Recent Progress in Lab-On-a-Chip Systems for the Monitoring of Metabolites for Mammalian and Microbial Cell Research
Abstract
1. Introduction
2. Optical Methods
2.1. Optical Detection of Intracellular Metabolites
2.2. Optical Detection of Extracellular Metabolites
2.2.1. Mammalian Cells
2.2.2. Microbial Cells
3. Electrochemical Methods for Extracellular Metabolites
3.1. Mammalian Cells
3.2. Microbial Cells
4. Achievements
5. Challenges and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Eibl, D.; Eibl, R.; Pörtner, R. Mammalian Cell Culture Technology: An Emerging Field. In Cell and Tissue Reaction Engineering: With a Contribution by Martin Fussenegger and Wilfried Weber; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3–11. [Google Scholar]
- Ziółkowska, K.; Kwapiszewski, R.; Brzózka, Z. Microfluidic devices as tools for mimicking the in vivo environment. New J. Chem. 2011, 35, 979–990. [Google Scholar] [CrossRef]
- Francis, G.L. Albumin and mammalian cell culture: Implications for biotechnology applications. Cytotechnology 2010, 62, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kieninger, J.; Weltin, A.; Flamm, H.; Urban, G.A. Microsensor systems for cell metabolism—From 2D culture to organ-on-chip. Lab A Chip 2018, 18, 1274–1291. [Google Scholar] [CrossRef] [PubMed]
- Primiceri, E.; Chiriacò, M.S.; Rinaldi, R.; Maruccio, G. Cell chips as new tools for cell biology—Results, perspectives and opportunities. Lab A Chip 2013, 13, 3789–3802. [Google Scholar] [CrossRef] [PubMed]
- Altaf-Ul-Amin, M.; Kanaya, S.; Mohamed-Hussein, Z.-A. Investigating Metabolic Pathways and Networks. In Encyclopedia of Bioinformatics and Computational Biology; Ranganathan, S., Nakai, K., Schonbach, C., Eds.; Academic Press: Oxford, UK, 2019; pp. 489–503. [Google Scholar]
- Shi, J.; Tong, L.; Tong, W.; Chen, H.; Lan, M.; Sun, X.; Zhu, Y. Current progress in long-term and continuous cell metabolite detection using microfluidics. TrAC Trends Anal. Chem. 2019, 117, 263–279. [Google Scholar] [CrossRef]
- Husted, A.S.; Trauelsen, M.; Rudenko, O.; Hjorth, S.A.; Schwartz, T.W. GPCR-Mediated Signaling of Metabolites. Cell Metab. 2017, 25, 777–796. [Google Scholar] [CrossRef]
- Kraly, J.R.; Holcomb, R.E.; Guan, Q.; Henry, C.S. Review: Microfluidic applications in metabolomics and metabolic profiling. Anal. Chim. Acta 2009, 653, 23–35. [Google Scholar] [CrossRef]
- Dona, A.C.; Kyriakides, M.; Scott, F.; Shephard, E.A.; Varshavi, D.; Veselkov, K.; Everett, J.R. A guide to the identification of metabolites in NMR-based metabonomics/metabolomics experiments. Comput. Struct. Biotechnol. J. 2016, 14, 135–153. [Google Scholar] [CrossRef]
- Bhinderwala, F.; Wase, N.; DiRusso, C.; Powers, R. Combining Mass Spectrometry and NMR Improves Metabolite Detection and Annotation. J. Proteome Res. 2018, 17, 4017–4022. [Google Scholar] [CrossRef]
- Wilson, I.D. High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS)-Based Drug Metabolite Profiling. In Metabolic Profiling: Methods and Protocols; Metz, T.O., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 173–190. [Google Scholar]
- Tavares, A.J.; Doughan, S.; Noor, M.O.; DaCosta, M.V.; Piunno, P.A.E.; Krull, U.J. Chapter 8 Novel Lab-on-a-Chip Sensing Systems: Applications of Optical, Electrochemical, and Piezoelectric Transduction in Bioanalysis. In Microfluidics in Detection Science: Lab-on-a-chip Technologies; The Royal Society of Chemistry: London, UK, 2015; pp. 224–269. [Google Scholar]
- Solanki, S.; Pandey, C.M. Biological Applications of Microfluidics System. In Microfluidics for Biologists: Fundamentals and Applications; Dixit, C.K., Kaushik, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 191–221. [Google Scholar]
