Multi-Electrode Array with a Planar Surface for Cell Patterning by Microprinting
Abstract
1. Introduction
2. Materials and Methods
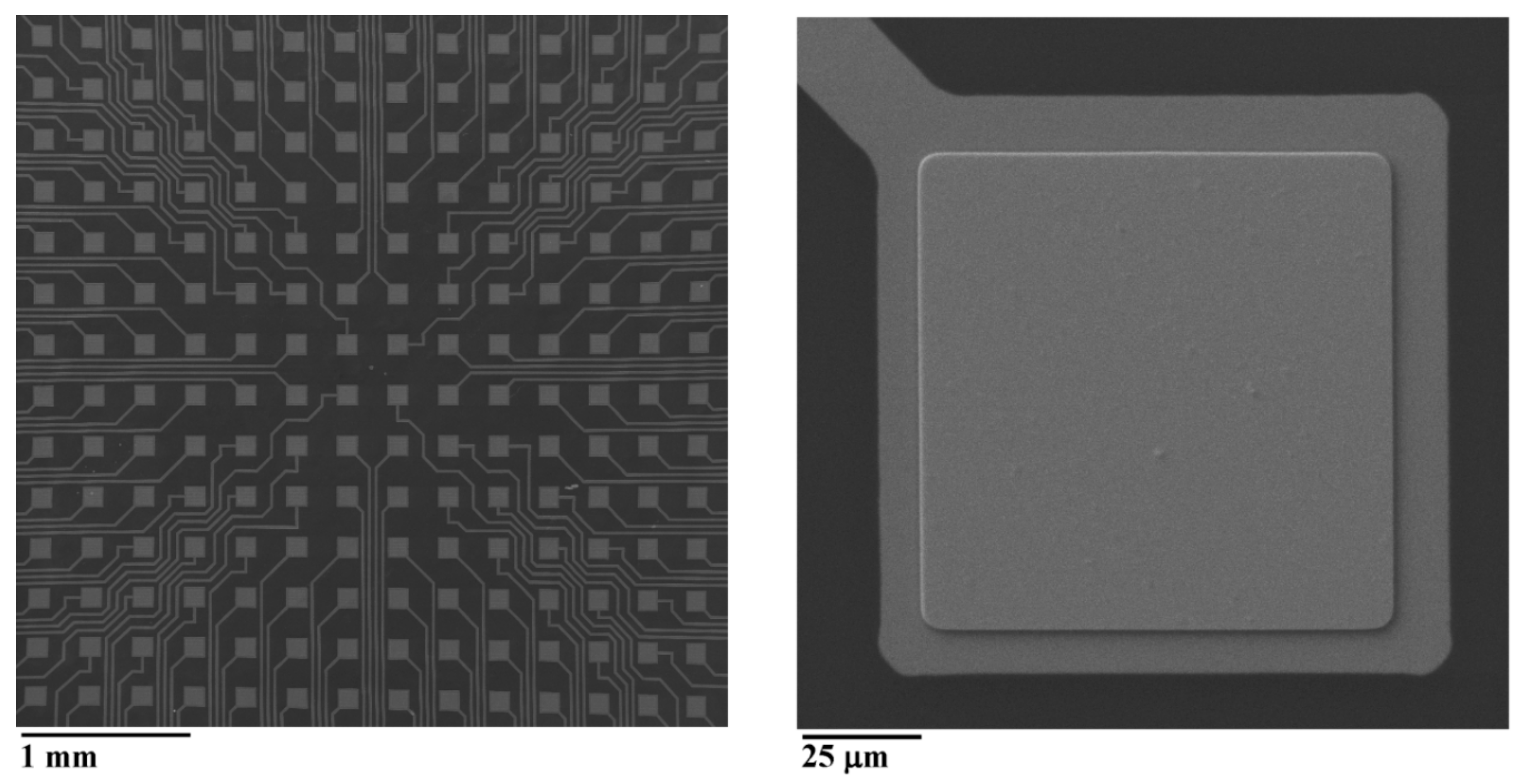
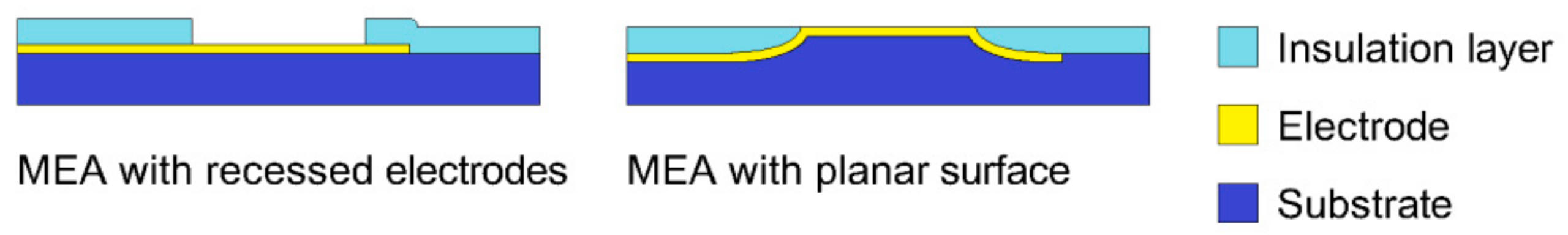
2.1. The Fabrication of MEA
2.2. Characterization of Planar Electrodes
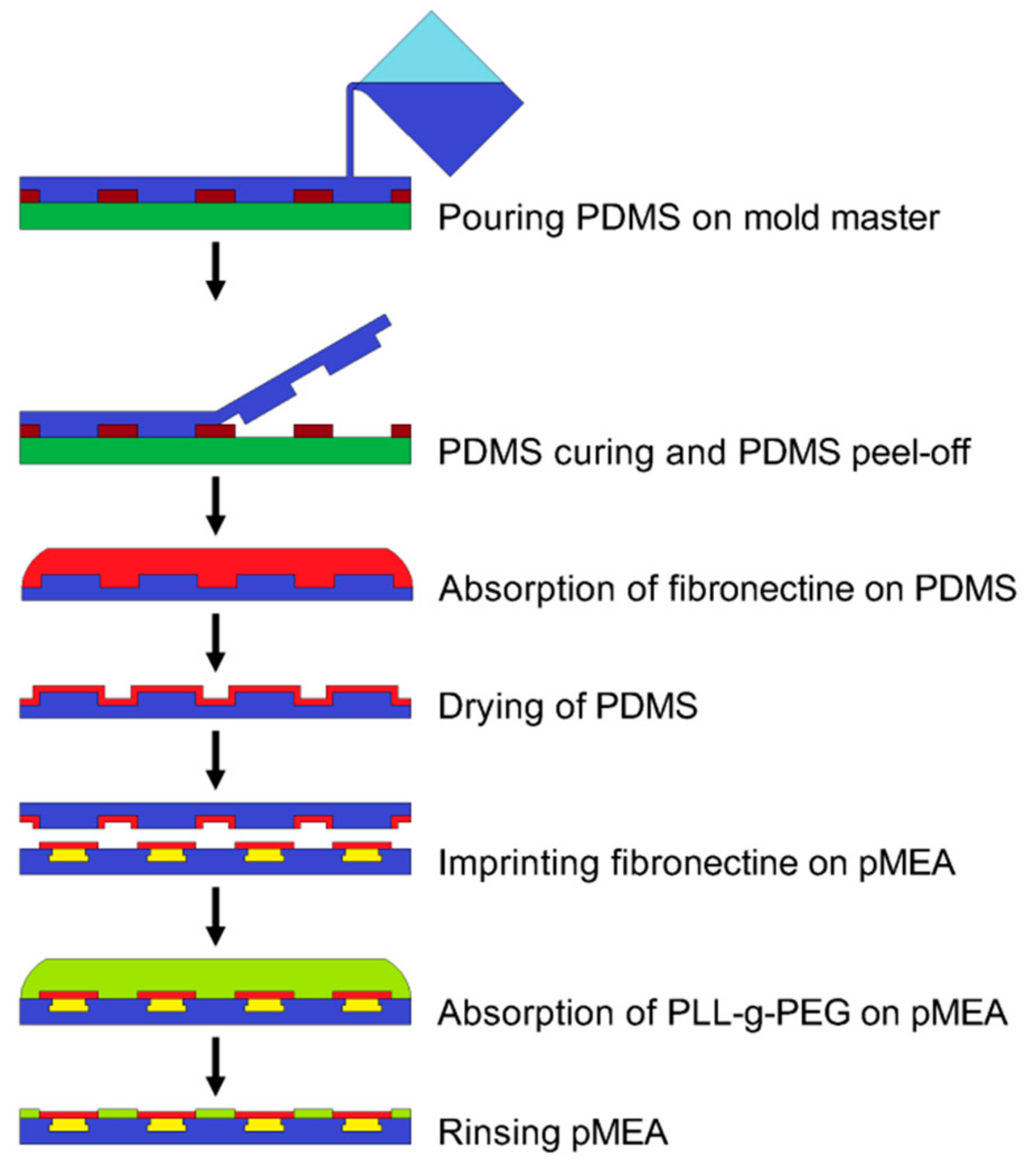
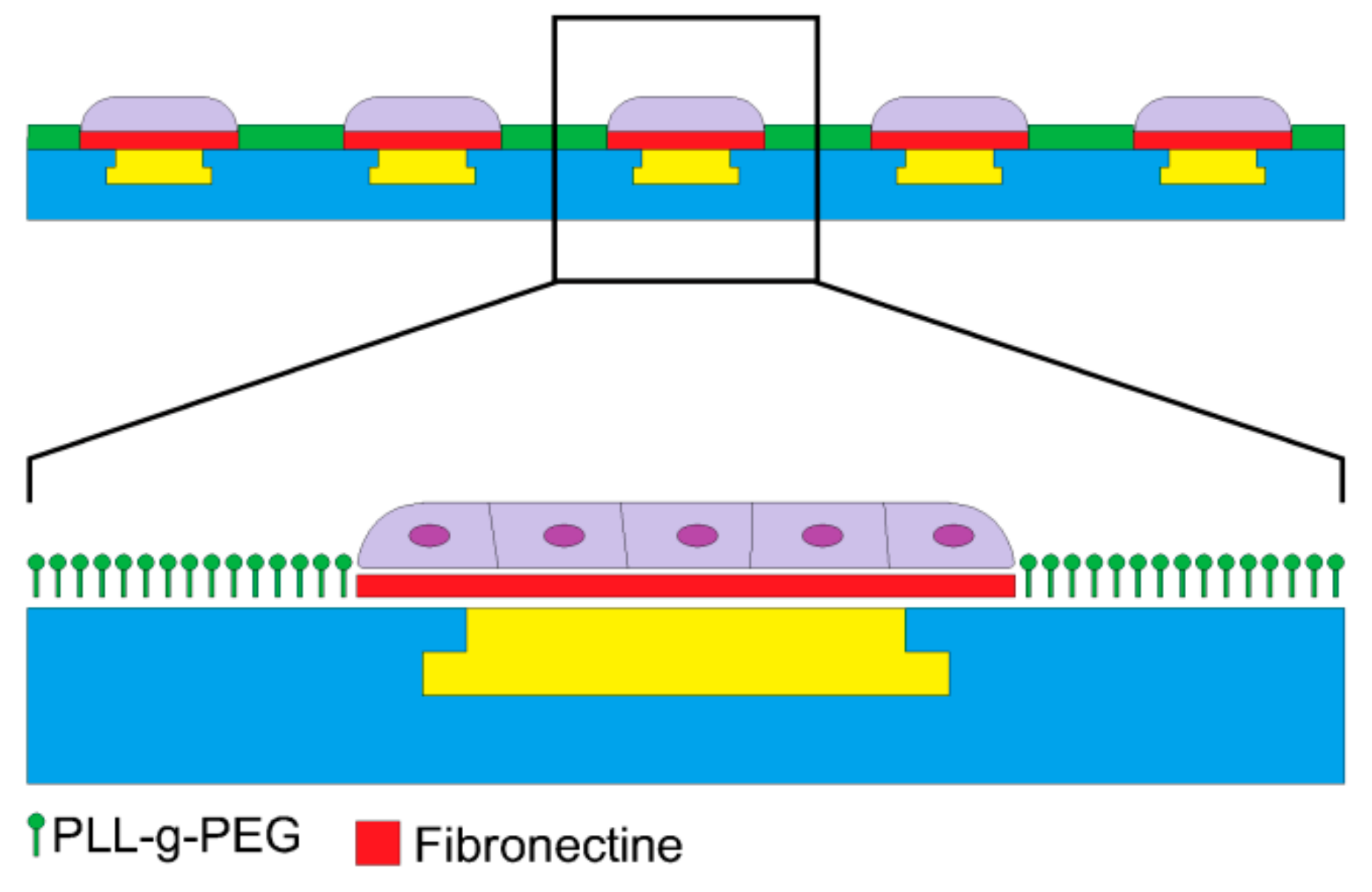
2.3. The Fabrication of the PDMS Stamp and Microprinting Method
2.4. HL-1 Cell Culturing, Measurements of Action Potentials, Staining, and Visualization
3. Results and Discussion
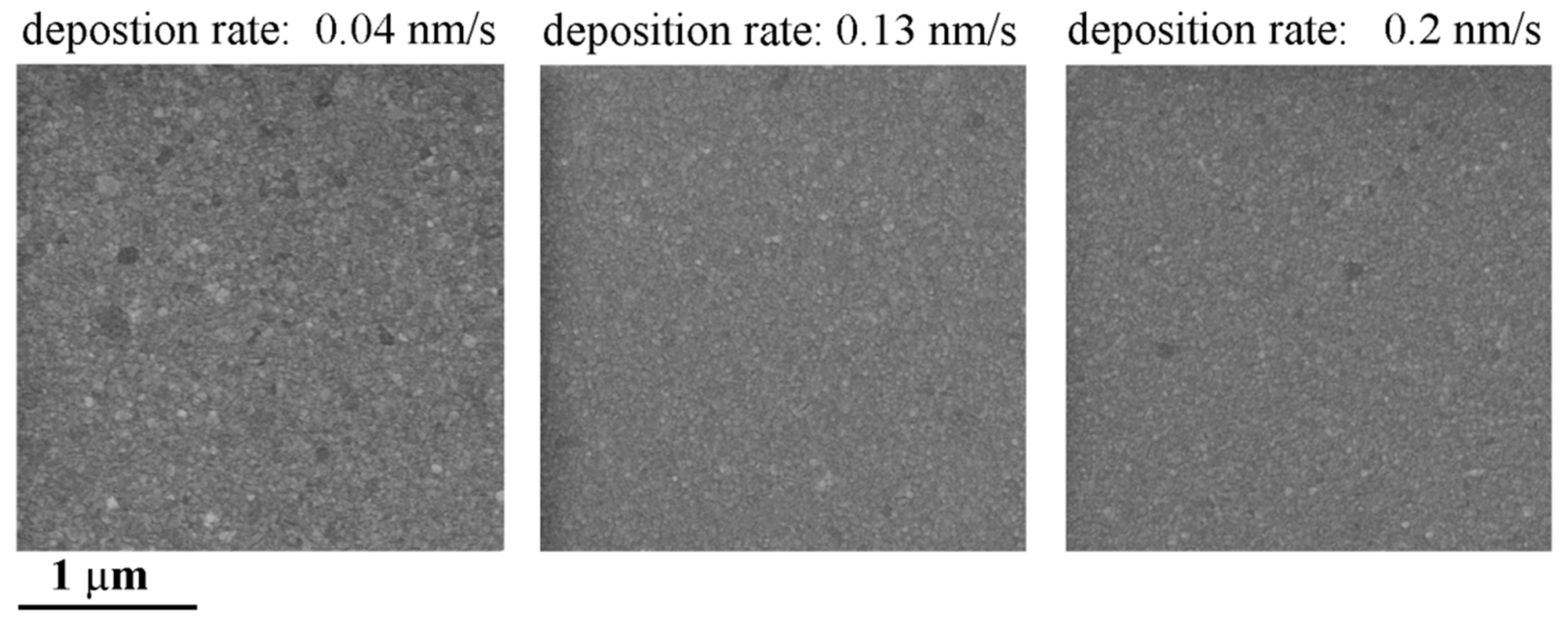
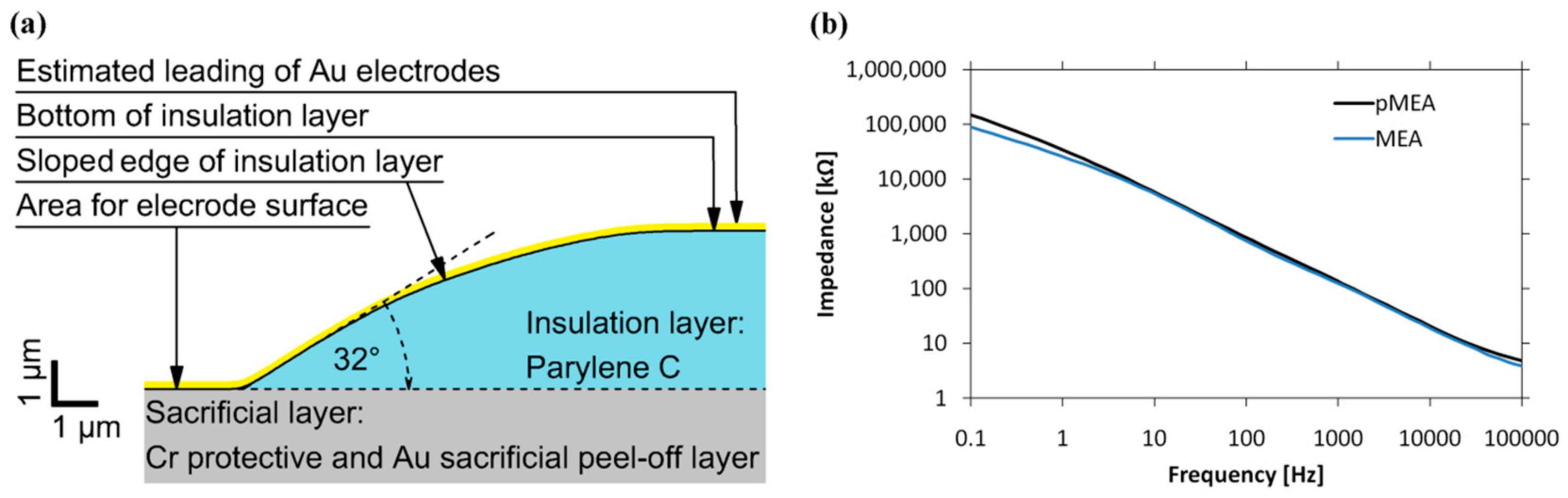
3.1. The Profile of the Sloped Edge of the Insulation Layer and the Impedance of the Planar Electrodes
3.2. Cell Patterning on pMEA by Microprinting
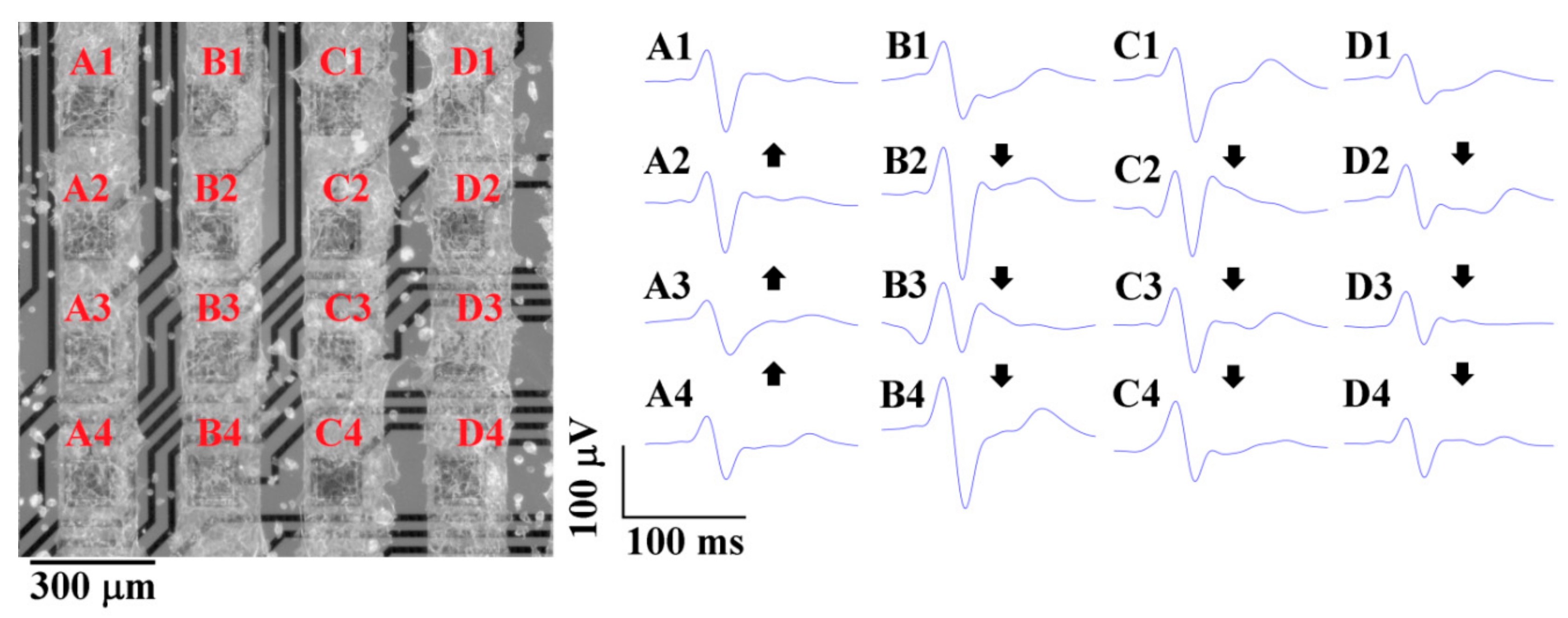
3.3. A Measure of HL-1 Cells’ Action Potential on the pMEA
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Obien, M.E.; Deligkaris, K.; Bullmann, T.; Bakkum, D.J.; Frey, U. Revealing Neuronal Function Through Microelectrode Array Recordings. Front. Neurosci. 