Direct Electrochemical Detection of Glutamate, Acetylcholine, Choline, and Adenosine Using Non-Enzymatic Electrodes
Abstract
1. Introduction
2. Neurotransmitter Characteristics
2.1. Neurotransmitter Detection
2.2. Challenges of Measuring Neurotransmitters
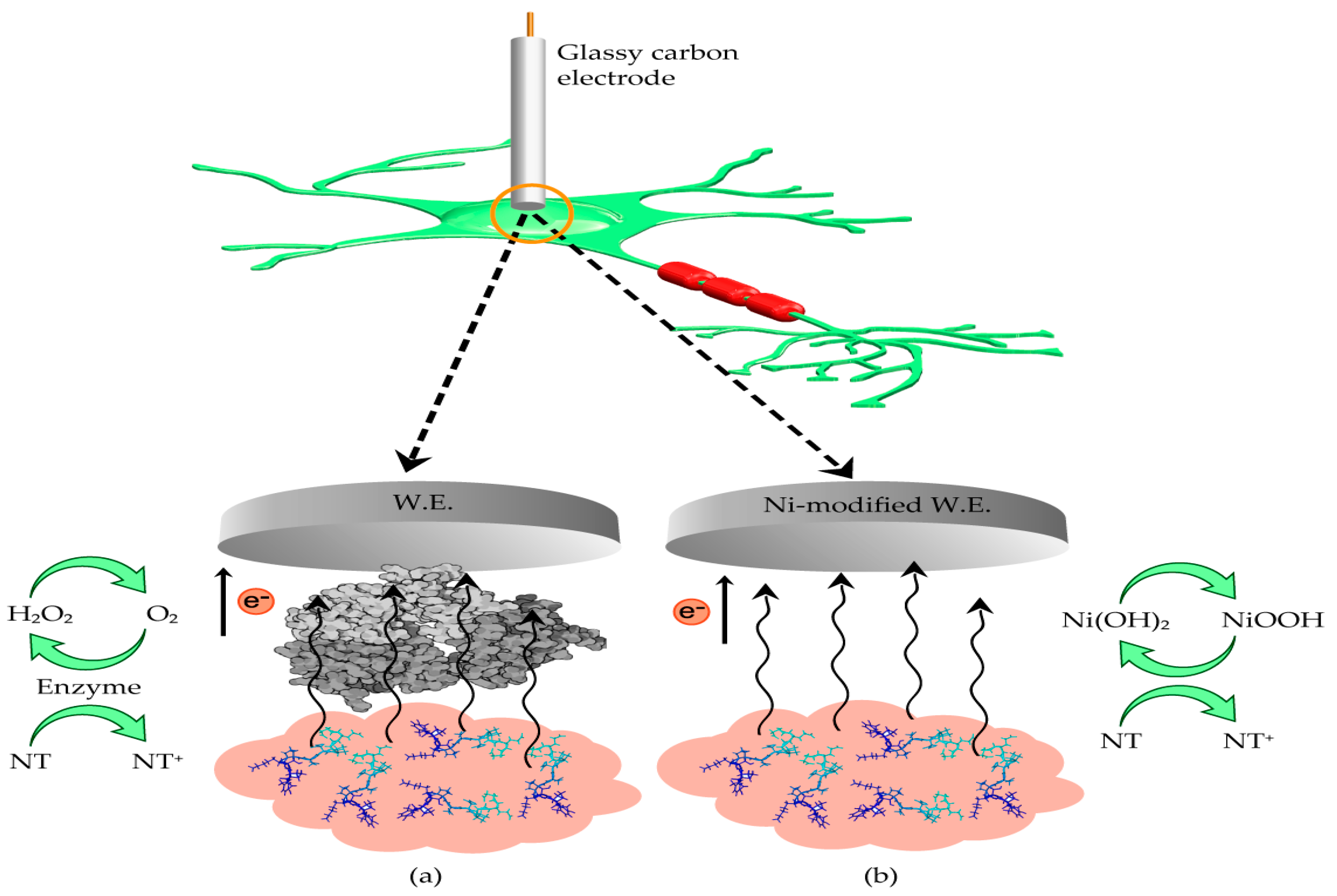
2.3. Enzymatic vs. Non-Enzymatic Electrochemical Neurotransmitter Biosensors
3. Materials for Non-electroactive Neurotransmitter Detection
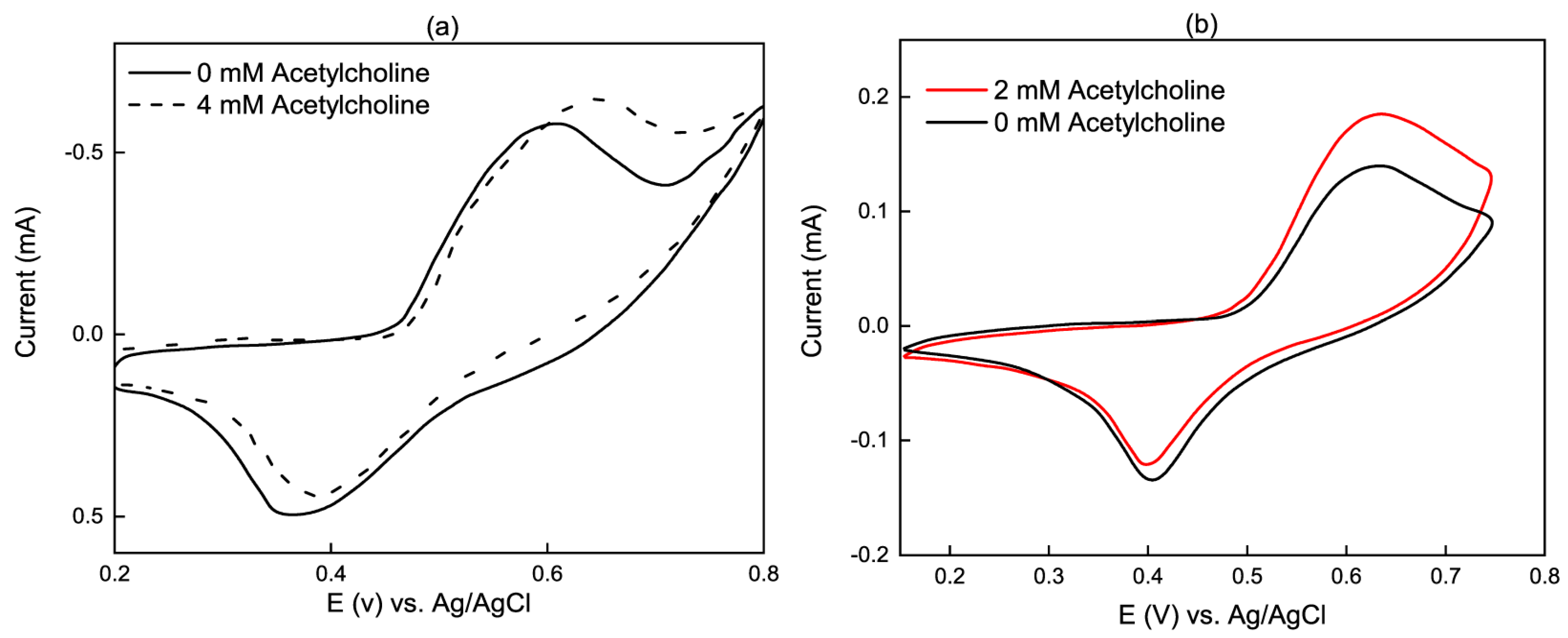
3.1. Electrochemical Reactions on Non-Enzymatic Electrodes
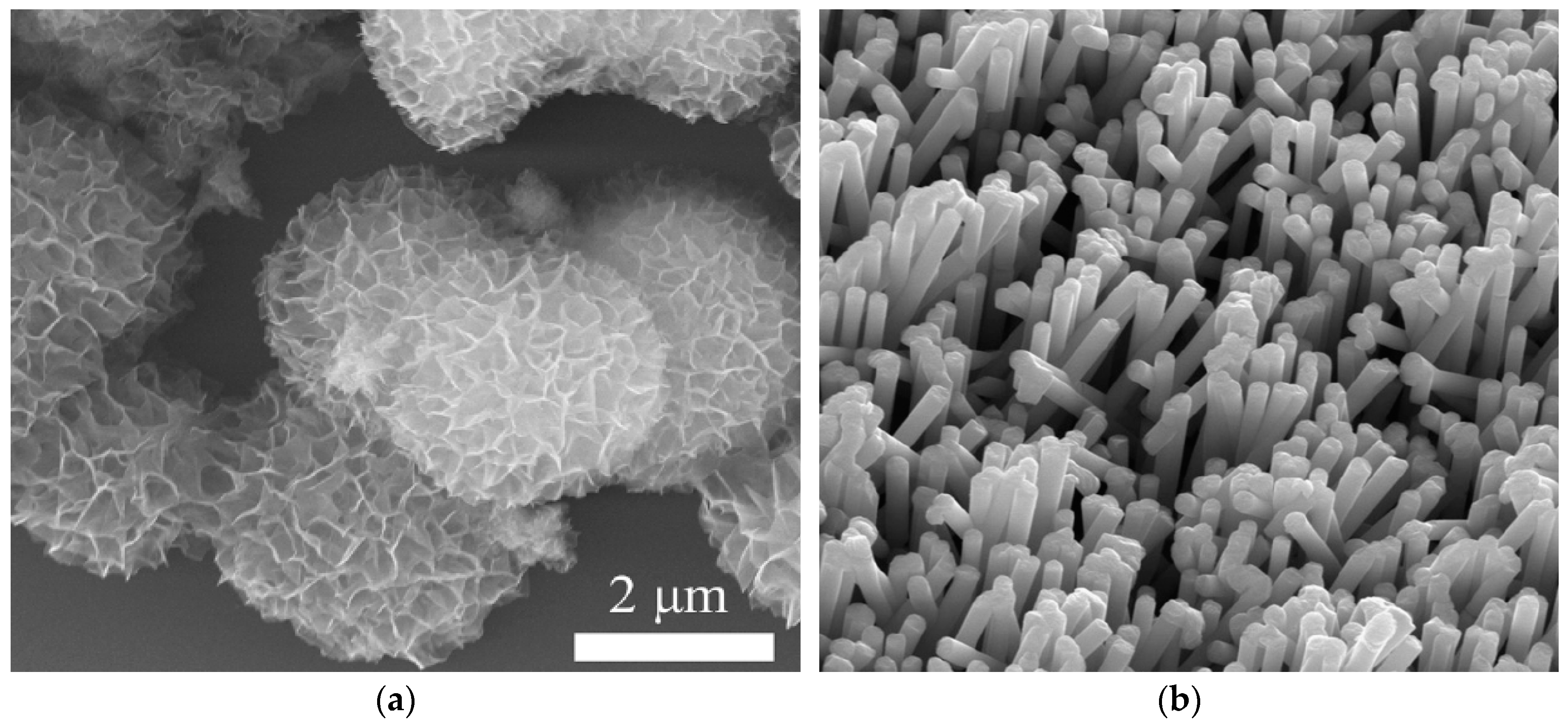
3.2. Commonly Used Materials
4. Enzymatic vs. Non-Enzymatic Neurotransmitter Sensors
5. In Vivo Applicability
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Tomkins, D.M.; Sellers, E.M. Addiction and the Brain: The Role of Neurotransmitters in the Cause and Treatment of Drug Dependence. Can. Med. Assoc. J. 2001, 164, 817–821. [Google Scholar]
- Moon, J.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y. Conducting Polymer-Based Electrochemical Biosensors for Neurotransmitters: A Review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Kavalali, E.T. The Mechanisms and Functions of Spontaneous Neurotransmitter Release. Nat. Rev. Neurosci. 2015, 16, 5–16. [Google Scholar]
- Pradhan, T.; Jung, H.S.; Jang, J.H.; Kim, T.W.; Kang, C.; Kim, J.S. Chemical Sensing of Neurotransmitters. Chem. Soc. Rev. 2014, 43, 4684–4713. [Google Scholar] [PubMed]
- Li, Y.; Li, S.; Zhang, Y.; Zhong, Y. Enzyme-Free Hydrogen Peroxide Sensor Based on Au@Ag@C Core-Double Shell Nanocomposites. Appl. Surf. Sci. 2015, 347, 428–434. [Google Scholar]
- Ikeno, S.; Haruyama, T. Biological Phosphate Ester Sensing using an Artificial Enzyme PMP Complex. Sens. Actuators B Chem. 2005, 108, 608–612. [Google Scholar]
- Bucher, E.S.; Wightman, R.M. Electrochemical Analysis of Neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239–261. [Google Scholar]
- Si, B.; Song, E. Recent Advances in the Detection of Neurotransmitters. Chemosensors 2018, 6, 1. [Google Scholar]
- Chandra, S.; Siraj, S.; Wong, D.K.Y. Recent Advances in Biosensing for Neurotransmitters and Disease Biomarkers using Microelectrodes. ChemElectroChem 2017, 4, 822–833. [Google Scholar]
