Recent Trends, Technical Concepts and Components of Computer-Assisted Orthopedic Surgery Systems: A Comprehensive Review
Abstract
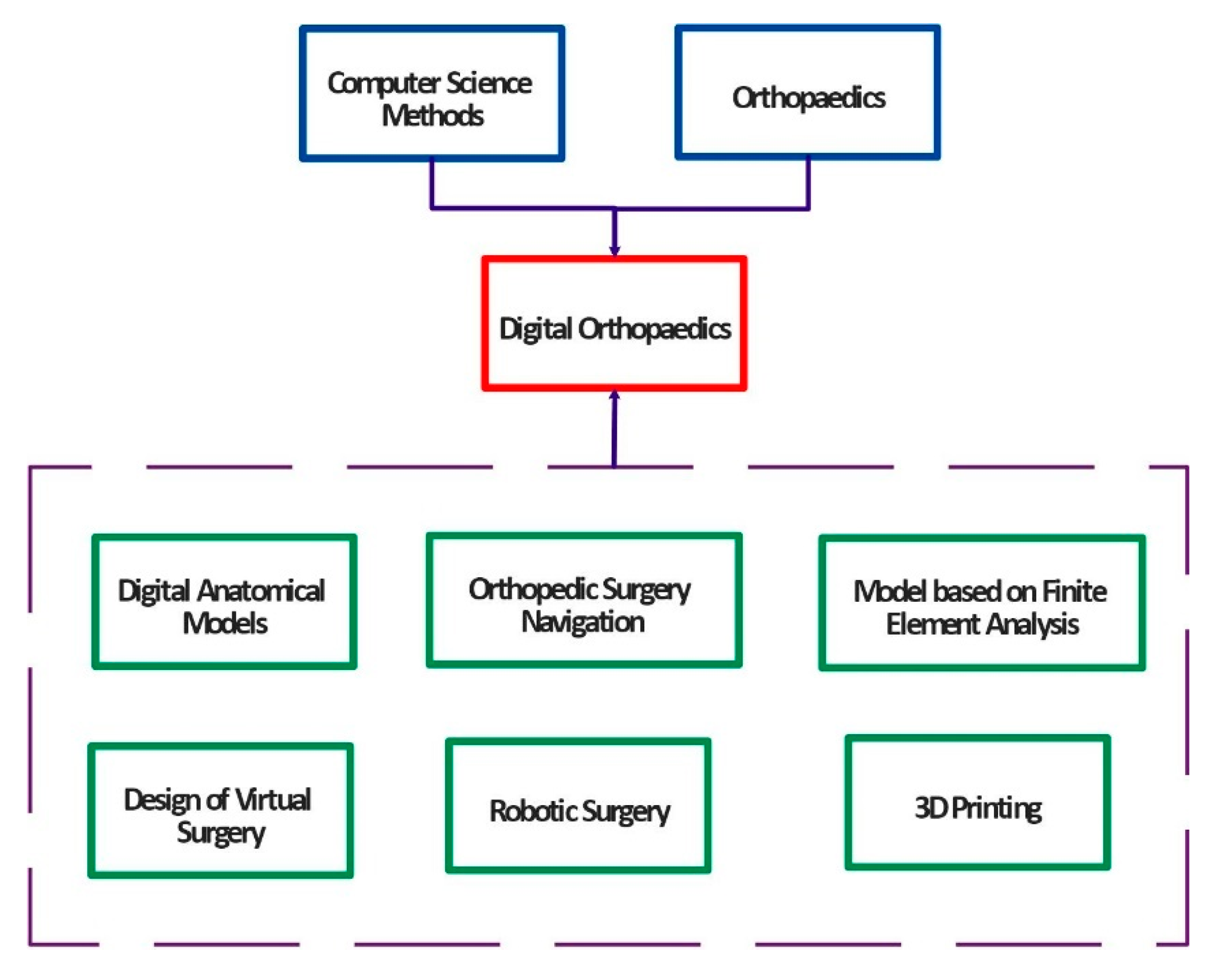
1. Introduction
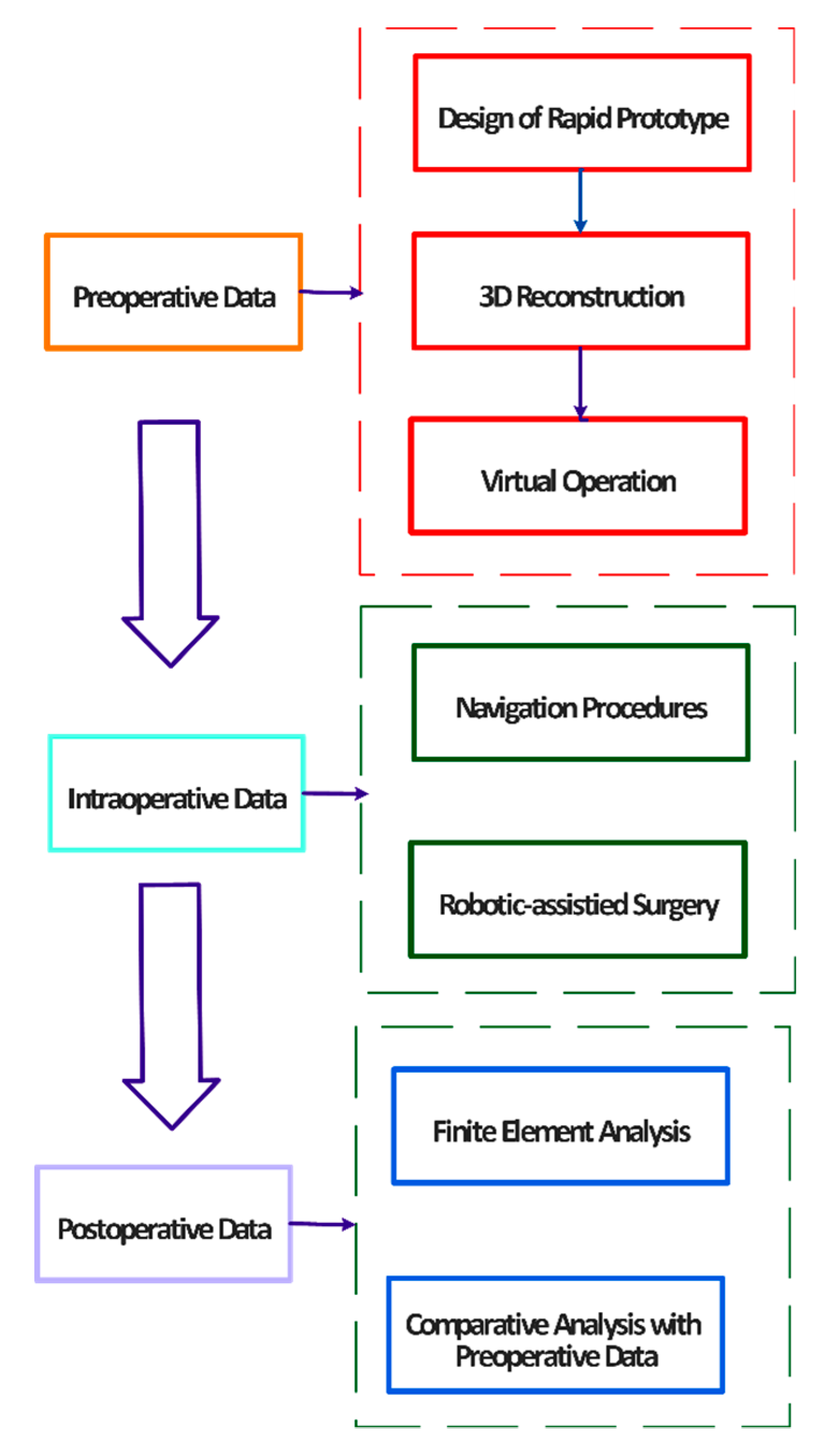
2. Essential Components and Types of CAOS Systems
2.1. Image Data Acquisition
2.2. Image Enhancement
2.3. Image Segmentation
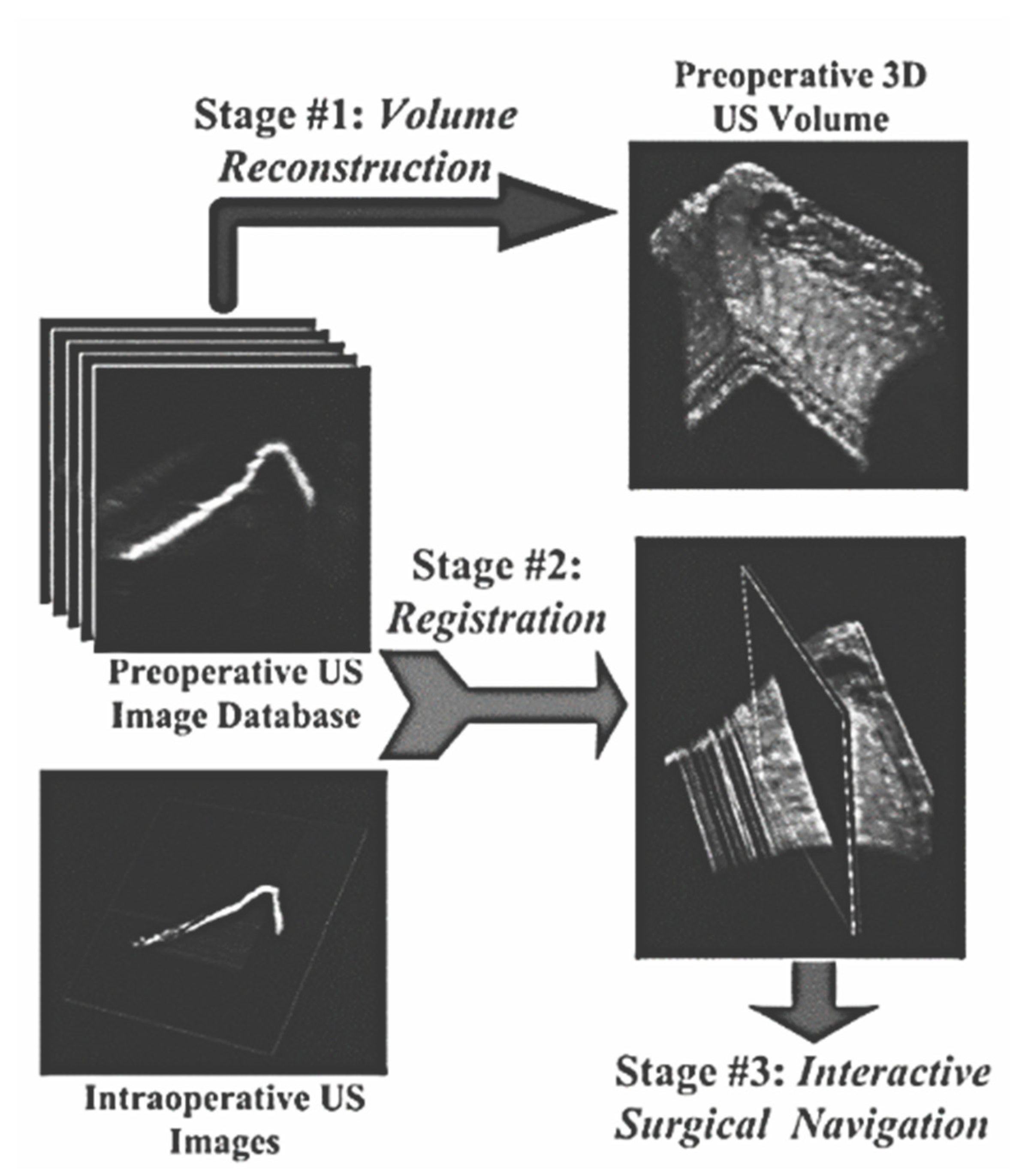
2.4. Image Registration
- Definition of the closest corresponding point on the target model for all the points and vertexes of the source models.