- Lei, K.F. Chapter 1 Materials and Fabrication Techniques for Nano- and Microfluidic Devices. In Microfluidics in Detection Science: Lab-on-a-Chip Technologies; The Royal Society of Chemistry: London, UK, 2015; pp. 1–28. [Google Scholar]
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Tsao, C.-W. Polymer Microfluidics: Simple, Low-Cost Fabrication Process Bridging Academic Lab Research to Commercialized Production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef]
- Nguyen, T.; Chidambara Vinayaka, A.; Duong Bang, D.; Wolff, A. A Complete Protocol for Rapid and Low-Cost Fabrication of Polymer Microfluidic Chips Containing Three-Dimensional Microstructures Used in Point-of-Care Devices. Micromachines 2019, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, B.; Yılmaz, F. Chapter 8–Lab-on-a-Chip Technology and Its Applications. In Omics Technologies and Bio-Engineering; Azevedo, D.B.V., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 145–153. [Google Scholar]
- Gabriel, E.F.M.; Lucca, B.G.; Duarte, G.R.M.; Coltro, W.K.T. Recent advances in toner-based microfluidic devices for bioanalytical applications. Anal. Methods 2018, 10, 2952–2962. [Google Scholar] [CrossRef]
- Yang, H.; Gijs, M.A.M. Micro-optics for microfluidic analytical applications. Chem. Soc. Rev. 2018, 47, 1391–1458. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.-I.; Tachikawa, K.; Manz, A. Microfluidics: Applications for analytical purposes in chemistry and biochemistry. Electrophoresis 2008, 29, 4443–4453. [Google Scholar] [CrossRef]
- Conde, J.P.; Madaboosi, N.; Soares, R.R.G.; Fernandes, J.T.S.; Novo, P.; Moulas, G.; Chu, V. Lab-on-chip systems for integrated bioanalyses. Essays Biochem. 2016, 60, 121–131. [Google Scholar]
- Capretto, L.; Carugo, D.; Mazzitelli, S.; Nastruzzi, C.; Zhang, X. Microfluidic and lab-on-a-chip preparation routes for organic nanoparticles and vesicular systems for nanomedicine applications. Adv. Drug Deliv. Rev. 2013, 65, 1496–1532. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X. Why microfluidics? Merits and trends in chemical synthesis. Lab A Chip 2017, 17, 3960–3978. [Google Scholar] [CrossRef]
- Pol, R.; Céspedes, F.; Gabriel, D.; Baeza, M. Microfluidic lab-on-a-chip platforms for environmental monitoring. TrAC Trends Anal. Chem. 2017, 95, 62–68. [Google Scholar] [CrossRef]
- Dhar, B.C.; Lee, N.Y. Lab-on-a-Chip Technology for Environmental Monitoring of Microorganisms. Biochip J. 2018, 12, 173–183. [Google Scholar] [CrossRef]
- Goel, S. Microfluidic Microbial Fuel Cell: On-chip Automated and Robust Method to Generate Energy. In Microbial Fuel Cell: A Bioelectrochemical System That Converts Waste to Watts; Das, D., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 229–247. [Google Scholar]
- Sugumar, D.; Kong, L. Lab-on-Chip Devices for Biodefense Applications. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: New York, NY, USA, 2015; pp. 1553–1557. [Google Scholar]
- Wu, M.Y.-C.; Hsu, M.-Y.; Chen, S.-J.; Hwang, D.-K.; Yen, T.-H.; Cheng, C.-M. Point-of-Care Detection Devices for Food Safety Monitoring: Proactive Disease Prevention. Trends Biotechnol. 2017, 35, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Atalay, Y.T.; Vermeir, S.; Witters, D.; Vergauwe, N.; Verbruggen, B.; Verboven, P.; Nicolaï, B.M.; Lammertyn, J. Microfluidic analytical systems for food analysis. Trends Food Sci. Technol. 2011, 22, 386–404. [Google Scholar] [CrossRef]
