A Hybrid Lab-on-a-Chip Injector System for Autonomous Carbofuran Screening
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Fabrication
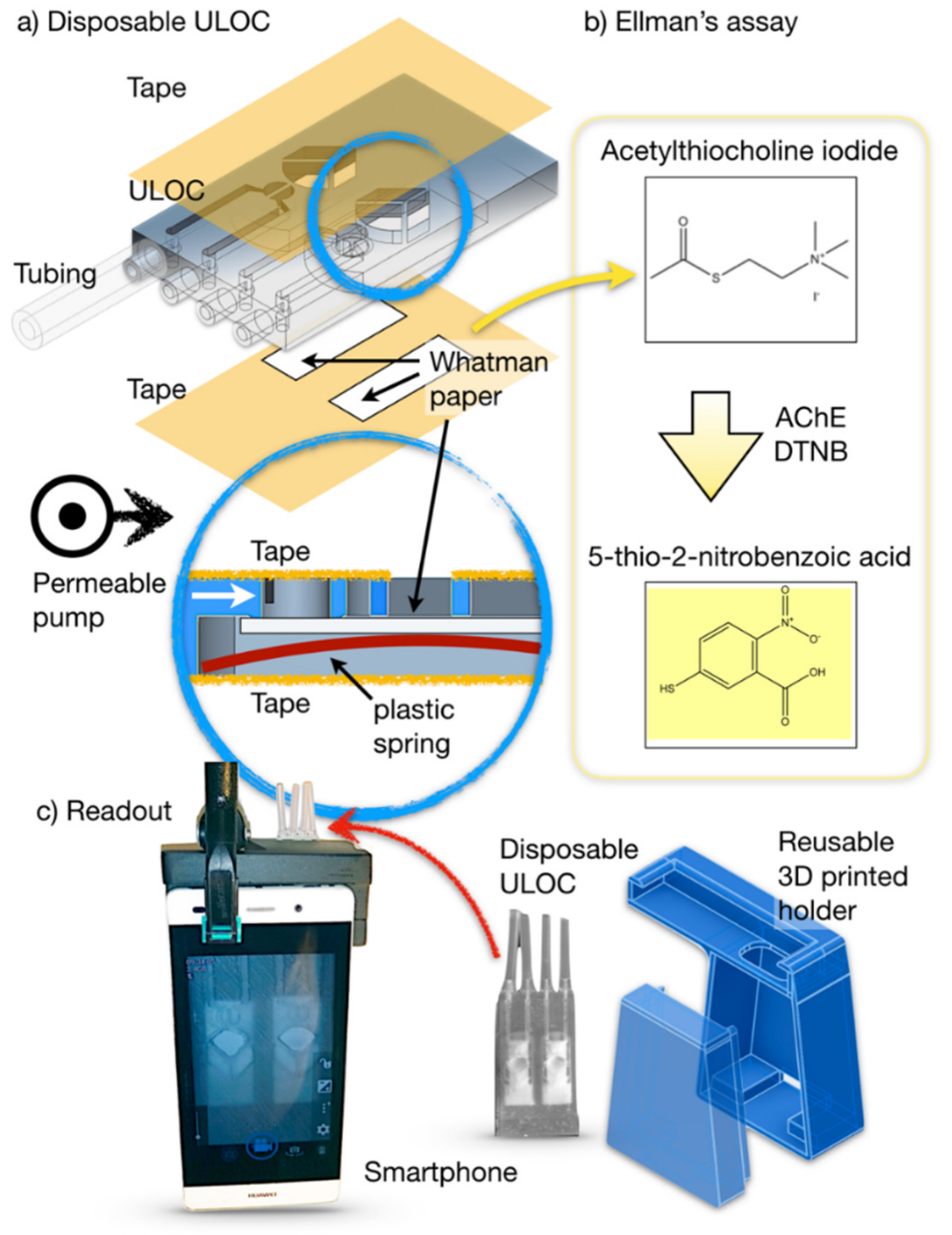
2.1.1. Unibody-LOC (ULOC) Device
2.1.2. Device Holder
2.2. Chemicals and Reagents
2.3. Enzyme Immobilization
2.4. Paper-Based AChE Assay
2.5. Smartphone Readout
2.6. Python Software and Graphical User Interface (GUI)
2.7. Food Samples
2.8. Food Samples Extraction
3. Results and Discussion
3.1. Hybrid Injector Architecture and Function
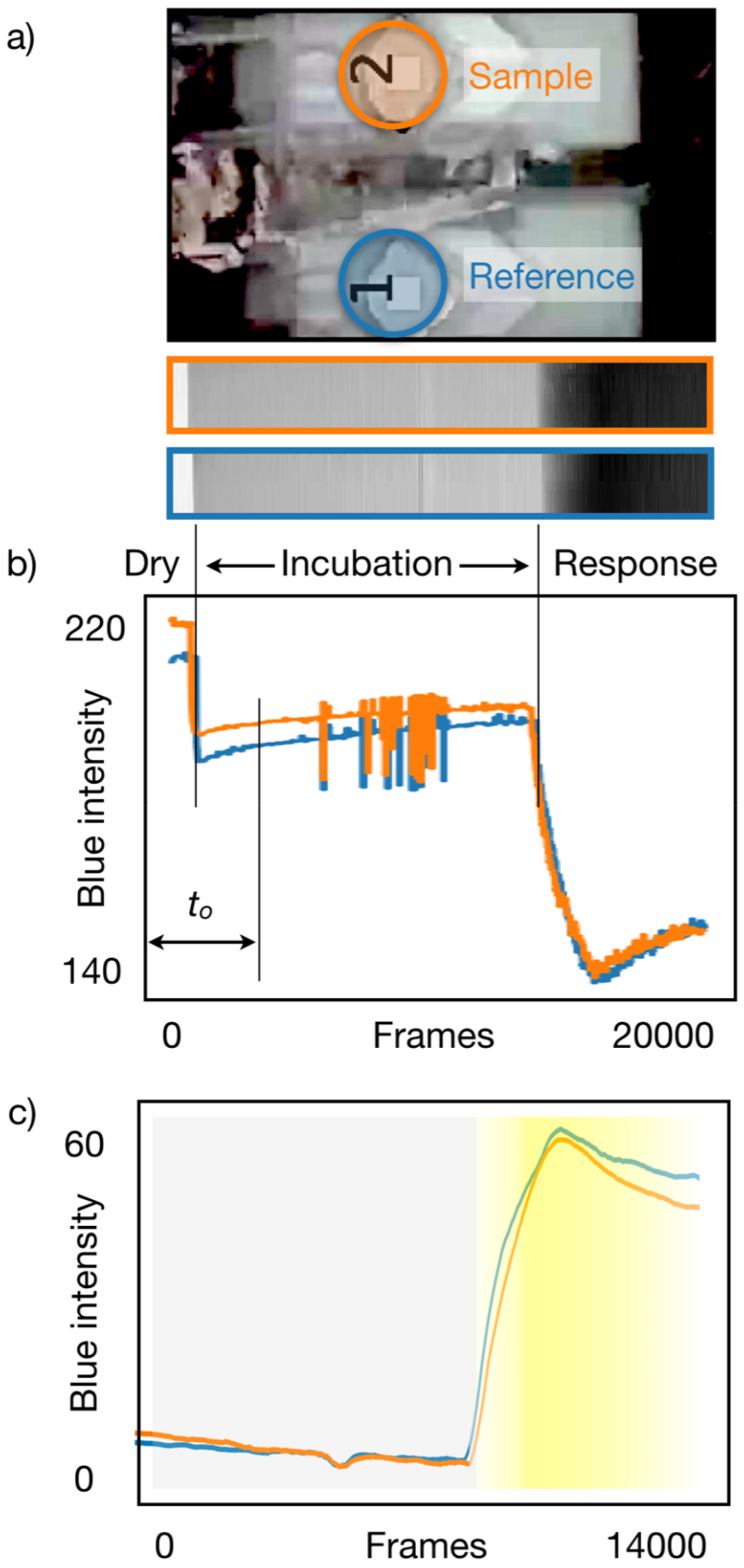
3.2. Data Acquisition
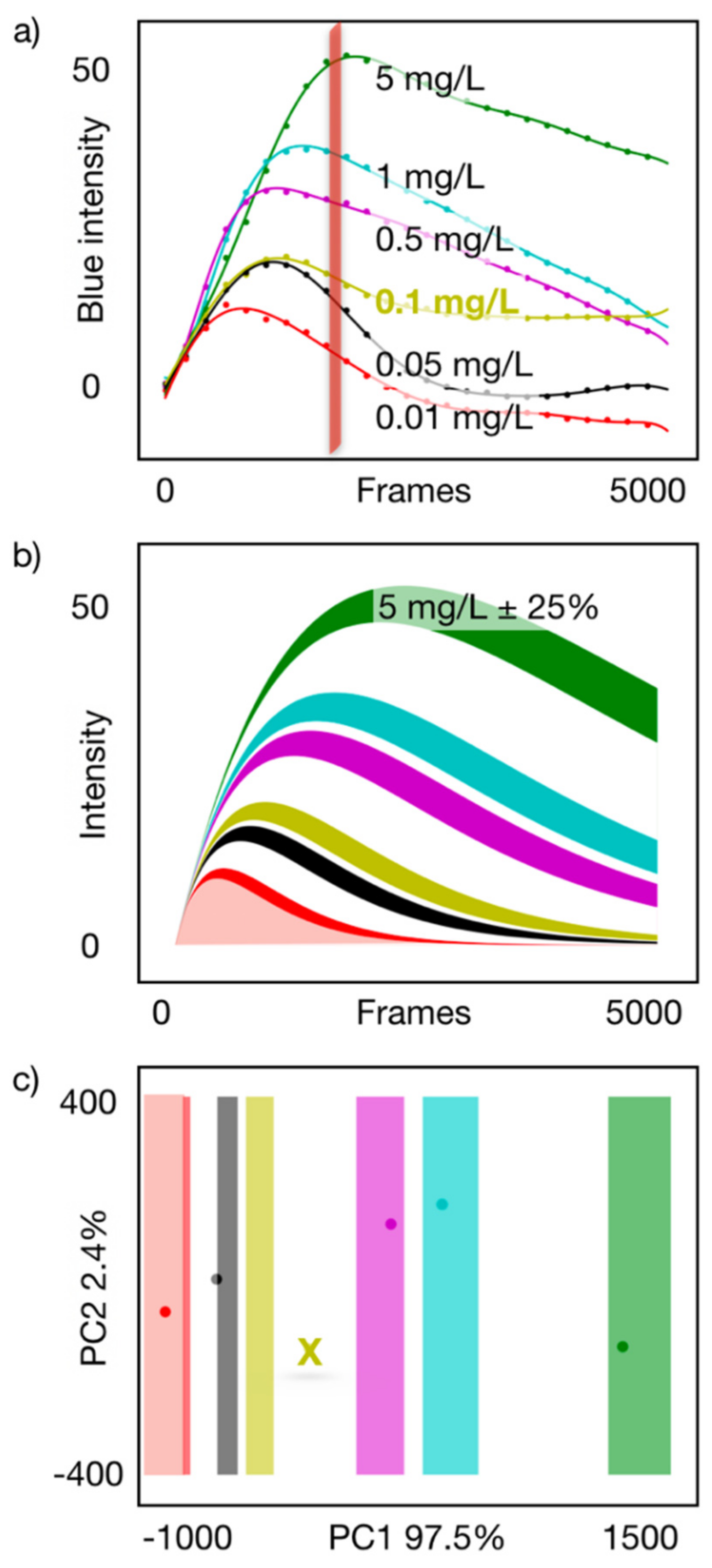
3.3. Semiquantification Using a PCA Acceptance/Rejection System
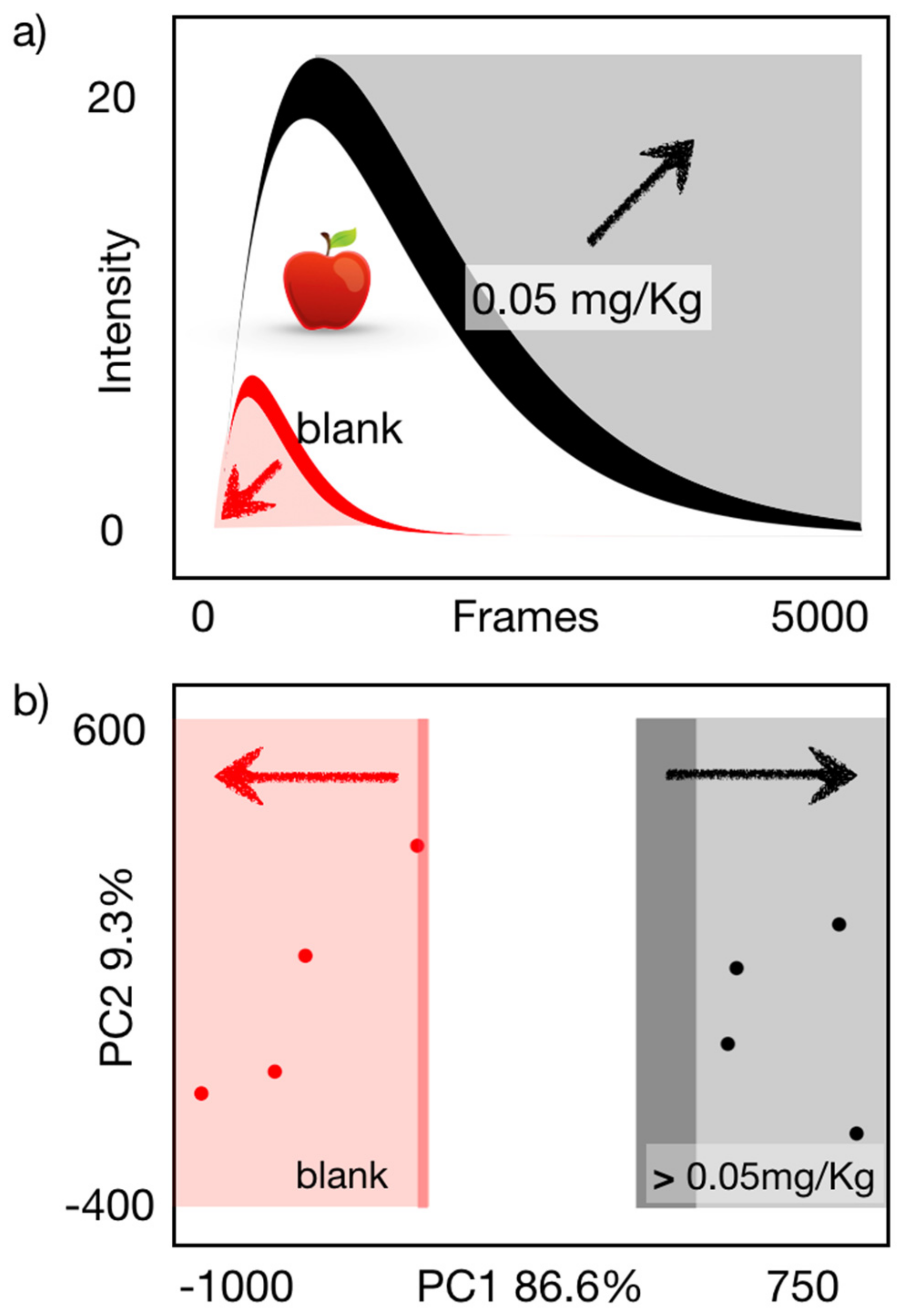
3.4. Binary Carbofuran Screening in Apple Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority (EFSA). The 2017 European Union report on pesticide residues in food. EFSA J. 2019, 17, e05743. [Google Scholar]
- Pundir, C.S.; Chauhan, N. Acetylcholinesterase inhibition-based biosensors for pesticide determination: A review. Anal. Biochem. 2012, 429, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Ensibi, C.; Hernández-Moreno, D.; Míguez Santiyán, M.P.; Daly Yahya, M.N.; Rodríguez, F.S.; Pérez-López, M. Effects of carbofuran and deltamethrin on acetylcholinesterase activity in brain and muscle of the common carp. Environ. Toxicol. 