- Patestas, M.; Gartner, L.P. A Textbook of Neuroanatomy; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Borisova, T.; Kucherenko, D.; Kucherenko, I.; Soldatkin, A.; Soldatkin, O.; Pastukhov, A.; Nazarova, A.; Galkin, M.; Borysov, A.; Krisanova, N.; et al. An Amperometric Glutamate Biosensor for Monitoring Glutamate Release from Brain Nerve Terminals and in Blood Plasma. Anal. Chim. Acta 2018, 1022, 113–123. [Google Scholar]
- Perry, E. Neurochemistry of Consciousness; John Benjamins Publishing Company: Philadelphia, PA, USA, 2002; Volume 36. [Google Scholar]
- Magar, H.S.; Ghica, M.E.; Abbas, M.N.; Brett, C.M.A. A Novel Sensitive Amperometric Choline Biosensor Based on Multiwalled Carbon Nanotubes and Gold Nanoparticles. Talanta 2017, 167, 462–469. [Google Scholar]
- Haskó, G.; Antonioli, L.; Cronstein, B.N. Adenosine Metabolism, Immunity and Joint Health. Biochem. Pharmacol. 2018, 151, 307–313. [Google Scholar] [PubMed]
- Batra, B.; Pundir, C.S. An Amperometric Glutamate Biosensor Based on Immobilization of Glutamate Oxidase onto Carboxylated Multiwalled Carbon Nanotubes/Gold Nanoparticles/Chitosan Composite Film Modified Au Electrode. Biosens. Bioelectron. 2013, 47, 496–501. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [PubMed]
- Farka, Z.; Juřík, T.; Kovář, D.; Trnková, L.; Skládal, P. Nanoparticle-Based Immunochemical Biosensors and Assays: Recent Advances and Challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical Sensor and Biosensor Platforms Based on Advanced Nanomaterials for Biological and Biomedical Applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarzadeh, A.; Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; Gharaatifar, N.; Hasanzadeh, M.; Baradaran, B.; de la Guardia, M. Nanomaterial-Based Biosensors for Detection of Pathogenic Virus. Trends Anal. Chem. 2017, 97, 445–457. [Google Scholar]
- Grumezescu, A. Nanobiosensors.; Elsevier Science: San Diego, CA, USA, 2016. [Google Scholar]
- Isoaho, N.; Peltola, E.; Sainio, S.; Wester, N.; Protopopova, V.; Wilson, B.P.; Koskinen, J.; Laurila, T. Carbon Nanostructure Based Platform for Enzymatic Glutamate Biosensors. J. Phys. Chem. C 2017, 121, 4618–4626. [Google Scholar]