- Kant, K.; Shahbazi, M.-A.; Dave, V.P.; Ngo, T.A.; Chidambara, V.A.; Than, L.Q.; Bang, D.D.; Wolff, A. Microfluidic devices for sample preparation and rapid detection of foodborne pathogens. Biotechnol. Adv. 2018, 36, 1003–1024. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, Y.; Li, Y.; Miao, Y.; Gao, S.; Lin, F.; Deng, Y.; Geng, L. Microfluidic chip coupled with optical biosensors for simultaneous detection of multiple analytes: A review. Biosens. Bioelectron. 2019, 126, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lin, J.-M. Development of cell metabolite analysis on microfluidic platform. J. Pharm. Anal. 2015, 5, 337–347. [Google Scholar] [CrossRef]
- Regtien, P.; Dertien, E. 7—Optical Sensors, in Sensors for Mechatronics, 2nd ed.; Regtien, P., Dertien, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 183–243. [Google Scholar]
- Whelan, J.; Craven, S.; Glennon, B. In situ Raman spectroscopy for simultaneous monitoring of multiple process parameters in mammalian cell culture bioreactors. Biotechnol. Prog. 2012, 28, 1355–1362. [Google Scholar] [CrossRef]
- Rowland-Jones, R.C.; van den Berg, F.; Racher, A.J.; Martin, E.B.; Jaques, C. Comparison of spectroscopy technologies for improved monitoring of cell culture processes in miniature bioreactors. Biotechnol. Prog. 2017, 33, 337–346. [Google Scholar] [CrossRef]
- Foley, R.; Hennessy, S.; Marison, I.W. Potential of Mid-Infrared Spectroscopy for On-Line Monitoring of Mammalian Cell Culture Medium Components. Appl. Spectrosc. 2012, 66, 33–39. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; Wang, X.; Xu, K.; Chen, Z.; Gong, X.; Liu, X.; Tong, L.; Tang, B. Simultaneous Determination of Superoxide and Hydrogen Peroxide in Macrophage RAW 264.7 Cell Extracts by Microchip Electrophoresis with Laser-Induced Fluorescence Detection. Anal. Chem. 2009, 81, 2193–2198. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Chen, Z.; Li, H.; Xu, K.; Zhang, L.; Tang, B. Electrokinetic gated injection-based microfluidic system for quantitative analysis of hydrogen peroxide in individual HepG2 cells. Lab A Chip 2011, 11, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, P.; Fan, Y.; Wang, X.; Xu, K.; Li, L.; Tang, B. Multicolor Fluorescence Detection-Based Microfluidic Device for Single-Cell Metabolomics: Simultaneous Quantitation of Multiple Small Molecules in Primary Liver Cells. Anal. Chem. 2016, 88, 8610–8616. [Google Scholar] [CrossRef] [PubMed]
- Koman, V.B.; von Moos, N.R.; Santschi, C.; Slaveykova, V.I.; Martin, O.J.F. New insights into ROS dynamics: A multi-layered microfluidic chip for ecotoxicological studies on aquatic microorganisms. Nanotoxicology 2016, 10, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jie, M.; He, Z.; Li, H.-F.; Lin, J.-M. Study of antioxidant effects on malignant glioma cells by constructing a tumor-microvascular structure on microchip. Anal. Chim. Acta 2017, 978, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Q.; Chen, P.; Li, Z.; Chen, Z.; Tang, B. Consecutive Gated Injection-Based Microchip Electrophoresis for Simultaneous Quantitation of Superoxide Anion and Nitric Oxide in Single PC-12 Cells. Anal. Chem. 2016, 88, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.Y.; Yin, X.F.; Fang, Z.L. Simultaneous determination of glutathione and reactive oxygen species in individual cells by microchip electrophoresis. Electrophoresis 2005, 26, 4759–4766. [Google Scholar] [CrossRef]
- Chin, L.K.; Yu, J.Q.; Fu, Y.; Yu, T.; Liu, A.Q.; Luo, K.Q. Production of reactive oxygen species in endothelial cells under different pulsatile shear stresses and glucose concentrations. Lab A Chip 2011, 11, 1856–1863. [Google Scholar] [CrossRef]
- Zuchowska, A.; Marciniak, K.; Bazylinska, U.; Jastrzebska, E.; Wilk, K.A.; Brzozka, Z. Different action of nanoencapsulated meso-tetraphenylporphyrin in breast spheroid co-culture and mono-culture under microfluidic conditions. Sens. Actuators B Chem. 2018, 275, 69–77. [Google Scholar] [CrossRef]
