Pungor, E. The theory of ion-selective electrode. Anal. Sci. 1998, 14, 249–256. [Google Scholar] [CrossRef]
- Eren, H.; Uzun, H.; Andac, M.; Bilir, S. Potentiometric monitoring of cobalt in beer sample by solid contact ion selective electrode. J. Food Drug Anal. 2014, 22, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Berlin, H. Ion-Selective Microelectrodes Principles: Design and Application; Springer: New York, NY, USA, 1986. [Google Scholar]



| Interfering Ions | Slope | R2 | logKi,j |
|---|---|---|---|
| NO3− | −32.38 | 0.99 | −0.10 |
| SO42− | −32.38 | 0.98 | −1.06 |
| CH3COO− | −29.04 | 0.99 | −0.21 |
| Cl− | −23.43 | 0.93 | 1.11 |
| CO32− | −26.45 | 0.98 | −0.53 |
| PO43− | −26.07 | 0.96 | −1.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Chen, X.-G.; Tao, C.; Huang, Y.; Ye, Y.; Wang, Q.; Zhou, Y.; Jin, Q.; Cai, W. An All-Solid-State Silicate Ion-Selective Electrode Using PbSiO3 as a Sensitive Membrane. Sensors 2019, 19, 525. https://doi.org/10.3390/s19030525
Wu R, Chen X-G, Tao C, Huang Y, Ye Y, Wang Q, Zhou Y, Jin Q, Cai W. An All-Solid-State Silicate Ion-Selective Electrode Using PbSiO3 as a Sensitive Membrane. Sensors. 2019; 19(3):525. https://doi.org/10.3390/s19030525
Chicago/Turabian StyleWu, Rongrong, Xue-Gang Chen, Chunhui Tao, Yuanfeng Huang, Ying Ye, Qiujin Wang, Yifan Zhou, Quan Jin, and Wei Cai. 2019. "An All-Solid-State Silicate Ion-Selective Electrode Using PbSiO3 as a Sensitive Membrane" Sensors 19, no. 3: 525. https://doi.org/10.3390/s19030525
APA StyleWu, R., Chen, X.-G., Tao, C., Huang, Y., Ye, Y., Wang, Q., Zhou, Y., Jin, Q., & Cai, W. (2019). An All-Solid-State Silicate Ion-Selective Electrode Using PbSiO3 as a Sensitive Membrane. Sensors, 19(3), 525. https://doi.org/10.3390/s19030525