- Jesus, M.D.L.F.; Grazu, V. Nanobiotechnology: Inorganic Nanoparticles Vs Organic Nanoparticles; Elsevier: London, UK, 2012; Volume 4. [Google Scholar]
- Wang, L.; Chen, X.; Liu, C.; Yang, W. Non-Enzymatic Acetylcholine Electrochemical Biosensor Based on Flower-Like NiAl Layered Double Hydroxides Decorated with Carbon Dots. Sens. Actuators B Chem. 2016, 233, 199–205. [Google Scholar]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for Biosensing Applications: A Review. Front. Chem. 2014, 2, 63. [Google Scholar] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their Applications—A Review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, L.; Duan, H. Nanomaterial Based Electrochemical Sensors for in Vitro Detection of Small Molecule Metabolites. Biotechnol. Adv. 2016, 34, 234–249. [Google Scholar] [CrossRef]
- Jamal, M.; Hasan, M.; Mathewson, A.; Razeeb, K.M. Disposable Sensor Based on Enzyme-Free Ni Nanowire Array Electrode to Detect Glutamate. Biosens. Bioelectron. 2013, 40, 213–218. [Google Scholar]
- Ahmad, R.; Tripathy, N.; Ahn, M.; Bhat, K.S.; Mahmoudi, T.; Wang, Y.; Yoo, J.; Kwon, D.; Yang, H.; Hahn, Y. Highly Efficient Non-Enzymatic Glucose Sensor Based on CuO Modified Vertically-Grown ZnO Nanorods on Electrode. Sci. Rep. 2017, 7, 1–10. [Google Scholar]
- Shen, Y.; Sheng, Q.; Zheng, J. A High-Performance Electrochemical Dopamine Sensor Based on a Platinum–nickel Bimetallic Decorated Poly(Dopamine)-Functionalized Reduced Graphene Oxide Nanocomposite. Anal. Methods 2017, 9, 4566–4573. [Google Scholar]
- Jamal, M.; Chakrabarty, S.; Shao, H.; McNulty, D.; Yousuf, M.A.; Furukawa, H.; Khosla, A.; Razeeb, K.M. A Non Enzymatic Glutamate Sensor Based on Nickel Oxide Nanoparticle. Microsyst. Technol. 2018, 24, 4217–4223. [Google Scholar] [CrossRef]
- Ju, J.; Bai, J.; Bo, X.; Guo, L. Non-Enzymatic Acetylcholine Sensor Based on Ni–Al Layered Double Hydroxides/Ordered Mesoporous Carbon. Electrochim. Acta 2012, 78, 569–575. [Google Scholar] [CrossRef]
- Tang, L.; Zhu, Y.; Xu, L.; Yang, X.; Li, C. Amperometric Glutamate Biosensor Based on Self-Assembling Glutamate Dehydrogenase and Dendrimer-Encapsulated Platinum Nanoparticles onto Carbon Nanotubes. Talanta 2007, 73, 438–443. [Google Scholar] [PubMed]