- Jang, S.; Lee, B.; Jeong, H.-H.; Jin, S.H.; Jang, S.; Kim, S.G.; Jung, G.Y.; Lee, C.-S. On-chip analysis, indexing and screening for chemical producing bacteria in a microfluidic static droplet array. Lab A Chip 2016, 16, 1909–1916. [Google Scholar] [CrossRef]
- Abatemarco, J.; Sarhan, M.F.; Wagner, J.M.; Lin, J.-L.; Liu, L.; Hassouneh, W.; Yuan, S.-F.; Alper, H.S.; Abate, A.R. RNA-aptamers-in-droplets (RAPID) high-throughput screening for secretory phenotypes. Nat. Commun. 2017, 8, 332. [Google Scholar] [CrossRef]
- Remiszewska, E.; Malecha, K.; Kruk, J.; Jankowska-Śliwińska, J.; Torbicz, W.; Samluk, A.; Pluta, K.D.; Pijanowska, D.G. Enzymatic method of urea determination in LTCC microfluidic system based on absorption photometry. Sens. Actuators B Chem. 2019, 285, 375–384. [Google Scholar] [CrossRef]
- Liu, B.-F.; Ozaki, M.; Hisamoto, H.; Luo, Q.; Utsumi, Y.; Hattori, T.; Terabe, S. Microfluidic Chip toward Cellular ATP and ATP-Conjugated Metabolic Analysis with Bioluminescence Detection. Anal. Chem. 2005, 77, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Stybayeva, G.; Macal, M.; Ramanculov, E.; George, M.D.; Dandekar, S.; Revzin, A. A microdevice for multiplexed detection of T-cell-secreted cytokines. Lab A Chip 2008, 8, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.; Rahimian, A.; Stybayeva, G.; Gao, Y.; Kwa, T.; Water, J.V.D.; Revzin, A. Reconfigurable microfluidic device with integrated antibody arrays for capture, multiplexed stimulation, and cytokine profiling of human monocytes. Biomicrofluidics 2015, 9, 044115. [Google Scholar] [CrossRef]
- Son, K.J.; Gheibi, P.; Stybayeva, G.; Rahimian, A.; Revzin, A. Detecting cell-secreted growth factors in microfluidic devices using bead-based biosensors. Microsyst. Nanoeng. 2017, 3, 17025. [Google Scholar] [CrossRef]
- Lin, X.; Leung, K.-H.; Lin, L.; Lin, L.; Lin, S.; Leung, C.-H.; Ma, D.-L.; Lin, J.-M. Determination of cell metabolite VEGF165 and dynamic analysis of protein–DNA interactions by combination of microfluidic technique and luminescent switch-on probe. Biosens. Bioelectron. 2016, 79, 41–47. [Google Scholar] [CrossRef]
- Cedillo-Alcantar, D.F.; Han, Y.D.; Choi, J.; Garcia-Cordero, J.L.; Revzin, A. Automated Droplet-Based Microfluidic Platform for Multiplexed Analysis of Biochemical Markers in Small Volumes. Anal. Chem. 2019, 91, 5133–5141. [Google Scholar] [CrossRef]
- Beneyton, T.; Thomas, S.; Griffiths, A.D.; Nicaud, J.-M.; Drevelle, A.; Rossignol, T. Droplet-based microfluidic high-throughput screening of heterologous enzymes secreted by the yeast Yarrowia lipolytica. Microb. Cell Factories 2017, 16, 18. [Google Scholar] [CrossRef]
- Son, K.J.; Shin, D.-S.; Kwa, T.; You, J.; Gao, Y.; Revzin, A. A microsystem integrating photodegradable hydrogel microstructures and reconfigurable microfluidics for single-cell analysis and retrieval. Lab A Chip 2015, 15, 637–641. [Google Scholar] [CrossRef]
- Wasalathanthri, D.P.; Malla, S.; Bist, I.; Tang, C.K.; Faria, R.C.; Rusling, J.F. High-throughput metabolic genotoxicity screening with a fluidic microwell chip and electrochemiluminescence. Lab A Chip 2013, 13, 4554–4562. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. (Maywood) 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Disease 2016, 7, e2253. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M. Chapter 6—Capillary Electrophoresis: Principles and Instrumentation. In Advanced Topics in Forensic DNA Typing: Methodology; Butler, J.M., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 141–165. [Google Scholar]