- Definition and computing of the rigid-body transformation based on geometric transformation, including translation and rotation. The purpose of this procedure is to minimize the average distance between corresponding points.
- Application of the transformation for all the source points.
- Repetition of the previous steps until the average distance is lower than the preset threshold.
2.5. Definition of Anatomical Landmarks
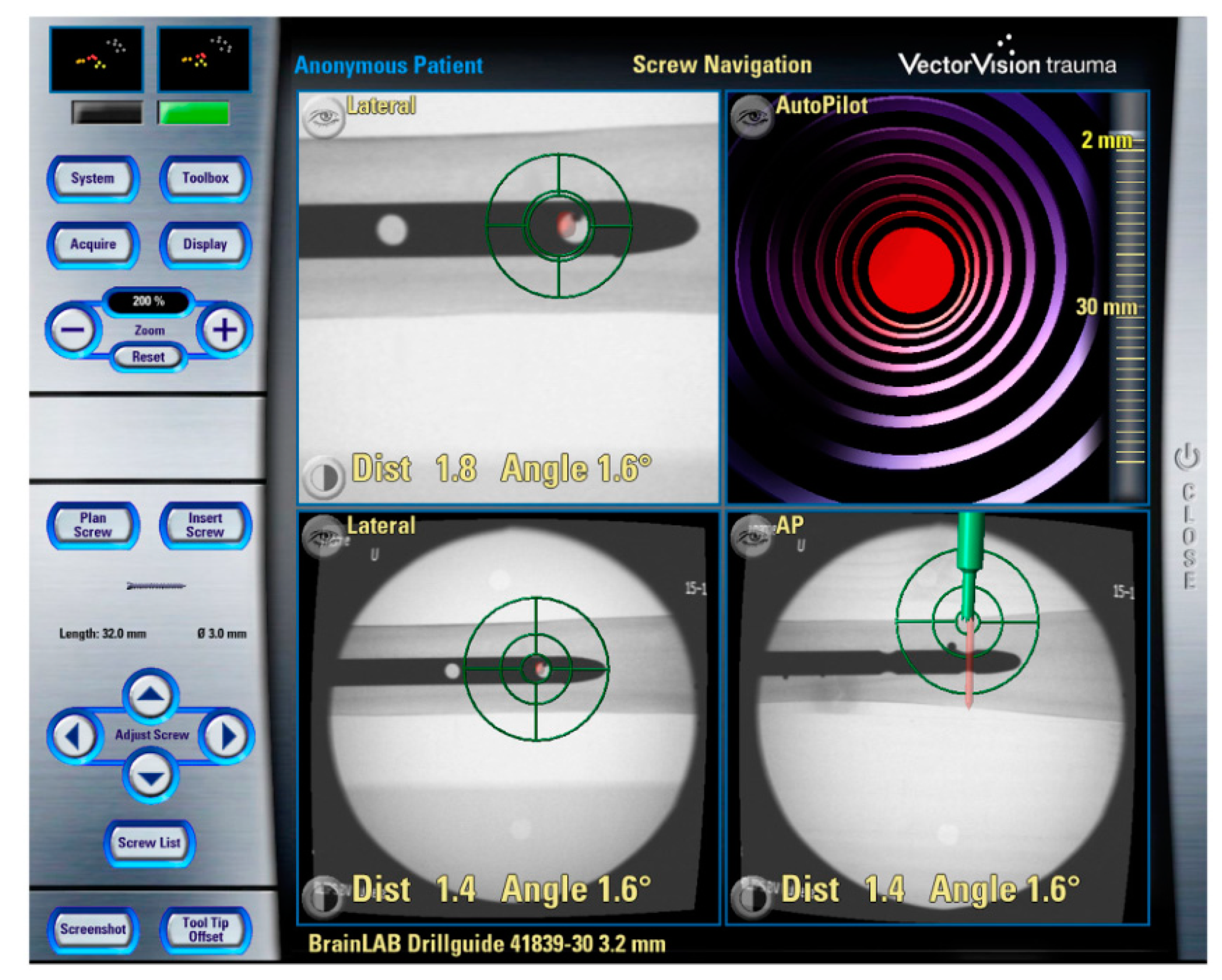
2.6. Introduction to Navigation Systems
2.7. 3D image-Based Navigation
2.8. Fluoroscopic Navigation
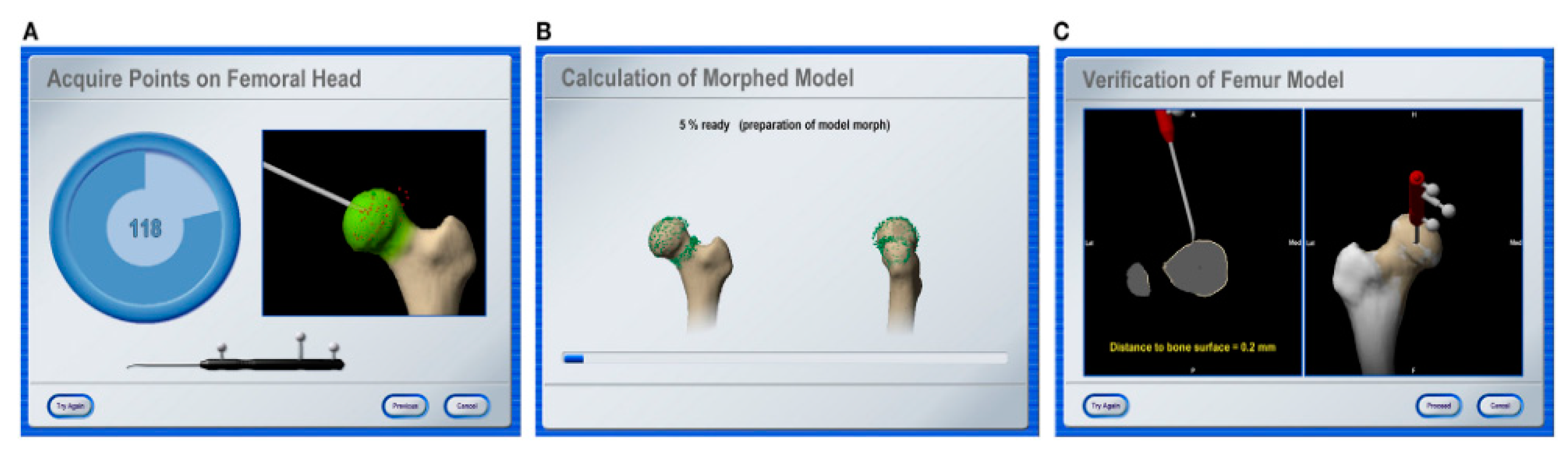
2.9. Imageless Navigation
2.10. Ultrasound-Based Navigation
2.11. Overall Comparison of Surgical Navigation
2.12. A Role of Medical Robots in Orthopedic Assist Surgery
3. CAOS Systems from the Biomechanical Perspective
- 3D modelling using parametric modelling programs,
- structural analysis using the finite element method,
- experimental testing of internal fixation devices,
- parametric optimization of orthopedic tools.
3.1. Percutaneous Screws
- proposal for the application of fixation devices using various imaging techniques,
- application of a guide wire,
- measurement of the desired screw length,
- hole drilling for the screw insertion,
- application of the percutaneous screw.
3.2. Pedicle Screws
3.3. Intraosseous Screws
3.4. Schanz Screws
3.5. Cannulated Screws
3.6. Complex-Shaped Implants and Plates
3.7. Materials of Orthopedic Implants
- chemically inert,
- completely biocompatible,
- higher strength,
- high resistance against fatigue,
- complete corrosion-proof,
- inexpensive.