- Batra, B.; Kumari, S.; Pundir, C.S. Construction of Glutamate Biosensor Based on Covalent Immobilization of Glutmate Oxidase on Polypyrrole Nanoparticles/Polyaniline Modified Gold Electrode. Enzym. Microb. Technol. 2014, 57, 69–77. [Google Scholar]
- Chang, C.; Chang, K.; Hsu, W.; Chen, H.; Chen, C. Determination of Glutamate Pyruvate Transaminase Activity in Clinical Specimens using a Biosensor Composed of Immobilized L-Glutamate Oxidase in a Photo-Crosslinkable Polymer Membrane on a Palladium-Deposited Screen-Printed Carbon Electrode. Anal. Chim. Acta 2003, 481, 199–208. [Google Scholar]
- Heli, H.; Hajjizadeh, M.; Jabbari, A.; Moosavi-Movahedi, A.A. Copper Nanoparticles-Modified Carbon Paste Transducer as a Biosensor for Determination of Acetylcholine. Biosens. Bioelectron. 2009, 24, 2328–2333. [Google Scholar]
- Kanik, F.E.; Kolb, M.; Timur, S.; Bahadir, M.; Toppare, L. An Amperometric Acetylcholine Biosensor Based on a Conducting Polymer. Int. J. Biol. Macromol. 2013, 59, 111–118. [Google Scholar]
- Chauhan, N.; Chawla, S.; Pundir, C.S.; Jain, U. An Electrochemical Sensor for Detection of Neurotransmitter-Acetylcholine using Metal Nanoparticles, 2D Material and Conducting Polymer Modified Electrode. Biosens. Bioelectron. 2017, 89, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Tunç, A.T.; Aynacı Koyuncu, E.; Arslan, F. Development of an Acetylcholinesterase-Choline Oxidase Based Biosensor for Acetylcholine Determination. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Sattarahmady, N.; Heli, H.; Moosavi-Movahedi, A.A. An Electrochemical Acetylcholine Biosensor Based on Nanoshells of Hollow Nickel Microspheres-Carbon Microparticles-Nafion Nanocomposite. Biosens. Bioelectron. 2010, 25, 2329–2335. [Google Scholar] [CrossRef]
- Sattarahmady, N.; Heli, H.; Vais, R.D. An Electrochemical Acetylcholine Sensor Based on Lichen-Like Nickel Oxide Nanostructure. Biosens. Bioelectron. 2013, 48, 197–202. [Google Scholar]
- Blanco, S.; Vargas, R.; Mostany, J.; Borrás, C.; Scharifker, B.R. A Novel Nickel Nanowire Amperometric Sensor: Direct Current Vs. Alternating Current Strategies for Ethanol, Acetaldehyde and Acetylcholine Detection. J. Electroanal. Chem. 2015, 740, 61–67. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Beenakumari, K.S. Electrodeposited Nickel/Platinum Alloy as a Biosensor for Acetyl Choline. Electroanalysis 2006, 18, 465–470. [Google Scholar]