2015, 8, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Spira, M.E.; Hai, A. Multi-Electrode Array Technologies for Neuroscience and Cardiology. Nat. Nanotechnol. 2013, 8, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Degenaar, P.; Pioufle, B.L.; Griscom, L.; Tbrier, A.; Akagi, Y.; Morita, Y.; Murakami, Y.; Yokoyama, K.; Fujita, H.; Tamiya, E. A Method for Micrometer Resolution Patterning of Primary Culture Neurons for SPM Analysis. J. Biochem. 2001, 130, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Griscom, L.; Degenaar, P.; LePioufle, B.; Tamiya, E.; Fujita, H. Techniques for patterning and guidance of primary culture neurons on micro-electrode arrays. Sens. Actuator B Chem. 2002, 83, 15–21. [Google Scholar] [CrossRef]
- Morin, F.; Nishimura, N.; Griscom, L.; LePioufle, B.; Fujita, H.; Takamura, Y.; Tamiya, E. Constraining the connectivity of neuronal networks cultured on microelectrode arrays with microfluidic techniques: A step towards neuron-based functional chips. Biosens. Bioelectron. 2006, 21, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A Microfluidic Culture Platform for CNS Axonal Injury, Regeneration and Transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef]
- Forró, C.; Thompson-Steckel, G.; Weaver, S.; Weydert, S.; Ihle, S.; Dermutz, H.; Aebersold, M.J.; Pilz, R.; Demkó, L.; Vörös, J. Modular Microstructure Design to Build Neuronal Networks of Defined Functional Connectivity. Biosens. Bioelectron. 2018, 122, 75–87. [Google Scholar] [CrossRef]
- James, C.D.; Davis, R.; Meyer, M.; Turner, A.; Turner, S.; Withers, G.; Kam, L.; Banker, G.; Craighead, H.; Issacson, M.; et al. Aligned Microcontact Printing of Micrometer-Scale Poly-L-Lysine Structures for Controlled Growth of Cultured Neurons on Planar Microelectrode Arrays. IEEE Trans. Biomed. Eng. 2000, 47, 17–21. [Google Scholar] [CrossRef]
- Nam, Y.; Branch, D.W.; Wheeler, B.C. Epoxy-Silane Linking of Biomolecules Is Simple and Effective for Patterning Neuronal Cultures. Biosens. Bioelectron. 2006, 22, 589–597. [Google Scholar] [CrossRef]