4. Discussion and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Kowal, J.; Langlotz, F.; Nolte, L.P. Basics of computer-assisted orthopaedic surgery. Navig. Mis Orthop. Surg. 2007, 2–8. [Google Scholar] [CrossRef]
- Sugano, N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin. Orthop. Surg. 2013, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Nolte, L.P. Computer-Assisted Orthopedic Surgery: Current State and Future Perspective. Front. Surg. 2015, 2, 66. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.-X.; Yan, Y.-B. Current status and progress of digital orthopaedics in China. J. Orthop. Transl. 2014, 2, 107–117. [Google Scholar] [CrossRef]
- Karhade, A.V.; Schwab, J.H.; Bedair, H.S. Development of Machine Learning Algorithms for Prediction of Sustained Postoperative Opioid Prescriptions After Total Hip Arthroplasty. J. Arthroplast. 2019, 34, 2272–2277. [Google Scholar] [CrossRef]
- Reina, N. Connected orthopedics and trauma surgery: New perspectives. Orthop. Traumatol. Surg. Res. 2019, 105, S15–S22. [Google Scholar] [CrossRef]
- Trauner, K.B. The Emerging Role of 3D Printing in Arthroplasty and Orthopedics. J. Arthroplast. 2018, 33, 2352–2354. [Google Scholar] [CrossRef]
- Ackerman, J.D.; Keller, K.; Fuchs, H. Real-time anatomical 3D image extraction for laparoscopic surgery. Stud. Health Technol. Inform. 2001, 81, 18–22. [Google Scholar] [CrossRef]
- Amiot, L.P.; Labelle, H.; Deguise, J.A.; Sati, M.; Brodeur, P.; Rivard, C.H. Computer-assisted pedicle screw fixation: A feasibility study. Spine 1995, 20, 1208–1212. [Google Scholar] [CrossRef]
- Bargar, W.L.; Bauer, A.; Börner, M. Primary and revision total hip replacement using the ROBODOC® system. Clin. Orthop. Relat. Res. 1998, 354, 82–91. [Google Scholar] [CrossRef]
- Bolger, C.; Wigfield, C. Image-guided surgery: Applications to the cervical and thoracic spine and a review of the first 120 procedures. J. Neurosurg. Spine 2009, 92, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A. A computerized tomography-computer graphics approach to stereotaxic localization. J. Neurosurg. 2009, 50, 715–720. [Google Scholar] [CrossRef] [PubMed]
- DiGioia, A.M.; Jaramaz, B.; Plakseychuk, A.Y.; Moody, J.E.; Nikou, C.; LaBarca, R.S.; Picard, F. Comparison of a mechanical acetabular alignment guide with computer placement of the socket. J. Arthroplast. 2002, 17, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, A.; Lavallee, S.; Cinquin, P. Automated 3-dimensional computed tomographic and fluoroscopic image registration. Comput. Aided Surg. 1998, 3, 11–19. [Google Scholar] [CrossRef]
- Lavallee, S.; Troccaz, J.; Gaborit, L.; Cinquin, P.; Benabid, A.L.; Hoffmann, D. Image guided operating robot: A clinical application in stereotactic neurosurgery. In Proceedings of the 1992 IEEE International Conference on Robotics and Automation, Washington, DC, USA, 27–30 September 2003; pp. 618–624. [Google Scholar] [CrossRef]
- Langlotz, F.; Nolte, L.P. Computer-Assisted Orthopaedic Surgery: From Theory to the Operating Room. Tech. Orthop. 2003, 18, 140–148. [Google Scholar] [CrossRef]
- Russakoff, D.B.; Rohlfing, T.; Adler, J.R.; Maurer, C.R. Intensity-based 2D-3D spine image registration incorporating a single fiducial marker. Acad. Radiol. 2005, 12, 287–294. [Google Scholar] [CrossRef]
- Rohlfing, T.; West, J.B.; Beier, J.; Liebig, T.; Taschner, C.A.; Thomale, U.W. Registration of functional and anatomical MRI: Accuracy assessment and application in navigated neurosurgery. Comput. Aided Surg. 2000, 5, 414–425. [Google Scholar] [CrossRef]
- Lim, D.; Lin, F.; Wixson, R.; Hendrix, R.; MacDonald, M.; Makhsous, M. Accuracy of Imageless Computer Assisted Navigation System through in Total Hip Arthroplasty in vitro and in vivo Studies. World Congr. Med. Phys. Biomed. Eng. 2006, 3044–3047. [Google Scholar] [CrossRef]
- Auricchio, A.; Sorgente, A.; Soubelet, E.; Regoli, F.; Spinucci, G.; Vaillant, R.; Moccetti, T. Accuracy and usefulness of fusion imaging between three-dimensional coronary sinus and coronary veins computed tomographic images with projection images obtained using fluoroscopy. Europace 2009, 11, 1483–1490. [Google Scholar] [CrossRef]
- Kenngott, H.G.; Wagner, M.; Nickel, F.; Wekerle, A.L.; Preukschas, A.; Apitz, M.; Müller-Stich, B.P. Computer-assisted abdominal surgery: New technologies. Langenbeck Arch. Surg. 2015, 400, 273–281. [Google Scholar] [CrossRef]
- Shen, D.; Wu, G.; Suk, H.I. Deep Learning in Medical Image Analysis. Annu. Rev. Biomed. Eng. 2017, 19, 221–248. [Google Scholar] [CrossRef] [PubMed]
- Szymkuć, S.; Gajewska, E.P.; Klucznik, T.; Molga, K.; Dittwald, P.; Startek, M.; Grzybowski, B.A. Computer-Assisted Synthetic Planning: The End of the Beginning. Angew. Chem. Int. Ed. 2016, 55, 5904–5937. [Google Scholar] [CrossRef] [PubMed]
- Bucholz, R.D.; Laycock, K.A. Image-guided surgery. In Biomedical Photonics Handbook: Therapeutics and Advanced Biophotonics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; Volume 3, pp. 219–238. [Google Scholar] [CrossRef]
- Swienckowski, J.J.; Bono, F.S.; Spagnuolo, M.W. Unicompartmental replacement arthroplasty: A review. Minerva Ortop. E Traumatol. 2005, 56, 49–63. [Google Scholar]
- Lin, H.H.; Lo, L.J. Three-dimensional computer-assisted surgical simulation and intraoperative navigation in orthognathic surgery: A literature review. J. Formos. Med. Assoc. 2015, 114, 300–307. [Google Scholar] [CrossRef]
- Dai, Y.; Angibaud, L.; Jung, A.; Hamad, C.; Bertrand, F.; Liu, D.; Huddleston, J. Accuracy of a computer-assisted surgical system for total knee arthroplasy: A review of surgical parameters on 4000+ clinical cases. J. Orthop. Res. 2017, 99, 20. [Google Scholar]
- Nysjö, J. Interactive 3D Image Analysis for Cranio-Maxillofacial Surgery Planning and Orthopedic Applications. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, 2016. [Google Scholar]
- Gérard, G. Knee Periprosthetic Infections: CAOS Use in One Stage Procedures. EPiC Ser. Health Sci. 2017, 1, 391–394. [Google Scholar] [CrossRef]
- Adams, L.; Krybus, W.; Meyer-Ebrecht, D.; Rueger, R.; Gilsbach, J.M.; Moesges, R.; Schloendorff, G. Computer-Assisted Surgery. IEEE Comput. Graph. Appl. 1990, 10, 43–51. [Google Scholar] [CrossRef]
- Jung, R.E.; Schneider, D.; Ganeles, J.; Wismeijer, D.; Zwahlen, M.; Hämmerle, C.H.F.; Tahmaseb, A. Computer technology applications in surgical implant dentistry: A systematic review. Int. J. Oral Maxillofac. Implant. 2009, 24, 92–109. [Google Scholar]
- Conole, G.; Warburton, B. A review of computer-assisted assessment. Res. Learn. Technol. 2005, 13, 17–31. [Google Scholar] [CrossRef]
- Contreras Ortiz, S.H.; Chiu, T.; Fox, M.D. Ultrasound image enhancement: A review. Biomed. Signal Process. Control 2012, 7, 419–428. [Google Scholar] [CrossRef]
- Park, S.C.; Park, M.K.; Kang, M.G. Super-resolution image reconstruction: A technical overview. IEEE Signal Process. Mag. 2003, 20, 21–36. [Google Scholar] [CrossRef]
- Bedi, S.S.; Khandelwal, R. Various Image Enhancement Techniques-A Critical Review. International J. Adv. Res. Comput. Commun. Eng. 2013, 2, 1605–1609. [Google Scholar]
- Subburaj, K.; Ravi, B.; Agarwal, M.G. Automated 3D geometric reasoning in computer assisted joint reconstructive surgery. In Proceedings of the 2009 IEEE International Conference on Automation Science and Engineering, Bangalore, India, 22–25 August 2009; pp. 367–372. [Google Scholar] [CrossRef]
- Hernandez, D.; Garimella, R.; Eltorai, A.E.M.; Daniels, A.H. Computer-assisted orthopaedic surgery. Orthop. Surg. 2017, 9, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden-van der Zwaag, H.M.J.; Wolterbeek, R.; Nelissen, R.G.H.H. Computer assisted orthopedic surgery; its influence on prosthesis size in total knee replacement. Knee 2008, 15, 281–285. [Google Scholar] [CrossRef]
- Jordan, A.H.; Audia, P.G. Self-enhancement and learning from performance feedback. Acad. Manag. Rev. 2012, 37, 211–231. [Google Scholar] [CrossRef]
- Beghdadi, A.; Larabi, M.C.; Bouzerdoum, A.; Iftekharuddin, K.M. A survey of perceptual image processing methods. Signal Process. Image Commun. 2013, 28, 811–831. [Google Scholar] [CrossRef]
- Shukla, K.N.; Potnis, A.; Dwivedy, P. A Review on Image Enhancement Techniques. Int. J. Eng. Appl. Comput. Sci. 2017, 2, 232–235. [Google Scholar] [CrossRef]
- Eklund, A.; Dufort, P.; Forsberg, D.; LaConte, S.M. Medical image processing on the GPU Past, present and future. Med. Image Anal. 2013, 17, 1073–1094. [Google Scholar] [CrossRef]
- Siu, W.C.; Hung, K.W. Review of image interpolation and super-resolution. In Proceedings of the 2012 Asia Pacific Signal and Information Processing Association Annual Summit and Conference, Hollywood, CA, USA, 3–6 December 2012; pp. 1–10. [Google Scholar]
- Aganj, I.; Yeo, B.T.T.; Sabuncu, M.R.; Fischl, B. On removing interpolation and resampling artifacts in rigid image registration. IEEE Trans. Image Process. 2013, 22, 816–827. [Google Scholar] [CrossRef]
- Wu, Z. A review of statistical methods for preprocessing oligonucleotide microarrays. Stat. Methods Med. Res. 2009, 18, 533–541. [Google Scholar] [CrossRef]
- Kumar, G.; Bhatia, P.K. A detailed review of feature extraction in image processing systems. In Proceedings of the International Conference on Advanced Computing and Communication Technologies, ACCT, Washington, DC, USA, 8–9 February 2014; pp. 5–12. [Google Scholar] [CrossRef]
- Egmont-Petersen, M.; De Ridder, D.; Handels, H. Image processing with neural networks—A review. Pattern Recognit. 2002, 35, 2279–2301. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, J.; Kaur, J. Survey of Contrast Enhancement Techniques based on Histogram Equalization. Int. J. Adv. Comput. Sci. Appl. 2013, 2, 137–141. [Google Scholar] [CrossRef]
- Gupta, S.; Kaur, Y. Review of Different Local and Global Contrast Enhancement Techniques for a Digital Image. Int. J. Comput. Appl. 2014, 100, 18–23. [Google Scholar] [CrossRef]