- Lin, S.; Liu, C.; Chou, T. Amperometric Acetylcholine Sensor Catalyzed by Nickel Anode Electrode. Biosens. Bioelectron. 2004, 20, 9–14. [Google Scholar] [PubMed]
- Ampurdanés, J.; Crespo, G.A.; Maroto, A.; Sarmentero, M.A.; Ballester, P.; Rius, F.X. Determination of Choline and Derivatives with a Solid-Contact Ion-Selective Electrode Based on Octaamide Cavitand and Carbon Nanotubes. Biosens. Bioelectron. 2009, 25, 344–349. [Google Scholar] [PubMed]
- Chen, Y.; Chen, H.; Tsai, R.; Lee, R.; Hua, M. A Colloidal Suspension of Nanostructured Poly(N-Butyl Benzimidazole)-Graphene Sheets with High Oxidase Yield for Analytical Glucose and Choline Detections. Anal. Chim. Acta 2013, 792, 101–109. [Google Scholar] [PubMed]
- Zhang, Z.; Wang, X.; Yang, X. A Sensitive Choline Biosensor using Fe3O4 Magnetic Nanoparticles as Peroxidase Mimics. Analyst 2011, 136, 496–4965. [Google Scholar]
- Zhang, L.; Chen, J.; Wang, J.; Wang, Y.; Yu, L.; Peng, H.; Zhu, J. Improved Enzyme Immobilization for Enhanced Bioelectrocatalytic Activity of Choline Sensor and Acetylcholine Sensor. Sens. Actuators B Chem. 2014, 193, 904–910. [Google Scholar]
- Sattarahmady, N.; Heli, H.; Dehdari Vais, R. A Flower-Like Nickel Oxide Nanostructure: Synthesis and Application for Choline Sensing. Talanta 2014, 119, 207–213. [Google Scholar] [PubMed]
- Carballo, R.; Dall’Orto, V.C.; Rezzano, I. Poly[Ni(II)Protoporphyrin IX] Modified Electrode for Amperometric Detection of Acetylcholine (Ach) and Choline (Ch). Anal. Lett. 2007, 40, 1962–1971. [Google Scholar]
- Lin, S.; Chou, T. Amperometric Acetylcholine Sensor using Electrochemical Deposition Ni Electrode. J. Electrochem. Soc. 2005, 152, H53. [Google Scholar]
- Mashhadizadeh, M.; Naseri, N.; Mehrgardi, M. A Simple Non-Enzymatic Strategy for Adenosine Triphosphate Electrochemical Aptasensor using Silver Nanoparticle-Decorated Graphene Oxide. J. Iran. Chem. Soc. 2017, 14, 2007–2016. [Google Scholar]
- Llaudet, E.; Hatz, S.; Droniou, M.; Dale, N. Microelectrode Biosensor for Real-Time Measurement of ATP in Biological Tissue. Anal. Chem. 2005, 77, 3267–3273. [Google Scholar] [CrossRef]