2014, 29, 386–393. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance carbofuran. EFSA J. 2006, 4, 90r. [Google Scholar] [CrossRef][Green Version]
- Martínez-del-Río, J.; Martínez Vidal, J.L.; Garrido Frenich, A. Economic evaluation of pesticide-residue analysis of vegetables. TrAC Trends Anal. Chem. 2013, 44, 90–97. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Nelis, J.L.D.; Ross, G.M.S.; Jafari, S.; Guercetti, J.; Kopper, K.; Zhao, Y.; Rafferty, K.; Salvador, J.P.; Migliorelli, D.; et al. Critical assessment of recent trends related to screening and confirmatory analytical methods for selected food contaminants and allergens. TrAC Trends Anal. Chem. 2019, 121, 115688. [Google Scholar] [CrossRef]
- Rejczak, T.; Tuzimski, T. Recent trends in sample preparation and liquid chromatography/mass spectrometry for pesticide residue analysis in food and related matrixes. J. AOAC Int. 2015, 98, 1143–1162. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Tsagkaris, A.S.; Zhao, Y.; Lou-Franco, J.; Nolan, P.; Zhou, H.; Cao, C.; Rafferty, K.; Hajslova, J.; Elliott, C.T.; et al. The end user sensor tree: An end-user friendly sensor database. Biosens. Bioelectron. 2019, 130, 245–253. [Google Scholar] [CrossRef]