- Singh, A.; Singh, M.; Kaur, M. Study of Various Image Enhancement Techniques-A Review. Int. J. Comput. Sci. Mob. Comput. 2013, 2, 186–191. [Google Scholar]
- Kong, N.S.P.; Ibrahim, H.; Hoo, S.C. A Literature Review on Histogram Equalization and Its Variations for Digital Image Enhancement. Int. J. Innov. Manag. Technol. 2013, 4, 386–389. [Google Scholar] [CrossRef]
- Li, H.; Liu, F. Image denoising via sparse and redundant representations over learned dictionaries in wavelet domain. In Proceedings of the 5th International Conference on Image and Graphics, ICIG 2009, Xi’an, China, 20–23 September 2009; pp. 754–758. [Google Scholar] [CrossRef]
- Balafar, M.A.; Ramli, A.R.; Saripan, M.I.; Mashohor, S. Review of brain MRI image segmentation methods. Artif. Intell. Rev. 2010, 33, 261–274. [Google Scholar] [CrossRef]
- Smistad, E.; Falch, T.L.; Bozorgi, M.; Elster, A.C.; Lindseth, F. Medical image segmentation on GPUs—A comprehensive review. Med. Image Anal. 2015, 20, 1–18. [Google Scholar] [CrossRef]
- Kaur, D.; Kaur, Y. Various Image Segmentation Techniques: A Review. Int. J. Comput. Sci. Mob. Comput. (IJCSMC) 2014, 3, 809–814. [Google Scholar]
- Guo, Y.; Liu, Y.; Oerlemans, A.; Lao, S.; Wu, S.; Lew, M.S. Deep learning for visual understanding: A review. Neurocomputing 2016, 187, 27–48. [Google Scholar] [CrossRef]
- Vala, H.J.; Baxi, A. A Review on Otsu Image Segmentation Algorithm. Int. J. Adv. Res. Comput. Eng. Technol. 2013, 2, 387–389. [Google Scholar]
- Hamuda, E.; Glavin, M.; Jones, E. A survey of image processing techniques for plant extraction and segmentation in the field. Comput. Electron. Agric. 2016, 125, 184–199. [Google Scholar] [CrossRef]
- Işin, A.; Direkoǧlu, C.; Şah, M. Review of MRI-based Brain Tumor Image Segmentation Using Deep Learning Methods. Proc. Comput. Sci. 2016, 102, 317–324. [Google Scholar] [CrossRef]
- Havaei, M.; Davy, A.; Warde-Farley, D.; Biard, A.; Courville, A.; Bengio, Y.; Larochelle, H. Brain tumor segmentation with Deep Neural Networks. Med. Image Anal. 2017, 35, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Wajid, S.K.; Hussain, A.; Huang, K. Three-Dimensional Local Energy-Based Shape Histogram (3D-LESH): A Nov. Feature Extr. Tech. Expert Syst. Appl. 2018, 112, 388–400. [Google Scholar] [CrossRef]
- Rai, H.M.; Chatterjee, K. Hybrid adaptive algorithm based on wavelet transform and independent component analysis for denoising of MRI images. Meas. J. Int. Meas. Confed. 2019, 144, 72–82. [Google Scholar] [CrossRef]
- Wu, Z.; Fu, J.; Wang, Z.; Li, X.; Li, J.; Pei, Y.; Fan, H. Three-dimensional virtual bone bank system for selecting massive bone allograft in orthopaedic oncology. Int. Orthop. 2015, 39, 1151–1158. [Google Scholar] [CrossRef]
- Fanti, Z.; Torres, F.; Arámbula Cosío, F. Preliminary results in large bone segmentation from 3D freehand ultrasound. IX Int. Semin. Med. Inf. Process. Anal. 2013, 8922, 89220F. [Google Scholar] [CrossRef]
- Lázár, I.; Hajdu, A. Segmentation of retinal vessels by means of directional response vector similarity and region growing. Comput. Biol. Med. 2015, 66, 209–221. [Google Scholar] [CrossRef]
- Klintström, B.; Klintström, E.; Smedby, Ö.; Moreno, R. Feature space clustering for trabecular bone segmentation. Lect. Notes Comput. Sci. (Incl. Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinform.) 2017, 10270, 65–75. [Google Scholar] [CrossRef]
- Bertasius, G.; Torresani, L.; Yu, S.X.; Shi, J. Convolutional random walk networks for semantic image segmentation. In Proceedings of the 30th IEEE Conference on Computer Vision and Pattern Recognition, CVPR, Honolulu, HI, USA, 21–26 July 2017; pp. 6137–6145. [Google Scholar] [CrossRef]
- Meila, M.; Shi, J. A random walks view of spectral segmentation. In Proceedings of the AI and STATISTICS (AISTATS), Key West, FL, USA, 4–7 January 2001; pp. 1–4. [Google Scholar]
- Shamir, A. A survey on mesh segmentation techniques. Comput. Graph. Forum 2008, 27, 1539–1556. [Google Scholar] [CrossRef]
- Lv, J.; Chen, X.; Huangy, J.; Bao, H. Semi-supervised mesh segmentation and labeling. Eur. Symp. Geom. Process. 2012, 31, 2241–2248. [Google Scholar] [CrossRef]
- Mesejo, P.; Ibáñez, Ó.; Cordón, Ó.; Cagnoni, S. A survey on image segmentation using metaheuristic-based deformable models: State of the art and critical analysis. Appl. Sof. Comput. J. 2016, 44, 1–29. [Google Scholar] [CrossRef]
- Zhang, S.; Zhan, Y.; Dewan, M.; Huang, J.; Metaxas, D.N.; Zhou, X.S. Deformable segmentation via sparse shape representation. Lect. Notes Comput. Sci. (Incl. Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinform.) 2011, 6892, 451–458. [Google Scholar] [CrossRef]
- Möller, M.; Lymburner, L.; Volk, M. The comparison index: A tool for assessing the accuracy of image segmentation. Int. J. Appl. Earth Obs. Geoinf. 2007, 9, 311–321. [Google Scholar] [CrossRef]
- Badrinarayanan, V.; Kendall, A.; Cipolla, R. SegNet: A Deep Convolutional Encoder-Decoder Architecture for Image Segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Sotiras, A.; Davatzikos, C.; Paragios, N. Deformable medical image registration: A survey. IEEE Trans. Med. Imaging 2013, 32, 1153–1190. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Hamarneh, G. Medical image registration: A review. Med. Imaging Technol. Appl. 2013, 1, 619–660. [Google Scholar] [CrossRef]
- Alam, F.; Rahman, S.U.; Ullah, S.; Gulati, K. Medical image registration in image guided surgery: Issues, challenges and research opportunities. Biocybern. Biomed. Eng. 2018, 38, 71–89. [Google Scholar] [CrossRef]
- Alam, F.; Rahman, S.U. Intrinsic registration techniques for medical images: A state-of-the-art review. J. Postgrad. Med. Inst. 2016, 30, 119–132. [Google Scholar]
- Maintz, J.B.A.; Viergever, M.A. An Overview of Medical Image Registration Methods; Utrecht University Repository: Utrecht, The Netherlands, 1996; Volume 12, pp. 1–22. [Google Scholar]
- Alam, F.; Rahman, S.U.; Khusro, S.; Ullah, S.; Khalil, A. Evaluation of medical image registration techniques based on nature and domain of the transformation. J. Med. Imaging Radiat. Sci. 2016, 47, 178–193. [Google Scholar] [CrossRef]
- Motai, Y.; Siddique, N.A.; Yoshida, H. Heterogeneous data analysis: Online learning for medical-image-based diagnosis. Pattern Recognit. 2017, 63, 612–624. [Google Scholar] [CrossRef]
- Wan, R.; Li, M. An overview of medical image registration. In Proceedings of the 5th International Conference on Computational Intelligence and Multimedia Applications (ICCIMA 2003), Xi’an, China, 20 October 2003; pp. 385–390. [Google Scholar] [CrossRef]
- Alam, F.; Rahman, S.U. Challenges and Solutions in Multimodal Medical Image Subregion Detection and Registration. J. Med. Imaging Radiat. Sci. 2019, 50, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Hassan, M.; Khan, A.; Chaudhry, A. Robust fuzzy RBF network based image segmentation and intelligent decision making system for carotid artery ultrasound images. Neurocomputing 2015, 151, 745–755. [Google Scholar] [CrossRef]
- Markelj, P.; Tomaževič, D.; Likar, B.; Pernuš, F. A review of 3D/2D registration methods for image-guided interventions. Med. Image Anal. 2012, 16, 642–661. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, J.; Li, W.; Hu, T.; Wang, P. Deep learning on point clouds and its application: A survey. Sensors 2019, 19, 4188. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.R.S.; Arivazhagan, S. Survey of Medical Image Registration. J. Biomed. Eng. Technol. 2013, 1, 8–25. [Google Scholar] [CrossRef]
- Kutyniok, G.; Ma, J.; März, M. Mathematical methods in medical image processing. Quantif. Biophys. Param. Med. Imaging 2018, 153–166. [Google Scholar] [CrossRef]
- Suetens, P. Fundamentals of medical imaging. Fundam. Med. Imaging 2017, 6, 9375–9389. [Google Scholar] [CrossRef]
- Ker, J.; Wang, L.; Rao, J.; Lim, T. Deep Learning Applications in Medical Image Analysis. IEEE Access 2017, 6, 9375–9389. [Google Scholar] [CrossRef]
- Bouaziz, S.; Tagliasacchi, A.; Pauly, M. Sparse iterative closest point. Eur. Symp. Geom. Process. 2013, 32, 113–123. [Google Scholar] [CrossRef]
- Maier-Hein, L.; Franz, A.M.; Dos Santos, T.R.; Schmidt, M.; Fangerau, M.; Meinzer, H.P.; Fitzpatrick, J.M. Convergent iterative closest-point algorithm to accomodate anisotropic and inhomogenous localization error. IEEE Trans. Pattern Anal. Mach. Intell. 2012, 34, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Serafin, J.; Grisetti, G. NICP: Dense normal based point cloud registration. In Proceedings of the 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Hamburg, Germany, 28 September–2 October 2015; pp. 742–749. [Google Scholar] [CrossRef]
- Marani, R.; Renò, V.; Nitti, M.; D’Orazio, T.; Stella, E. A Modified Iterative Closest Point Algorithm for 3D Point Cloud Registration. Comput.-Aided Civil Infrastruct. Eng. 2016, 31, 515–534. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Campbell, D.; Jia, Y. Go-ICP: A Globally Optimal Solution to 3D ICP Point-Set Registration. IEEE Trans. Pattern Anal. Mach. Intell. 2016, 38, 2241–2254. [Google Scholar] [CrossRef]
- Angibaud, L.; Silver, X.; Gulbransen, S.; Stulberg, B. Accuracy of a Novel Computer-Assisted Guidance System for Total Knee Arthroplasty. Bone Jt. J. Orthop. Proc. Suppl. 2013, 95, 107. [Google Scholar]
- Weil, Y.; Mosheiff, R.; Joskowicz, L.; Liebergall, M. Principles of computer-aided surgery in trauma surgery. Navig. MIS Orthop. Surg. 2007, 476–485. [Google Scholar] [CrossRef]
- Bae, D.K.; Song, S.J. Computer assisted navigation in knee arthroplasty. Clin. Orthop. Surg. 2011, 3, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Kumta, S.M. Use of Computer Navigation in Orthopedic Oncology. Curr. Surg. Rep. 2014, 2, 47. [Google Scholar] [CrossRef]
- Fontana, E.J.; Benzinger, T.; Cobbs, C.; Henson, J.; Fouke, S.J. The evolving role of neurological imaging in neuro-oncology. J. Neuro-Oncol. 2014, 119, 491–502. [Google Scholar] [CrossRef]