- Kueng, A.; Kranz, C.; Mizaikoff, B. Amperometric ATP Biosensor Based on Polymer Entrapped Enzymes. Biosens. Bioelectron. 2004, 19, 1301–1307. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Didukh, D.Y.; Soldatkin, O.O.; Soldatkin, A.P. Amperometric Biosensor System for Simultaneous Determination of Adenosine-5′-Triphosphate and Glucose. Anal. Chem. 2014, 86, 5455–5462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Jiang, B.; Xiang, Y.; Chai, Y.; Yuan, R. Aptamer/Quantum Dot-Based Simultaneous Electrochemical Detection of Multiple Small Molecules. Anal. Chim. Acta 2011, 688, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Liu, J.; He, D.; He, X.; Wang, K.; Shi, H.; Wen, L. Aptamer/Target Binding-Induced Triple Helix Forming for Signal-on Electrochemical Biosensing. Talanta 2015, 143, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Shibli, S.M.A.; Beenakumari, K.S.; Suma, N.D. Nano Nickel Oxide/Nickel Incorporated Nickel Composite Coating for Sensing and Estimation of Acetylcholine. Biosens. Bioelectron. 2006, 22, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Pemberton, R.M.; Fielden, P.R.; Hart, J.P. The Design, Development and Application of Electrochemical Glutamate Biosensors. Trends Anal. Chem. 2016, 79, 106–113. [Google Scholar] [CrossRef]
- Keihan, A.H.; Sajjadi, S.; Sheibani, N.; Moosavi-Movahedi, A.A. A Highly Sensitive Choline Biosensor Based on Bamboo-Like Multiwall Carbon Nanotubes/Ionic Liquid/Prussian Blue Nanocomposite. Sens. Actuators B Chem. 2014, 204, 694–703. [Google Scholar]
- Soldatkin, O.O.; Soldatkin, A.P.; Schuvailo, O.M.; Marinesco, S.; Cespuglio, R. Microbiosensor Based on Glucose Oxidase and Hexokinase Co-Immobilised on Platinum Microelectrode for Selective ATP Detection. Talanta 2009, 78, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Bolat, E.Ö.; Tığ, G.A.; Pekyardımcı, Ş. Fabrication of an Amperometric Acetylcholine Esterase-Choline Oxidase Biosensor Based on MWCNTs-Fe3O4NPs-CS Nanocomposite for Determination of Acetylcholine. J. Electroanal. Chem. 2017, 785, 241–248. [Google Scholar]
- Yabuki, S. Supporting Materials that Improve the Stability of Enzyme Membranes. Anal. Sci. 2014, 30, 213–217. [Google Scholar]