- Karachaliou, C.-E.; Nikolaki, E.; Livaniou, E. Development of antibodies and immunoassays for carbamate pesticides. Curr. Org. Chem. 2017, 21, 2632–2639. [Google Scholar] [CrossRef]
- Wang, M.; Huang, J.; Wang, M.; Zhang, D.; Chen, J. Electrochemical nonenzymatic sensor based on CoO decorated reduced graphene oxide for the simultaneous determination of carbofuran and carbaryl in fruits and vegetables. Food Chem. 2014, 151, 191–197. [Google Scholar] [CrossRef]
- Jirasirichote, A.; Punrat, E.; Suea-Ngam, A.; Chailapakul, O.; Chuanuwatanakul, S. Voltammetric detection of carbofuran determination using screen-printed carbon electrodes modified with gold nanoparticles and graphene oxide. Talanta 2017, 175, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Wang, Q.; Liu, L. Nanomaterials-based optical techniques for the detection of acetylcholinesterase and pesticides. Sensors 2015, 15, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Nery, E.W.; Kubota, L.T. Sensing approaches on paper-based devices: A review. Anal. Bioanal. Chem. 2013, 405, 7573–7595. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wong, J.X.H.; Cui, C.; Li, X.; Yu, H.Z. A smartphone-readable barcode assay for the detection and quantitation of pesticide residues. Analyst 2015, 140, 5518–5525. [Google Scholar] [CrossRef] [PubMed]