- Jun, S.B.; Hynd, M.R.; Dowell-Mesfin, N.; Smith, K.L.; Turner, J.N.; Shain, W.; Kim, S.J. Low-Density Neuronal Networks Cultured Using Patterned Poly-L-Lysine on Microelectrode Arrays. J. Neurosci. Methods 2007, 160, 317–326. [Google Scholar] [CrossRef]
- Jungblut, M.; Knoll, W.; Thielemann, C.; Pottek, M. Triangular Neuronal Networks on Microelectrode Arrays: An Approach to Improve the Properties of Low-Density Networks for Extracellular Recording. Biomed. Microdevices 2009, 11, 1269–1278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marconi, E.; Nieus, T.; Maccione, A.; Valente, P.; Simi, A.; Messa, M.; Dante, S.; Baldelli, P.; Berdondini, L.; Benfenati, F. Emergent Functional Properties of Neuronal Networks with Controlled Topology. PLoS ONE 2012, 7, e34648. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in The Design of Surfaces That Prevent Fouling by Proteins, Bacteria, And Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Théry, M.; Piel, M. Adhesive micropatterns for cells: A microcontact printing protocol. Cold Spring Harb. Protoc. 2009, 4. [Google Scholar] [CrossRef]
- Kim, J.M.; Im, C.; Lee, W.R. Plateau-Shaped Flexible Polymer Microelectrode Array for Neural Recording. Polymers 2017, 9, 690. [Google Scholar] [CrossRef]
- Claycomb, W.C.; Lanson, N.A., Jr.; Stallworth, B.S.; Egeland, D.B.; Delcarpio, J.B.; Bahinski, A.; Izzo, N.J., Jr. HL-1 Cells: A Cardiac Muscle Cell Line That Contracts and Retains Phenotypic Characteristics of The Adult Cardiomyocyte. Proc. Natl. Acad. Sci. USA 1998, 95, 2979–2984. [Google Scholar] [CrossRef]
- White, S.M.; Constantin, P.E.; Claycomb, W.C. Cardiac Physiology at The Cellular Level: Use of Cultured HL-1 Cardiomyocytes for Studies of Cardiac Muscle Cell Structure and Function. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H823–H829. [Google Scholar] [CrossRef]
- Gizurarson, S.; Shao, Y.; Miljanovic, A.; Råmunddal, T.; Borén, J.; Bergfeldt, L.; Omerovic, E. Electrophysiological Effects of Lysophosphatidylcholine on HL-1 Cardiomyocytes Assessed with A Microelectrode Array System. Cell. Physiol. Biochem. 2012, 30, 477–488. [Google Scholar] [CrossRef]