- Soldatkina, O.; Soldatkin, O.; Kasap, B.; Kucherenko, D.; Kucherenko, I.; Kurc, B.; Dzyadevych, S. A Novel Amperometric Glutamate Biosensor Based on Glutamate Oxidase Adsorbed on Silicalite. Nanoscale Res. Lett. 2017, 12, 1–8. [Google Scholar]
- Govindarajan, S.; McNeil, C.J.; Lowry, J.P.; McMahon, C.P.; O’Neill, R.D. Highly Selective and Stable Microdisc Biosensors for L-Glutamate Monitoring. Sens. Actuators B Chem. 2013, 178, 606–614. [Google Scholar]
- Bai, W.; Zhu, W.; Ning, Y.; Li, P.; Zhao, Y.; Yang, N.; Chen, X.; Jiang, Y.; Yang, W.; Jiang, D.; et al. Dramatic Increases in Blood Glutamate Concentrations are Closely Related to Traumatic Brain Injury-Induced Acute Lung Injury. Sci. Rep. 2017, 7, 5380–5389. [Google Scholar]
- Wahono, N.; Qin, S.; Oomen, P.; Cremers, T.I.F.; de Vries, M.G.; Westerink, B.H.C. Evaluation of Permselective Membranes for Optimization of Intracerebral Amperometric Glutamate Biosensors. Biosens. Bioelectron. 2012, 33, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Song, Y.; Wang, L.; Zhang, S.; Luo, J.; Xu, S.; Cai, X. An Implantable Microelectrode Array for Simultaneous L-Glutamate and Electrophysiological Recordings in Vivo. Microsyst. Nanoeng. 2015, 1, 15002. [Google Scholar] [CrossRef]
- Al-Omair, M.A.; Touny, A.H.; Al-Odail, F.A.; Saleh, M.M. Electrocatalytic Oxidation of Glucose at Nickel Phosphate Nano/Micro Particles Modified Electrode. Electrocatalysis 2017, 8, 340–350. [Google Scholar] [CrossRef]
- Niu, X.; Li, X.; Pan, J.; He, Y.; Qiu, F.; Yan, Y. Recent Advances in Non-Enzymatic Electrochemical Glucose Sensors Based on Non-Precious Transition Metal Materials: Opportunities and Challenges. RSC Adv. 2016, 6, 84893–88495. [Google Scholar] [CrossRef]

| Ref. | Electrode [Primary Electrolyte] | Sensitivity (µA mM−1 cm−2) | LOD (µM) | Linear Range (mM) | Stability (% Activity) | Enzymatic Sensors | ||
|---|---|---|---|---|---|---|---|---|
| Sensitivity (µA mM−1 cm−2) | LOD (µM) | Stability (%Activity) | ||||||
| Glutamate | ||||||||
| [28] | Pt-NiNAE [1 M NaOH] | 96 | 83 | 0.5–8.0 | 4 weeks (96%) | 433 [33] | 1 × 10−4 [34] | 20 weeks [35] |
| [31] | NiO/GCE [0.1 M NaOH] | 11 | 272 | 1.0–8.0 | 2 weeks (99%) | |||
| Acetylcholine | ||||||||