- Mezger, U.; Jendrewski, C.; Bartels, M. Navigation in surgery. Langenbeck Arch. Surg. 2013, 398, 501–514. [Google Scholar] [CrossRef]
- Botton-Divet, L.; Houssaye, A.; Herrel, A.; Fabre, A.-C.; Cornette, R. Tools for quantitative formdescription; an evaluation of different software packages for semi-landmark analysis. PeerJ 2015, 1–18. [Google Scholar]
- Schlicher, W.; Nielsen, I.; Huang, J.C.; Maki, K.; Hatcher, D.C.; Miller, A.J. Consistency and precision of landmark identification in three-dimensional cone beam computed tomography scans. Eur. J. Orthod. 2012, 34, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.J.; Kurta, I.C.; Jasani, V.; Jones, C.H.W.; Rahmatalla, A.; MacKenzie, G.; Dove, J. Assessment of CAOS as a training model in spinal surgery: A randomised study. Eur. Spine J. 2007, 16, 239–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Angibaud, L.; Dai, Y.; Jung, A.; Hamad, C.; Bertrand, F.; Huddleston, J.; Liu, D. Geographic variations in the surgical profiles of computer-assisted total knee arthroplasty. J. Orthop. Res. 2017, 99, 19. [Google Scholar]
- Torres, P.M.B.; Gonçalves, P.J.S.; Martins, J.M.M. 3D reconstruction and visualization of femur bone structures. Rom. Rev. Precis. Mech. Opt. Mechatron. 2012, 41, 51–56. [Google Scholar]
- Hafez, M.A.; DiGioia, A.M. Computer-assisted total hip arthroplasty: The present and the future. Future Rheumatol. 2006, 1, 121–131. [Google Scholar] [CrossRef]
- Akins, R.; Abdelgawad, A.A.; Kanlic, E.M. Computer Navigation in Orthopedic Trauma: Safer Surgeries with Less Irradiation and More Precision. J. Surg. Orthop. Adv. 2012, 21, 187–197. [Google Scholar] [CrossRef]
- Zaffagnini, S.; Urrizola, F.; Signorelli, C.; Grassi, A.; Di Sarsina, T.R.; Lucidi, G.A.; Muccioli, G.M.; Bonanzinga, T.; Marcacci, M. Current use of navigation system in ACL surgery: A historical review. Knee Surg. Sport. Traumatol. Arthrosc. 2016, 24, 3396–3409. [Google Scholar] [CrossRef]
- Joskowicz, L.; Hazan, E.J. Computer-aided orthopedic surgery: Incremental shift or paradigm change? Adv. Exp. Med. Biol. 2018, 1093, 21–30. [Google Scholar] [CrossRef]
- Mihai, S.; Filip, V. 3D modeling and performing of orthopedic implants by material deposition rapid prototyping. Rom. Rev. Precis. Mech. Opt. Mechatron. 2012, 41, 128–131. [Google Scholar]
- Stewart, C.; Akhavan, B.; Wise, S.G.; Bilek, M.M.M. A review of biomimetic surface functionalization for bone-integrating orthopedic implants: Mechanisms, current approaches, and future directions. Prog. Mater. Sci. 2019, 106, 100588. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Zheng, Y.; Tang, L.; Qin, Y.-X.; Zhu, D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Mirota, D.J.; Ishii, M.; Hager, G.D. Vision-Based Navigation in Image-Guided Interventions. Annu. Rev. Biomed. Eng. 2011, 13, 297–319. [Google Scholar] [CrossRef] [PubMed]
- Eggers, G.; Mühling, J.; Marmulla, R. Image-to-patient registration techniques in head surgery. Int. J. Oral Maxillofac. Surg. 2006, 35, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Plooij, J.M.; Maal, T.J.J.; Haers, P.; Borstlap, W.A.; Kuijpers-Jagtman, A.M.; Bergé, S.J. Digital three-dimensional image fusion processes for planning and evaluating orthodontics and orthognathic surgery. A systematic review. Int. J. Oral. Maxillofac. Surg. 2011, 40, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Sattler, T.; Torii, A.; Sivic, J.; Pollefeys, M.; Taira, H.; Okutomi, M.; Pajdla, T. Are large-scale 3D models really necessary for accurate visual localization? In Proceedings of the 30th IEEE Conference on Computer Vision and Pattern Recognition, CVPR 2017, Honolulu, HI, USA, 21–26 July 2017; pp. 6175–6184. [Google Scholar] [CrossRef]
- Mirota, D.J.; Wang, H.; Taylor, R.H.; Ishii, M.; Gallia, G.L.; Hager, G.D. A system for video-Based navigation for endoscopic endonasal skull base surgery. IEEE Trans. Med. Imaging 2012, 31, 963–976. [Google Scholar] [CrossRef]
- Mason, A.; Paulsen, R.; Babuska, J.M.; Rajpal, S.; Burneikiene, S.; Nelson, E.L.; Villavicencio, A.T. The accuracy of pedicle screw placement using intraoperative image guidance systems. J. Neurosurg. Spine 2013, 20, 196–203. [Google Scholar] [CrossRef]
- Moses, Z.B.; Mayer, R.R.; Strickland, B.A.; Kretzer, R.M.; Wolinsky, J.P.; Gokaslan, Z.L.; Baaj, A.A. Neuronavigation in minimally invasive spine surgery. Neurosurg. Focus 2013, 35, E12. [Google Scholar] [CrossRef]
- Axel, L.; Dougherty, L. MR imaging of motion with spatial modulation of magnetization. Radiology 1989, 171, 841–845. [Google Scholar] [CrossRef]
- Choi, S. Total hip arthroplasty. In Decision-Making in Orthopedic and Regional Anesthesiology: A Case-Based Approach; Cambridge University Press: Cambridge, UK, 2015; pp. 95–100. [Google Scholar] [CrossRef]
- Sperling, J.W.; Hawkins, R.J.; Walch, G.; Zuckerman, J.D. Complications in total shoulder arthroplasty. J. Bone Joint Surg. 2013, 95, 563–569. [Google Scholar] [CrossRef]
- Bryan, D.; Parvizi, J.; Austin, M.; Backe, H.; Valle, C.; Kolessar, D.J.; Kreuzer, S.; Malinzak, R.; Masri, B.; McGrory, B.J.; et al. Obesity and total joint arthroplasty. A literature based review. J. Arthrop. 2013, 28, 714–721. [Google Scholar] [CrossRef]
- Manrique, J.; Gomez, M.M.; Parvizi, J. Stiffness after total knee arthroplasty. J. Knee Surg. 2015, 28, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Helm, P.A.; Teichman, R.; Hartmann, S.L.; Simon, D. Spinal Navigation and Imaging: History, Trends, and Future. IEEE Trans. Med. Imaging 2015, 34, 1738–1746. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Suenaga, H.; Liao, H.; Hoshi, K.; Yang, L.; Kobayashi, E.; Sakuma, I. Real-time computer-generated integral imaging and 3D image calibration for augmented reality surgical navigation. Comput. Med. Imaging Grap. 2015, 40, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, J.; Ando, T.; Kubota, A.; Yamashita, H.; Sakuma, I.; Chiba, T.; Kobayashi, E. Vision-based endoscope tracking for 3D ultrasound image-guided surgical navigation. Comput. Med. Imaging Grap. 2015, 40, 205–216. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, Z. Recovering 3D planes from a single image via convolutional neural networks. Lect. Notes Comput. Sci. (Incl. Subser. Lectur. Notes Artif. Intell. Lectur. Notes Bioinform.) 2018, 11214, 84–103. [Google Scholar] [CrossRef]
- Suenaga, H.; Tran, H.H.; Liao, H.; Masamune, K.; Dohi, T.; Hoshi, K.; Takato, T. Vision-based markerless registration using stereo vision and an augmented reality surgical navigation system: A pilot study. BMC Med. Imaging 2015, 15, 51–62. [Google Scholar] [CrossRef]
- Havsteen, I.; Ohlhues, A.; Madsen, K.H.; Nybing, J.D.; Christensen, H.; Christensen, A. Are movement artifacts in magnetic resonance imaging a real problém?-a narrative review. Front. Neurol. 2017, 8, 232–240. [Google Scholar] [CrossRef]
- Ouyang, J.; Li, Q.; El Fakhri, G. Magnetic resonance-based motion correction for positron emission tomography imaging. Semin. Nucl. Med. 2013, 43, 60–67. [Google Scholar] [CrossRef]
- Von Jako, R.; Finn, M.A.; Yonemura, K.S.; Araghi, A.; Khoo, L.T.; Carrino, J.A.; Perez-Cruet, M. Minimally invasive percutaneous transpedicular screw fixation: Increased accuracy and reduced radiation exposure by means of a novel electromagnetic navigation system. Acta Neurochir. 2011, 153, 589–596. [Google Scholar] [CrossRef]
- Kawakami, Y.; Hiranaka, T.; Matsumoto, T.; Hida, Y.; Fukui, T.; Uemoto, H.; Doita, M.; Tsuji, M.; Kuroda, R. The accuracy of bone tunnel position using fluoroscopic-based navigation system in anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1503–1510. [Google Scholar] [CrossRef]
- Weil, Y.A.; Liebergall, M.; Mosheiff, R.; Khoury, A. Fluoroscopic Based Navigation in orthopaedic trauma—A review of a large center’s experience. Harefuah 2018, 157, 145–148. [Google Scholar]
- Wang, J.; Wang, Y.; Zhu, G.; Chen, X.; Zhao, X.; Qiao, H.; Fan, Y. Influence of the quality of intraoperative fluoroscopic images on the spatial positioning accuracy of a CAOS system. Int. J. Med. Robot. Comput. Assist. Surg. 2018, 14, 1898. [Google Scholar] [CrossRef]
- Takao, M.; Nishii, T.; Sakai, T.; Yoshikawa, H.; Sugano, N. Iliosacral screw insertion using CT-3D-fluoroscopy matching navigation. Injury 2014, 45, 988–994. [Google Scholar] [CrossRef]
- Uruc, V.; Ozden, R.; Dogramaci, Y.; Kalaci, A.; Dikmen, B.; Yildiz, O.S.; Yengil, E. The comparison of freehand fluoroscopic guidance and electromagnetic navigation for distal locking of intramedullary implants. Injury 2013, 44, 863–866. [Google Scholar] [CrossRef]
- Mendelsohn, D.; Strelzow, J.; Dea, N.; Ford, N.L.; Batke, J.; Pennington, A.; Street, J. Patient and surgeon radiation exposure during spinal instrumentation using intraoperative computed tomography-based navigation. Spine J. 2016, 16, 343–354. [Google Scholar] [CrossRef]
- Bourgeois, A.C.; Faulkner, A.R.; Bradley, Y.C.; Pasciak, A.S.; Barlow, P.B.; Gash, J.R.; Reid, W.S. Improved accuracy of minimally invasive transpedicular screw placement in the lumbar spine with 3-dimensional stereotactic image guidance: A comparative meta-analysis. J. Spinal Disord. Tech. 2012, 8, 324–329. [Google Scholar] [CrossRef]
- Hahn, P.; Oezdemir, S.; Komp, M.; Giannakopoulos, A.; Heikenfeld, R.; Kasch, R.; Merk, H.; Godolias, G.; Ruetten, S. A new electromagnetic navigation system for pedicle screws placement: A human cadaver study at the lumbar spine. PLoS ONE 2015, 10, e0133708. [Google Scholar] [CrossRef]
- Bandela, J.R.; Jacob, R.P.; Arreola, M.; Griglock, T.M.; Bova, F.; Yang, M. Use of CT-based intraoperative spinal navigation: Management of radiation exposure to operator, staff, and patients. World Neurosurg. 2013, 79, 390–394. [Google Scholar] [CrossRef]