- Amouzadeh Tabrizi, M.; Shamsipur, M.; Farzin, L. A high sensitive electrochemical aptasensor for the determination of VEGF(165) in serum of lung cancer patient. Biosens. Bioelectron. 2015, 74, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Keshet, R.; Szlosarek, P.; Carracedo, A.; Erez, A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat. Rev. Cancer 2018, 18, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent Compatibility of Poly(dimethylsiloxane)-Based Microfluidic Devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef]
- Chung, K.F. Chapter 27—Cytokines, in Asthma and COPD, 2nd ed.; Barnes, P.J., Ed.; Academic Press: Oxford, UK, 2009; pp. 327–341. [Google Scholar]
- Sanchez, S.; Rodríguez-Sanoja, R.; Ramos, A.; Demain, A.L. Our microbes not only produce antibiotics, they also overproduce amino acids. J. Antibiot. 2018, 71, 26–36. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Zhang, Y.; Liu, J. Advancing single-cell proteomics and metabolomics with microfluidic technologies. Analyst 2019, 144, 846–858. [Google Scholar] [CrossRef]
- Mongersun, A.; Smeenk, I.; Pratx, G.; Asuri, P.; Abbyad, P. Droplet Microfluidic Platform for the Determination of Single-Cell Lactate Release. Anal. Chem. 2016, 88, 3257–3263. [Google Scholar] [CrossRef]
- Chikahisa, S.; Séi, H. The Role of ATP in Sleep Regulation. Front. Neurol. 2011, 2, 87. [Google Scholar] [CrossRef]
- Idzko, M.; Ferrari, D.; Riegel, A.-K.; Eltzschig, H.K. Extracellular nucleotide and nucleoside signaling in vascular and blood disease. Blood 2014, 124, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, X.; Lin, Y. Dissecting the regulation and function of ATP at the single-cell level. PLoS Biol. 2018, 16, e3000095. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V.; Borm, P.J.; Jimenez, L.A.; Gilmour, P.S.; Schins, R.P.; Knaapen, A.M.; Rahman, I.; Faux, S.P.; Brown, D.M.; et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Radic. Biol. Med. 2003, 34, 1369–1382. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef] [PubMed]
- Fatoyinbo, H.O. Chapter 6 Introduction to Optofluidics for LOC Systems. In Microfluidics in Detection Science: Lab-on-a-Chip Technologies; The Royal Society of Chemistry: London, UK, 2015; pp. 153–191. [Google Scholar]
- Shetti, N.P.; Nayak, D.S.; Reddy, K.R.; Aminabhvi, T.M. Chapter 10—Graphene–Clay-Based Hybrid Nanostructures for Electrochemical Sensors and Biosensors. In Graphene-Based Electrochemical Sensors for Biomolecules; Pandikumar, A., Rameshkumar, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 235–274. [Google Scholar]
- Rackus, D.G.; Shamsi, M.H.; Wheeler, A.R. Electrochemistry, biosensors and microfluidics: A convergence of fields. Chem. Soc. Rev. 2015, 44, 5320–5340. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.H.; Danielsson, B.; Willander, M. ZnO Nanostructure-Based Intracellular Sensor. Sensors 2015, 15, 11787–11804. [Google Scholar] [CrossRef] [PubMed]
- Actis, P.; Tokar, S.; Clausmeyer, J.; Babakinejad, B.; Mikhaleva, S.; Cornut, R.; Takahashi, Y.; López Córdoba, A.; Novak, P.; Shevchuck, A.I.; et al. Electrochemical Nanoprobes for Single-Cell Analysis. ACS Nano 2014, 8, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Nashimoto, Y.; Taira, N.; Azcon, J.R.; Shiku, H. Intracellular Electrochemical Sensing. Electroanalysis 2018, 30, 2195–2209. [Google Scholar] [CrossRef]
- Cheah, L.-T.; Dou, Y.-H.; Seymour, A.-M.L.; Dyer, C.E.; Haswell, S.J.; Wadhawan, J.D.; Greenman, J. Microfluidic perfusion system for maintaining viable heart tissue with real-time electrochemical monitoring of reactive oxygen species. Lab A Chip 2010, 10, 2720–2726. [Google Scholar] [CrossRef]
- Matharu, Z.; Enomoto, J.; Revzin, A. Miniature Enzyme-Based Electrodes for Detection of Hydrogen Peroxide Release from Alcohol-Injured Hepatocytes. Anal. Chem. 2013, 85, 932–939. [Google Scholar] [CrossRef]