- No, H.-Y.; Kim, Y.A.; Lee, Y.T.; Lee, H.-S. Cholinesterase-based dipstick assay for the detection of organophosphate and carbamate pesticides. Anal. Chim. Acta 2007, 594, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Acetylcholinesterase Based Dipsticks with Indoxylacetate as a Substrate for Assay of Organophosphates and Carbamates. Anal. Lett. 2012, 45, 367–374. [Google Scholar] [CrossRef]
- Jang, Y.-H.; Hancock, M.J.; Kim, S.B.; Selimović, Š.; Sim, W.Y.; Bae, H.; Khademhosseini, A. An integrated microfluidic device for two-dimensional combinatorial dilution. Lab Chip 2011, 11, 3277–3286. [Google Scholar] [CrossRef]
- Kovacova, J.; Hrbek, V.; Kloutvorova, J.; Kocourek, V.; Drabova, L.; Hajslova, J. Assessment of pesticide residues in strawberries grown under various treatment regimes. Food Addit. Contam. Part A 2013, 30, 2123–2135. [Google Scholar] [CrossRef]
- Comina, G.; Suska, A.; Filippini, D. Towards autonomous lab-on-a-chip devices for cell phone biosensing. Biosens. Bioelectron. 2016, 77, 1153–1167. [Google Scholar] [CrossRef]
- Comina, G.; Suska, A.; Filippini, D. Low cost lab-on-a-chip prototyping with a consumer grade 3D printer. Lab Chip 2014, 14, 2978–2982. [Google Scholar] [CrossRef]