- Pandey, P.; Abugharbieh, R.; Hodgson, A.J. Trackerless 3D Ultrasound Stitching for Computer-Assisted Orthopaedic Surgery and Pelvic Fractures. CAOS 2017, 1, 318–321. [Google Scholar] [CrossRef]
- Varnavas, A.; Carrell, T.; Penney, G. Increasing the automation of a 2D-3D registration system. IEEE Trans. Med. Imaging 2013, 32, 387–399. [Google Scholar] [CrossRef]
- Villard, J.; Ryang, Y.M.; Demetriades, A.K.; Reinke, A.; Behr, M.; Preuss, A.; Meyer, B.; Ringel, F. Radiation exposure to the surgeon and the patient during posterior lumbar spinal instrumentation: A prospective randomized comparison of navigated versus non-navigated freehand techniques. Spine 2014, 39, 1004–1009. [Google Scholar] [CrossRef]
- Slomczykowski, M.A.; Hofstetter, R.; Sati, M.; Krettek, C.; Nolte, L.P. Novel computer-assisted fluoroscopy system for intraoperative guidance: Feasibility study for distal locking of femoral nails. J. Orthop. Trauma 2001, 15, 122–131. [Google Scholar] [CrossRef]
- Ryang, Y.M.; Villard, J.; Obermüller, T.; Friedrich, B.; Wolf, P.; Gempt, J.; Meyer, B. Learning curve of 3D fluoroscopy image-guided pedicle screw placement in the thoracolumbar spine. Spine J. 2015, 15, 467–476. [Google Scholar] [CrossRef]
- Wassilew, G.I.; Perka, C.; Janz, V.; König, C.; Asbach, P.; Hasart, O. Use of an Ultrasound-Based Navigation System for an Accurate Acetabular Positioning in Total Hip Arthroplasty. A Prospective, Randomized, Controlled Study. J. Arthrop. 2012, 27, 687–694. [Google Scholar] [CrossRef]
- Turley, G.A.; Ahmed, S.M.Y.; Williams, M.A.; Griffin, D.R. Validation of the femoral anteversion measurement method used in imageless navigation. Comput. Aided Surg. 2012, 17, 187–197. [Google Scholar] [CrossRef]
- Audenaert, E.; Smet, B.; Pattyn, C.; Khanduja, V. Imageless versus image-based registration in navigated arthroscopy of the hip. J. Bone Joint Surg. 2012, 94, 624–629. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Y.; Cai, L. Imageless navigation versus traditional method in total hip arthroplasty: A meta-analysis. Int. J. Surg. 2015, 21, 122–127. [Google Scholar] [CrossRef]
- Scholes, C.; Sahni, V.; Lustig, S.; Parker, D.A.; Coolican, M.R.J. Patient-specific instrumentation for total knee arthroplasty does not match the pre-operative plan as assessed by intra-operative computer-assisted navigation. Knee Surg. Sport. Traumatol. Arthrosc. 2014, 22, 660–665. [Google Scholar] [CrossRef]
- Weber, M.; Woerner, M.; Messmer, B.; Grifka, J.; Renkawitz, T. Navigation is Equal to Estimation by Eye and Palpation in Preventing Psoas Impingement in THA. Clin. Orthop. Relat. Res. 2017, 475, 196–203. [Google Scholar] [CrossRef][Green Version]
- Deep, K.; Shankar, S.; Mahendra, A. Computer assisted navigation in total knee and hip arthroplasty. SICOT-J 2017, 3, 50–56. [Google Scholar] [CrossRef]
- Härtl, R.; Lam, K.S.; Wang, J.; Korge, A.; Kandziora, F.; Audigé, L. Worldwide survey on the use of navigation in spine surgery. World Neurosurg. 2013, 79, 162–172. [Google Scholar] [CrossRef]
- Wasterlain, A.S.; Buza, J.A.; Thakkar, S.C.; Schwarzkopf, R.; Vigdorchik, J. Navigation and robotics in total hip arthroplasty. JBJS Rev. 2017, 5, 2. [Google Scholar] [CrossRef]
- Nam, D.; Maher, P.A.; Rebolledo, B.J.; Nawabi, D.H.; McLawhorn, A.S.; Pearle, A.D. Patient specific cutting guides versus an imageless, computer-assisted surgery system in total knee arthroplasty. Knee 2013, 20, 263–267. [Google Scholar] [CrossRef]
- Chen, T.K.; Abolmaesumi, P.; Pichora, D.R.; Ellis, R.E. A system for ultrasound-guided computer-assisted orthopaedic surgery. Comput. Aided Surg. 2005, 10, 281–292. [Google Scholar] [CrossRef]
- Gonçalves, P.J.S.; Torres, P.M.B.; Santos, F.; António, R.; Catarino, N.; Martins, J.M.M. A Vision System for Robotic Ultrasound Guided Orthopaedic Surgery. J. Int. Robot. Syst. Theory Appl. 2014, 77, 327–339. [Google Scholar] [CrossRef]
- Atesok, K.; Schemitsch, E. Computer-assisted Trauma surgery. J. Am. Acad. Orthop. Surg. 2010, 18, 247–258. [Google Scholar] [CrossRef]
- Mozes, A.; Chang, T.C.; Arata, L.; Zhao, W. Three-dimensional A-mode ultrasound calibration and registration for robotic orthopaedic knee surgery. Int. J. Med. Robot. Comput. Assist. Surg. 2010, 6, 91–101. [Google Scholar] [CrossRef]
- Vercruyssen, M.; Hultin, M.; Van Assche, N.; Svensson, K.; Naert, I.; Quirynen, M. Guided surgery: Accuracy and efficacy. Periodontology 2000, 66, 228–246. [Google Scholar] [CrossRef]
- Ohashi, H.; Matsuura, M.; Okamoto, Y.; Okajima, Y. Intra- and intersurgeon variability in image-free navigation system for THA. Clin. Orthop. Relat. Res. 2009, 467, 2305–2309. [Google Scholar] [CrossRef][Green Version]
- Ryan, J.A.; Jamali, A.A.; Bargar, W.L. Accuracy of computer navigation for acetabular component placement in. Clin. Orthop. Relat. Res. 2010, 468, 169–177. [Google Scholar] [CrossRef]
- Lin, F.; Lim, D.; Wixson, R.L.; Milos, S.; Hendrix, R.W.; Makhsous, M. Limitations of Imageless Computer-Assisted Navigation for Total Hip Arthroplasty. J. Arthrop. 2011, 26, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, E.; Bryant, A.; Tetsworth, K. Anterior Pelvic Soft Tissue Thickness Influences Acetabular Cup Positioning with Imageless Navigation. J. Arthrop. 2012, 27, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Stiehl, J.B.; Heck, D.A.; Jaramaz, B.; Amiot, L.-P. Comparison of fluoroscopic and imageless registration in surgical navigation of the acetabular component. Comput. Aided Surg. 2007, 12, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.P.; Kannan, V.; Dandachli, W.; Iranpour, F.; Brust, K.U.; Hart, A.J. Learning how to resurface cam-type femoral heads with acceptable accuracy and precision: The role of computed tomography-based navigation. J. Bone Joint Surg. 2008, 90, 57–64. [Google Scholar] [CrossRef]
- Pitto, R.P.; Malak, S.; Anderson, I.A. Accuracy of computer-assisted navigation for femoral head resurfacing decreases in hips with abnormal anatomy. Clin. Orthop. Relat. Res. 2009, 467, 2310–2317. [Google Scholar] [CrossRef][Green Version]
- Subramanian, P.; Wainwright, T.W.; Bahadori, S.; Middleton, R.G. A review of the evolution of robotic-assisted total hip arthroplasty. HIP Int. 2019, 29, 232–238. [Google Scholar] [CrossRef]
- Jia, Z.; Du, Z.; Wang, M. A novel finite element method based biomechanical model for HIT-robot assisted orthopedic surgery system. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology, New York, NY, USA, 31 August–3 September 2006; pp. 6505–6508. [Google Scholar]
- Wang, M. Development and validity of tissue biomechanics modeling for virtual robot assisted orthopedic surgery system. In Proceedings of the 3rd International Conference on Bioinformatics and Biomedical Engineering, iCBBE 2009, Beijing, China, 11–16 June 2009. [Google Scholar]
- Bai, L.; Yang, J.; Chen, X.; Sun, Y.; Li, X. Medical robotics in bone fracture reduction surgery: A review. Sensors 2019, 19, 3593. [Google Scholar] [CrossRef]
- Zhao, J.-X.; Li, C.; Ren, H.; Hao, M.; Zhang, L.-C.; Tang, P.-F. Evolution and Current Applications of Robot-Assisted Fracture Reduction: A Comprehensive Review. Ann. Biomed. Eng. 2019. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Tzioupis, C.C.; Pape, H.C.; Roberts, C.S. Percutaneous fixation of the pelvic ring: An update. J. Bone Jt. Surg. Br. Vol. 2007, 89, 145–154. [Google Scholar] [CrossRef]
- Saragaglia, D.; Rubens-Duval, B.; Gaillot, J.; Lateur, G.; Pailhé, R. Total knee arthroplasties from the origin to navigation: history, rationale, indications. Int. Orthopaedics 2019, 43, 597–604. [Google Scholar] [CrossRef]
- Abdelgawad, A.; Akins, R.; Kanlic, E.M. Use of computer assisted orthopedic surgery in pelvic and acetabular trauma. Acta Med. Acad. 2011, 40, 166–173. [Google Scholar] [CrossRef]
- Jabran, A.; Peach, C.; Ren, L. Biomechanical analysis of plate systems for proximal humerus fractures: A systematic literature review. Biomed. Eng. Online 2018, 17, 47–77. [Google Scholar] [CrossRef] [PubMed]
- Jabran, A.; Peach, C.; Zou, Z.; Ren, L. Biomechanical comparison of screw-based zoning of PHILOS and Fx proximal humerus plates. BMC Musculoskelet. Disord. 2018, 19, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Dirhold, B.M.; Citak, M.; Al-Khateeb, H.; Haasper, C.; Kendoff, D.; Krettek, C.; Citak, M. Current state of computer-assisted trauma surgery. Curr. Rev. Musculoskelet. Med. 2012, 5, 184–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanlić, E.M.; DeLaRosa, F.; Pirela-Cruz, M. Computer assisted orthopaedic surgery—CAOS. Bosn. J. Basic Med. Sci. 2006, 6, 7–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Porcellini, G.; Tarallo, L.; Novi, M.; Spiezia, F.; Catani, F. Technology applications in shoulder replacement. J. Orthop. Traumatol. 2019, 20, 27. [Google Scholar] [CrossRef]
- Biazzo, A.; Confalonieri, N. Computer-assisted surgery in total knee replacement: Advantages, surgical procedure and review of the literature. Acta Biomed. 2019, 90, 16–23. [Google Scholar]
- Weil, Y.A.; Liebergall, M.; Khoury, A. Computer assisted surgery for iliosacral screw placement-how far have we gone? J. Trauma Treat. 2016, 5, 345–352. [Google Scholar] [CrossRef]
- Jabran, A.; Peach, C.; Zou, Z.; Ren, L. Parametric Design Optimisation of Proximal Humerus Plates Based on Finite Element Method. Ann. Biomed. Eng. 2019, 47, 601–614. [Google Scholar] [CrossRef]
- Apostolov, P.; Burnev, M.; Milkov, P. Methods and techniques of percutaneous external fixation in pelvic fractures. J. IMAB Annu. Proc. 2011, 17, 166–171. [Google Scholar] [CrossRef][Green Version]
- Jabran, A.; Peach, C.; Zou, Z.; Ren, L. Hybrid blade and locking plate fixation for proximal humerus fractures: A comparative biomechanical analysis. Biomed. Eng. Online 2018, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Worth, A.J.; Crosse, K.R.; Kersley, A. Computer-Assisted Surgery Using 3D Printed Saw Guides for Acute Correction of Antebrachial Angular Limb Deformities in Dogs. Vet. Comp. Orthop. Traumatol. 2019, 32, 241–249. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office of Legal Affairs. Treaty Series: Treaties and International Agreements Registered or Filed and Recorded with the Secretariat of the United Nations; United Nations: New York, NY, USA, 1986. [Google Scholar]