- Law, J.K.; Yeung, C.K.; Hofmann, B.; Ingebrandt, S.; Rudd, J.A.; Offenhäusser, A.; Chan, M. The Use of Microelectrode Array (MEA) To Study the Protective Effects of Potassium Channel Openers on Metabolically Compromised HL-1 Cardiomyocytes. Physiol. Meas. 2009, 30, 155–167. [Google Scholar] [CrossRef]
- Garma, L.D.; Matino, L.; Melle, G.; Moia, F.; De Angelis, F.; Santoro, F.; Dipalo, M. Cost-Effective and Multifunctional Acquisition System For In Vitro Electrophysiological Investigations with Multi-Electrode Arrays. PLoS ONE 2019, 14, e0214017. [Google Scholar] [CrossRef]
- Mattox, D. Interface Formation and the adhesion of deposited thin films. In Monograph, R65852; Technical Information Division Sandia Corporation Albuquerque: Albuquerque, NM, USA, 1965; pp. 1–20. [Google Scholar]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014, 24, 035017. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G. Soft Lithography for Micro- and Nanoscale Patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Elbert, D.; Hubbell, J. Self-Assembly and Steric Stabilization at Heterogeneous, Biological Surfaces Using Adsorbing Block Copolymers. Chem. Biol. 1998, 5, 177–183. [Google Scholar] [CrossRef]