- Comina, G.; Suska, A.; Filippini, D. 3D printed unibody lab-on-a-chip: Features survey and check-valves integration. Micromachines 2015, 6, 437–451. [Google Scholar] [CrossRef]
- Comina, G.; Suska, A.; Filippini, D. Autonomous chemical sensing interface for universal cell phone readout. Angew. Chem. Int. Ed. 2015, 54, 8708–8712. [Google Scholar] [CrossRef] [PubMed]
- Suska, A.; Filippini, D. Autonomous lab-on-a-chip generic architecture for disposables with integrated actuation. Nature Sci. Rep. 2020. accepted. [Google Scholar]
- Kokalj, T.; Park, Y.; Vencelj, M.; Jenko, M.; Lee, L.P. Self-powered Imbibing Microfluidic Pump by Liquid Encapsulation: SIMPLE. Lab Chip 2014, 14, 4329–4333. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.-S.; Chang, T.-C.; Wang, S.-C. Gold nanoparticle-based colorimetric methods to determine protein contents in artificial urine using membrane micro-concentrators and mobile phone camera. Sens. Actuators B Chem. 2017, 239, 9–16. [Google Scholar] [CrossRef]
- Nouanthavong, S.; Nacapricha, D.; Henry, C.S.; Sameenoi, Y. Pesticide analysis using nanoceria-coated paper-based devices as a detection platform. Analyst 2016, 141, 1837–1846. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.; Park, S.J.; Kwon, C.; Noh, H. Development of Colorimetric Paper Sensor for Pesticide Detection Using Competitive-inhibiting Reaction. BioChip J. 2018, 12, 326–331. [Google Scholar] [CrossRef]