| [36] | Cu/CPE [0.1 M NaOH] | - | 39 | 0.12–2.68 | - | 2.19 [37] | 0.004 [38] | 2 weeks [39] |
| [40] | ns-Ni-CC [0.1 M NaOH] | 48.58 | 0.049 | 0.0002–0.828 | 5 weeks | |||
| [32] | NiAl-LDHs/OMC/GCE [0.1 M NaOH] | - | 0.042 | 0.002–4.92 | 3 weeks (93%) | |||
| [41] | NiO/CPE [0.1 M NaOH] | 392.4 | 26.7 | 0.25–5.88 | - | |||
| [23] | NiAl-LDH/CD/GCE [0.1 M KOH] | 133.2 | 1.7 | 0.005–6.88 | 8 weeks (95%) | |||
| [42] | NiNAE [0.1 KOH] | ~1400 | 0.84 | - | - | |||
| [43] | Ni/Pt-graphite [0.2 NaOH] | - | ~0.1 | 0–4 × 10−4 | - | |||
| [44] | Ni wire [0.2 NaOH] | ~9.5 | - | 0–1.7 | - | |||
| Choline | ||||||||
| [45] | Cavitand+SWCNT/GCE | - | 0.39 | 0.010–10.0 | 12 weeks | 495 [46] | 1 × 10−4 [47] | 13 weeks (95%) [48] |
| [49] | Flower-like NiO [0.1 NaOH] | 60.5 | 25.4 | 0.25–6.98 | 4 weeks | |||
| [50] | pNiPP [0.3 M NaCl] | ~6 | 18 | - | - | |||
| [51] | Ni [0.2 M NaOH] | ~97 | ~134 | |||||
| Adenosine | ||||||||
| [52] | Aptamer-AgNPs/graphene oxide | - | 0.005 | 1 × 10−5–85 × 10−5 | - | 250 [53] | 0.010 [54] | 7 weeks (57%) [55] |
| [56] | Aptamer/QDs [0.2 M acetate] | ~1.8 | 0.03 | 1 × 10−8–0.1 | - | |||
| [57] | Aptamer/MB/Au [0.3 M NaCl] | - | 0.0072 | 0–5 × 10−5 | - | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shadlaghani, A.; Farzaneh, M.; Kinser, D.; Reid, R.C. Direct Electrochemical Detection of Glutamate, Acetylcholine, Choline, and Adenosine Using Non-Enzymatic Electrodes. Sensors 2019, 19, 447. https://doi.org/10.3390/s19030447
Shadlaghani A, Farzaneh M, Kinser D, Reid RC. Direct Electrochemical Detection of Glutamate, Acetylcholine, Choline, and Adenosine Using Non-Enzymatic Electrodes. Sensors. 2019; 19(3):447. https://doi.org/10.3390/s19030447
Chicago/Turabian StyleShadlaghani, Arash, Mahsa Farzaneh, Dacen Kinser, and Russell C. Reid. 2019. "Direct Electrochemical Detection of Glutamate, Acetylcholine, Choline, and Adenosine Using Non-Enzymatic Electrodes" Sensors 19, no. 3: 447. https://doi.org/10.3390/s19030447
APA StyleShadlaghani, A., Farzaneh, M., Kinser, D., & Reid, R. C. (2019). Direct Electrochemical Detection of Glutamate, Acetylcholine, Choline, and Adenosine Using Non-Enzymatic Electrodes. Sensors, 19(3), 447. https://doi.org/10.3390/s19030447