- Carpi, N.; Piel, M.; Azioune, A.; Fink, J. Micropatterning on glass with deep UV. Protoc. Exch. 2011. [Google Scholar] [CrossRef]
- Tu, X.; Wei, J.; Wang, B.; Tang, Y.; Shi, J.; Chen, Y. Patterned Parylene C for Cell Adhesion, Spreading and Alignment Studies. Microelectron. Eng. 2017, 175, 56–60. [Google Scholar] [CrossRef]
- Chang, T.Y.; Yadav, V.G.; De Leo, S.; Mohedas, A.; Rajalingam, B.; Chen, C.L.; Selvarasah, S.; Dokmeci, M.R.; Khademhosseini, A. Cell and Protein Compatibility of Parylene-C Surfaces. Langmuir 2007, 23, 11718–11725. [Google Scholar] [CrossRef]
- Agladze, N.N.; Halaidych, O.V.; Tsvelaya, V.A.; Bruegmann, T.; Kilgus, C.; Sasse, P.; Agladze, K.I. Synchronization of excitable cardiac cultures of different origin. Biomater. Sci. 2017, 5, 1777–1785. [Google Scholar] [CrossRef]
- Dias, P.; Desplantez, T.; El-Harasis, M.A.; Chowdhury, R.A.; Ullrich, N.D.; Cabestrero de Diego, A.; Peters, N.S.; Severs, N.J.; MacLeod, K.T.; Dupont, E. Characterisation of connexin expression and electrophysiological properties in stable clones of the HL-1 myocyte cell line. PLoS ONE 2014, 9, e90266. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavík, J.; Skopalík, J.; Provazník, I.; Hubálek, J. Multi-Electrode Array with a Planar Surface for Cell Patterning by Microprinting. Sensors 2019, 19, 5379. https://doi.org/10.3390/s19245379
Slavík J, Skopalík J, Provazník I, Hubálek J. Multi-Electrode Array with a Planar Surface for Cell Patterning by Microprinting. Sensors. 2019; 19(24):5379. https://doi.org/10.3390/s19245379
Chicago/Turabian StyleSlavík, Jan, Josef Skopalík, Ivo Provazník, and Jaromír Hubálek. 2019. "Multi-Electrode Array with a Planar Surface for Cell Patterning by Microprinting" Sensors 19, no. 24: 5379. https://doi.org/10.3390/s19245379
APA StyleSlavík, J., Skopalík, J., Provazník, I., & Hubálek, J. (2019). Multi-Electrode Array with a Planar Surface for Cell Patterning by Microprinting. Sensors, 19(24), 5379. https://doi.org/10.3390/s19245379