- Fang, C.; Cai, H.; Kuong, E.; Chui, E.; Siu, Y.C.; Ji, T.; Drstvenšek, I. Surgical applications of three-dimensional printing in the pelvis and acetabulum: From models and tools to implants. Unfallchirurg 2019, 122, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Börm, W.; König, R.W.; Albrecht, A.; Richter, H.P.; Kast, E. Percutaneous transarticular atlantoaxial screw fixation using a cannulated screw system and image guidance. Minim. Invasive Neurosurg. 2004, 47, 111–114. [Google Scholar] [CrossRef]
- Amoretti, N.; Marcy, P.Y.; Hauger, O.; Browaeys, P.; Amoretti, M.E.; Hoxorka, I.; Boileau, P. Percutaneous screw fixation of a vertebral pedicle fracture under CT-guidance: A new technique. Eur. J. radiol. 2012, 81, 591–593. [Google Scholar] [CrossRef]
- Iorio, J.A.; Jakoi, A.M.; Rehman, S. Percutaneous Sacroiliac Screw Fixation of the Posterior Pelvic Ring. Orthop. Clin. North Am. 2015, 46, 511–521. [Google Scholar] [CrossRef]
- Klassen, P.D.; Baume, B.; Elsharkawy, A.E. Percutaneous posterior combined C2 translaminar and pedicle screws using Intraoperative O-arm Navigation in an atypical traumatic spondylolisthesis: Technical notes. Interdiscip. Neurosurg. 2017, 9, 39–40. [Google Scholar] [CrossRef]
- Askari, M.; Shin, A.Y. Extraction of cannulated percutaneous screw from scaphoid: A simplified technique. J. Hand Surg. 2012, 37, 1702–1705. [Google Scholar] [CrossRef]
- Biber, R.; Pauser, J.; Brem, M.; Bail, H.J. Bioabsorbable metal screws in traumatology: A promising innovation. Trauma Case Rep. 2017, 8, 11–15. [Google Scholar] [CrossRef]
- Chew, F.S. Musculoskeletal Imaging: The Essentials; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Acar, B.; Kose, O.; Kati, Y.A.; Egerci, O.F.; Turan, A.; Yuksel, H.Y. Comparison of volar versus dorsal screw fixation for scaphoid waist fractures: A finite element analysis. Orthop. Traumatol. Surg. Res. 2018, 104, 1107–1113. [Google Scholar] [CrossRef]
- Le, L.; Jabran, A.; Peach, C.; Ren, L. Effect of screw thread length on stiffness of proximal humerus locking plate constructs: A finite element study. Med. Eng. Phys. 2019, 63, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Vigdorchik, J.M.; Jin, X.; Sethi, A.; Herzog, D.T.; Oliphant, B.W.; Yang, K.H.; Vaidya, R. A biomechanical study of standard posterior pelvic ring fixation versus a posterior pedicle screw construct. Injury 2015, 46, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chaudhari, R.; Wu, C.; Mehbod, A.A.; Erkan, S.; Transfeldt, E.E. Biomechanical evaluation of an expandable meshed bag augmented with pedicle or facet screws for percutaneous lumbar interbody fusion. Spine J. 2010, 10, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, B.T.; Nalley, C.; Gaskins, R.B., III; Gutierrez, S.; Alexander, G.E., III; Anijar, L.; Santoni, B.G. Biomechanical analysis of impending femoral neck fractures: The role of percutaneous cement augmentation for osteolytic lesions. Clin. Biomech. 2014, 29, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Ropars, M.; Mitton, D.; Skalli, W. Minimally invasive screw plates for surgery of unstable intertrochanteric femoral fractures: A biomechanical comparative study. Clin. Biomech. 2008, 23, 1012–1017. [Google Scholar] [CrossRef]
- Park, Y.; Ha, J.W.; Lee, Y.T.; Sung, N.Y. Percutaneous placement of pedicle screws in overweight and obese patients. Spine J. 2011, 11, 919–924. [Google Scholar] [CrossRef]
- Weninger, P.; Dall’Ara, E.; Leixnering, M.; Pezzei, C.; Hertz, H.; Drobetz, H.; Zysset, P. Volar fixed-angle plating of extra-articular distal radius fractures—A biomechanical analysis comparing threaded screws and smooth pegs. J. Trauma Acute Care Surg. 2010, 69, E46–E55. [Google Scholar] [CrossRef]
- Yao, J.; Park, M.J.; Patel, C.S. Biomechanical comparison of volar locked plate constructs using smooth and threaded locking pegs. Orthopedic 2014, 37, E169–E173. [Google Scholar] [CrossRef]
- Chudik, S.C.; Weinhold, P.; Dahners, L.E. Fixed-angle plate fixation in simulated fractures of the proximal humerus: A biomechanical study of a new device. J. Shoulder Elb. Surg. 2003, 12, 578–588. [Google Scholar] [CrossRef]
- Siffri, P.C.; Peindl, R.D.; Coley, E.R.; Norton, J.; Connor, P.M.; Kellam, J.F. Biomechanical analysis of blade plate versus locking plate fixation for a proximal humerus fracture: Comparison using cadaveric and synthetic humeri. J. Orthop. Trauma 2006, 20, 547–554. [Google Scholar] [CrossRef]
- La Rosa, G.; Clienti, C.; Mineo, R.; Audenino, A. Experimental analysis of pedicle screws. Proc. Struct. Integr. 2016, 2, 1244–1251. [Google Scholar] [CrossRef][Green Version]
- Clin, J.; Le Navéaux, F.; Driscoll, M.; Mac-Thiong, J.M.; Labelle, H.; Parent, S.; Serhan, H. Biomechanical Comparison of the Load-Sharing Capacity of High and Low Implant Density Constructs with Three Types of Pedicle Screws for the Instrumentation of Adolescent Idiopathic Scoliosis. Spine Deform. 2019, 7, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Lonstein, J.E.; Denis, F.; Perra, J.H.; Pinto, M.R.; Smith, M.D.; Winter, R.B. Complications associated with pedicle screws. JBJS 1999, 81, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Carreau, J.H.; Bastrom, T.; Petcharaporn, M.; Schulte, C.; Marks, M.; Illés, T.; Newton, P.O. Computer-generated, three-dimensional spine model from biplanar radiographs: A validity study in idiopathic scoliosis curves greater than 50 degrees. Spine Deform. 2014, 2, 81–88. [Google Scholar] [CrossRef]
- Humbert, L.; De Guise, J.A.; Aubert, B.; Godbout, B.; Skalli, W. 3D reconstruction of the spine from biplanar X-rays using parametric models based on transversal and longitudinal inferences. Med. Eng. Phys. 2009, 31, 681–687. [Google Scholar] [CrossRef]
- Shirazi-Adl, S.A.; Shrivastava, S.C.; Ahmed, A.M. Stress analysis of the lumbar disc-body unit in compression. A three-dimensional nonlinear finite element study. Spine 1984, 9, 120–134. [Google Scholar] [CrossRef]
- Bharucha, N.J.; Lonner, B.S.; Auerbach, J.D.; Kean, K.E.; Trobisch, P.D. Low-density versus high-density thoracic pedicle screw constructs in adolescent idiopathic scoliosis: Do more screws lead to a better outcome? Spine J. 2013, 13, 375–381. [Google Scholar] [CrossRef]
- Larson, A.N.; Polly, D.W., Jr.; Diamond, B.; Ledonio, C.; Richards, B.S., III; Emans, J.B.; Sucato, D.J.; Johnston, C.E. Minimize Implants Maximize Outcomes Study Group. Does higher anchor density result in increased curve correction and improved clinical outcomes in adolescent idiopathic scoliosis? Spine 2014, 39, 571–578. [Google Scholar] [CrossRef]
- Gotfryd, A.O.; Avanzi, O. Randomized clinical study on surgical techniques with different pedicle screw densities in the treatment of adolescent idiopathic scoliosis types Lenke 1A and 1B. Spine Deform. 2013, 1, 272–279. [Google Scholar] [CrossRef]
- Larson, A.N.; Aubin, C.E.; Polly, D.W., Jr.; Ledonio, C.G.; Lonner, B.S.; Shah, S.A.; Richards, B.S., III; Erickson, M.A.; Emans, J.B.; Weinstein, S.L.; et al. Are more screws better? A systematic review of anchor density and curve correction in adolescent idiopathic scoliosis. Spine Deform. 2013, 1, 237–247. [Google Scholar] [CrossRef]
- Kubiak, A.J.; Lindqvist-Jones, K.; Dearn, K.D.; Shepherd, D.E. Comparison of the mechanical properties of two designs of polyaxial pedicle screw. Eng. Fail. Anal. 2019, 95, 96–106. [Google Scholar] [CrossRef]
- Arslan, A.K.; Demir, T.; Örmeci, M.F.; Camuşcu, N.; Türeyen, K. Postfusion pullout strength comparison of a novel pedicle screw with classical pedicle screws on synthetic foams. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2013, 227, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Gelgor, I.E.; Karaman, A.I.; Buyukyilmaz, T. Comparison of 2 distalization systems supported by intraosseous screws. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 161E1–161E8. [Google Scholar] [CrossRef]
- Amasyalı, M.; Sabuncuoğlu, F.A.; Oflaz, U. Intraoral Molar Distalization with Intraosseous Mini Screw. Turk. J. Orthod. 2018, 31, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Cullen, P.M. Intraosseous cannulation in children. Anaesth. Intensiv. Care Med. 2014, 15, 567–569. [Google Scholar] [CrossRef]
- Rony, L.; Lancigu, R.; Hubert, L. Intraosseous metal implants in orthopedics: A review. Morphologie 2018, 102, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Chen, Y.M.; Biao, M.N.; Zhang, X.D.; Yang, B.C. Bio-functionalization of biomedical metals. Mater. Sci. Eng. C 2017, 70, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Sampatacos, N.; Getelman, M.H.; Henninger, H.B. Biomechanical comparison of two techniques for arthroscopic suprapectoral biceps tenodesis: Interference screw versus implant-free intraosseous tendon fixation. J. Shoulder Elb. Surg. 2014, 23, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Hordyk, P.J.; Fuerbringer, B.A.; Roukis, T.S. Clinical Management of Acute, Closed Displaced Intra-Articular Calcaneal Fractures. Clin. Podiatry Med. Surg. 2019, 36, 163–171. [Google Scholar] [CrossRef]
- Scott, R.T.; Hyer, C.F.; DeMill, S.L. Screw fixation diameter for fifth metatarsal Jones fracture: A cadaveric study. J. Foot Ankl. Surg. 2015, 54, 227–229. [Google Scholar] [CrossRef]
- Roukis, T.S. Closed Manipulation, Intraosseous Reduction, and Rigid Internal Fixation for Displaced Intra-Articular Calcaneal Fractures. Clin. Podiatry Med. Surg. 2019, 36, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A. Additive manufacturing applications in orthopaedics: A review. J. Clin. Orthop. Trauma 2018, 9, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Solomin, L. (Ed.) The Basic Principles of External Skeletal Fixation Using the Ilizarov and Other Devices; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Garg, S.; Quick, H.D.; Kim, E.B.; Erickson, M.A. Use of Activity Trackers in Orthopaedics. J. Am. Acad. Orthop. Surg. 2019, 27, e859–e866. [Google Scholar] [CrossRef] [PubMed]