- Liu, W.; Kou, J.; Xing, H.; Li, B. Paper-based chromatographic chemiluminescence chip for the detection of dichlorvos in vegetables. Biosens. Bioelectron. 2014, 52, 76–81. [Google Scholar] [CrossRef]
- Xie, C.; Li, H.; Li, S.; Gao, S. Surface molecular imprinting for chemiluminescence detection of the organophosphate pesticide chlorpyrifos. Microchim. Acta 2011, 174, 311. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsagkaris, A.S.; Pulkrabova, J.; Hajslova, J.; Filippini, D. A Hybrid Lab-on-a-Chip Injector System for Autonomous Carbofuran Screening. Sensors 2019, 19, 5579. https://doi.org/10.3390/s19245579
Tsagkaris AS, Pulkrabova J, Hajslova J, Filippini D. A Hybrid Lab-on-a-Chip Injector System for Autonomous Carbofuran Screening. Sensors. 2019; 19(24):5579. https://doi.org/10.3390/s19245579
Chicago/Turabian StyleTsagkaris, Aristeidis S., Jana Pulkrabova, Jana Hajslova, and Daniel Filippini. 2019. "A Hybrid Lab-on-a-Chip Injector System for Autonomous Carbofuran Screening" Sensors 19, no. 24: 5579. https://doi.org/10.3390/s19245579
APA StyleTsagkaris, A. S., Pulkrabova, J., Hajslova, J., & Filippini, D. (2019). A Hybrid Lab-on-a-Chip Injector System for Autonomous Carbofuran Screening. Sensors, 19(24), 5579. https://doi.org/10.3390/s19245579