- Giordano, V.; Godoy-Santos, A.L.; Belangero, W.D.; Pires, R.E.S.; Labronici, P.J.; Koch, H.A. Finite element analysis of the equivalent stress distribution in Schanz screws during the use of a femoral fracture distractor. Revista Brasileira de Ortopedia 2017, 52, 396–401. [Google Scholar] [CrossRef]
- Sonohata, M.; Kitajima, M.; Kawano, S.; Tanaka, R.; Mawatari, M. Total hip arthroplasty with femoral subtrochanteric osteotomy after Schanz osteotomy. J. Orthop. Sci. 2016, 21, 469–474. [Google Scholar] [CrossRef]
- Frydrýšek, K.; Jořenek, J.; Učeň, O.; Kub’n, T.; Žilka, L.; Pleva, L. Design of external fixators used in traumatology and orthopaedics–treatment of fractures of pelvis and its acetabulum. Procedia Eng. 2012, 48, 164–173. [Google Scholar] [CrossRef][Green Version]
- Evans, M.; Spencer, M.; Wang, Q.; White, S.H.; Cunningham, J.L. Design and testing of external fixator bone screws. J. Biomed. Eng. 1990, 12, 457–462. [Google Scholar] [CrossRef]
- Tomanec, F.; Rusnáková, S.; Kalová, M.; Maňas, L. Innovation of ilizarov stabilization device with the design changes. MM Sci. J. 2019, 3, 2732–2738. [Google Scholar] [CrossRef]
- Qiao, F.; Li, D.; Jin, Z.; Gao, Y.; Zhou, T.; He, J.; Cheng, L. Application of 3D printed customized external fixator in fracture reduction. Injury 2015, 46, 1150–1155. [Google Scholar] [CrossRef]
- Heidari, B.S.; Oliaei, E.; Shayesteh, H.; Davachi, S.M.; Hejazi, I.; Seyfi, J.; Bahrami, M.; Rashedi, H. Simulation of mechanical behavior and optimization of simulated injection molding process for PLA based antibacterial composite and nanocomposite bone screws using central composite design. J. Mech. Behav. Biomed. Mater. 2017, 65, 160–176. [Google Scholar] [CrossRef]
- Heidari, B.S.; Davachi, S.M.; Moghaddam, A.H.; Seyfi, J.; Hejazi, I.; Sahraeian, R.; Rashedi, H. Optimization simulated injection molding process for ultrahigh molecular weight polyethylene nanocomposite hip liner using response surface methodology and simulation of mechanical behavior. J. Mech. Behav. Biomed. Mater. 2018, 81, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, G.H.; Manafi, A.R.; Najafi, F.; Najafi, M.A. Treatment of intertrochanteric fractures in elderly highrisk patients: Dynamic hip screw vs. external fixation. Injury 2014, 45, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, B.; Bohay, D.; Early, J.S.; Jennings, M.; Pomeroy, G.; Schuberth, J.M.; Wukich, D.K. Cannulated Screws. J. Foot Ankle Surg. 2019, 58, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Capuder, K.; Gill, C.; Hafez, J.; Kawalec, J.; Hetherington, V. Effect of repeated cycles of steam sterilization on the integrity of cannulated surgical screws. Foot 2019, 39, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Kemker, B.; Magone, K.; Owen, J.; Atkinson, P.; Martin, S.; Atkinson, T. A sliding hip screw augmented with 2 screws is biomechanically similar to an inverted triad of cannulated screws in repair of a Pauwels type-III fracture. Injury 2017, 48, 1743–1748. [Google Scholar] [CrossRef]
- Mei, J.; Liu, S.; Jia, G.; Cui, X.; Jiang, C.; Ou, Y. Finite element analysis of the effect of cannulated screw placement and drilling frequency on femoral neck fracture fixation. Injury 2014, 45, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Panteli, M.; Rodham, P.; Giannoudis, P.V. Biomechanical rationale for implant choices in femoral neck fracture fixation in the non-elderly. Injury 2015, 46, 445–452. [Google Scholar] [CrossRef]
- Tolunay, T.; Arslan, K.; Yaman, O.; Dalbayrak, S.; Demir, T. Biomechanical performance of various cement-augmented cannulated pedicle screw designs for osteoporotic bones. Spine Deform. 2015, 3, 205–210. [Google Scholar] [CrossRef]
- Chen, L.H.; Tai, C.L.; Lai, P.L.; Lee, D.M.; Tsai, T.T.; Fu, T.S.; Niu, C.C.; Chen, W.J. Pullout strength for cannulated pedicle screws with bone cement augmentation in severely osteoporotic bone: Influences of radial hole and pilot hole tapping. Clin. Biomech. 2009, 24, 613–618. [Google Scholar] [CrossRef]
- Shih, K.S.; Hsu, C.C.; Hou, S.M.; Yu, S.C.; Liaw, C.K. Comparison of the bending performance of solid and cannulated spinal pedicle screws using finite element analyses and biomechanical tests. Med. Eng. Phys. 2015, 37, 879–884. [Google Scholar] [CrossRef]
- Gruszka, D.; Nowak, T.E.; Tkacz, T.; Wagner, D.; Rommens, P.M. Complex radial head and neck fractures treated with modern locking plate fixation. J. Shoulder Elb. Surg. 2019, 28, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.; Phasuk, K.; Polido, W.D.; Lin, W.S. Consideration for Contemporary Implant Surgery. Dent. Clin. 2019, 63, 309–329. [Google Scholar] [CrossRef] [PubMed]
- Jabran, A.; Ren, L.; Peach, C.; Zou, Z. A Methodology for Biomechanical Assessment of Proximal Humerus Fractures Using an Integrated Experimental and Computational Framework. Proc. CIRP 2016, 49, 139–142. [Google Scholar] [CrossRef]
- LaMartina, J., II; Christmas, K.N.; Simon, P.; Streit, J.J.; Allert, J.W.; Clark, J.; Frankle, M.A. Difficulty in decision making in the treatment of displaced proximal humerus fractures: The effect of uncertainty on surgical outcomes. J. Shoulder Elb. Surg. 2018, 27, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Varga, P.; Inzana, J.A.; Gueorguiev, B.; Südkamp, N.P.; Windolf, M. Validated computational framework for efficient systematic evaluation of osteoporotic fracture fixation in the proximal humerus. Med. Eng. Phys. 2018, 57, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gong, C.; Chen, X.; Sun, Y.; Zhang, J.; Cai, L.; Zhu, S.; Xie, S.Q. Additive manufacturing of customized metallic orthopedic implants: Materials, structures, and surface modifications. Metals 2019, 9, 1004. [Google Scholar] [CrossRef]
- Li, J.-W.; Du, C.-F.; Yuchi, C.-X.; Zhang, C.-Q. Application of Biodegradable Materials in Orthopedics. J. Med Biol. Eng. 2019, 39, 633–645. [Google Scholar] [CrossRef]
- Shafaghi, R.; Rodriguez, O.; Schemitsch, E.H.; Zalzal, P.; Waldman, S.D.; Papini, M.; Towler, M.R. A review of materials for managing bone loss in revision total knee arthroplasty. Mater. Sci. Eng. C 2019, 104, 109941. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar]
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterial 2019, 219, 119366. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Fan, M.; Liu, B.; He, D.; Tian, W. Comparison of the clinical accuracy between point-to-point registration and auto-registration using an active infrared navigation system. Spine 2018, 43, E1329–E1333. [Google Scholar] [CrossRef] [PubMed]
- Wein, W.; Karamalis, A.; Baumgartner, A.; Navab, N. Automatic bone detection and soft tissue aware ultrasound–CT registration for computer-aided orthopedic surgery. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Hu, L.; Li, C.; Wang, T.; Zhao, L.; Li, Y.; Mao, Z.; Liu, D.; Zhang, L.; He, C.; et al. Advancing computer-assisted orthopaedic surgery using a hexapod device for closed diaphyseal fracture reduction. Int. J. Med. Robot. Comput. Assist. Surg. 2015, 11, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.V.; Deakin, A.H.; Nicol, A.C.; Picard, F. Measuring the positional accuracy of computer assisted surgical tracking systems. Comput. Aided Surg. 2010, 15, 13–18. [Google Scholar] [CrossRef]
- Gao, L.; Madry, H.; Chugaev, D.V.; Denti, M.; Frolov, A.; Burtsev, M.; Magnitskaya, N.; Mukhanov, V.; Neyret, P.; Solomin, L.N.; et al. Advances in modern osteotomies around the knee: Report on the Association of Sports Traumatology, Arthroscopy, Orthopaedic surgery, Rehabilitation (ASTAOR) Moscow International Osteotomy Congress 2017. J. Exp. Orthop. 2019, 6, 9. [Google Scholar] [CrossRef]
- Picard, F.; Deakin, A.H.; Riches, P.E.; Deep, K.; Baines, J. Computer assisted orthopaedic surgery: Past, present and future. Med. Eng. Phys. 2019, 72, 55–65. [Google Scholar] [CrossRef]
- Sugano, N. Computer Assisted Orthopaedic Surgery for Hip and Knee: Current State of the Art in Clinical Application and Basic Research; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–206. [Google Scholar]
- Karunaratne, S.; Duan, M.; Pappas, E.; Fritsch, B.; Boyle, R.; Gupta, S.; Stalley, P.; Horsley, M.; Steffens, D. The effectiveness of robotic hip and knee arthroplasty on patient-reported outcomes: A systematic review and meta-analysis. Int. Orthop. 2019, 43, 1283–1295. [Google Scholar] [CrossRef]
- Sugano, N. Computer-assisted orthopedic surgery. J. Orthop. Sci. 2003, 8, 442–448. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubicek, J.; Tomanec, F.; Cerny, M.; Vilimek, D.; Kalova, M.; Oczka, D. Recent Trends, Technical Concepts and Components of Computer-Assisted Orthopedic Surgery Systems: A Comprehensive Review. Sensors 2019, 19, 5199. https://doi.org/10.3390/s19235199
Kubicek J, Tomanec F, Cerny M, Vilimek D, Kalova M, Oczka D. Recent Trends, Technical Concepts and Components of Computer-Assisted Orthopedic Surgery Systems: A Comprehensive Review. Sensors. 2019; 19(23):5199. https://doi.org/10.3390/s19235199
Chicago/Turabian StyleKubicek, Jan, Filip Tomanec, Martin Cerny, Dominik Vilimek, Martina Kalova, and David Oczka. 2019. "Recent Trends, Technical Concepts and Components of Computer-Assisted Orthopedic Surgery Systems: A Comprehensive Review" Sensors 19, no. 23: 5199. https://doi.org/10.3390/s19235199
APA StyleKubicek, J., Tomanec, F., Cerny, M., Vilimek, D., Kalova, M., & Oczka, D. (2019). Recent Trends, Technical Concepts and Components of Computer-Assisted Orthopedic Surgery Systems: A Comprehensive Review. Sensors, 19(23), 5199. https://doi.org/10.3390/s19235199