- Li, Y.; Sella, C.; Lemaître, F.; Guille-Collignon, M.; Amatore, C.; Thouin, L. Downstream Simultaneous Electrochemical Detection of Primary Reactive Oxygen and Nitrogen Species Released by Cell Populations in an Integrated Microfluidic Device. Anal. Chem. 2018, 90, 9386–9394. [Google Scholar] [CrossRef] [PubMed]
- Flamm, H.; Kieninger, J.; Weltin, A.; Urban, G.A. Superoxide microsensor integrated into a Sensing Cell Culture Flask microsystem using direct oxidation for cell culture application. Biosens. Bioelectron. 2015, 65, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Arbault, S.; Chen, Y.; Crozatier, C.; Tapsoba, I. Electrochemical detection in a microfluidic device of oxidative stress generated by macrophage cells. Lab A Chip 2007, 7, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Kwa, T.; Zhou, Q.; Gao, Y.; Rahimian, A.; Kwon, L.; Liu, Y.; Revzin, A. Reconfigurable microfluidics with integrated aptasensors for monitoring intercellular communication. Lab A Chip 2014, 14, 1695–1704. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Matharu, Z.; Rahimian, A.; Revzin, A. Detecting multiple cell-secreted cytokines from the same aptamer-functionalized electrode. Biosens. Bioelectron. 2015, 64, 43–50. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Mousavi Shaegh, S.A.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef]
- Matharu, Z.; Patel, D.; Gao, Y.; Haque, A.; Zhou, Q.; Revzin, A. Detecting Transforming Growth Factor-β Release from Liver Cells Using an Aptasensor Integrated with Microfluidics. Anal. Chem. 2014, 86, 8865–8872. [Google Scholar] [CrossRef]
- Zhou, Q.; Kwa, T.; Gao, Y.; Liu, Y.; Rahimian, A.; Revzin, A. On-chip regeneration of aptasensors for monitoring cell secretion. Lab A Chip 2014, 14, 276–279. [Google Scholar] [CrossRef]
- Riahi, R.; Shaegh, S.A.M.; Ghaderi, M.; Zhang, Y.S.; Shin, S.R.; Aleman, J.; Massa, S.; Kim, D.; Dokmeci, M.R.; Khademhosseini, A. Automated microfluidic platform of bead-based electrochemical immunosensor integrated with bioreactor for continual monitoring of cell secreted biomarkers. Sci. Rep. 2016, 6, 24598. [Google Scholar] [CrossRef]
- Cai, X.; Klauke, N.; Glidle, A.; Cobbold, P.; Smith, G.L.; Cooper, J.M. Ultra-Low-Volume, Real-Time Measurements of Lactate from the Single Heart Cell Using Microsystems Technology. Anal. Chem. 2002, 74, 908–914. [Google Scholar] [CrossRef]
- Cheng, W.; Klauke, N.; Sedgwick, H.; Smith, G.L.; Cooper, J.M. Metabolic monitoring of the electrically stimulated single heart cell within a microfluidic platform. Lab A Chip 2006, 6, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, L.; Chen, Z.; Lin, Y.; Yu, P.; Mao, L. Continuous Electrochemical Monitoring of Extracellular Lactate Production from Neonatal Rat Cardiomyocytes following Myocardial Hypoxia. Anal. Chem. 2012, 84, 5285–5291. [Google Scholar] [CrossRef] [PubMed]
- Weltin, A.; Slotwinski, K.; Kieninger, J.; Moser, I.; Jobst, G.; Wego, M.; Ehret, R.; Urban, G.A. Cell culture monitoring for drug screening and cancer research: A transparent, microfluidic, multi-sensor microsystem. Lab A Chip 2014, 14, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Prill, S.; Jaeger, M.S.; Duschl, C. Long-term microfluidic glucose and lactate monitoring in hepatic cell culture. Biomicrofluidics 2014, 8, 034102. [Google Scholar] [CrossRef]
- Talaei, S.; van der Wal, P.D.; Ahmed, S.; Liley, M.; de Rooij, N.F. Enzyme SU-8 microreactors: Simple tools for cell-culture monitoring. Microfluid. Nanofluidics 2015, 19, 351–361. [Google Scholar] [CrossRef]
- Misun, P.M.; Rothe, J.; Schmid, Y.R.F.; Hierlemann, A.; Frey, O. Multi-analyte biosensor interface for real-time monitoring of 3D microtissue spheroids in hanging-drop networks. Microsyst. Nanoeng. 2016, 2, 16022. [Google Scholar] [CrossRef]
- Mross, S.; Zimmermann, T.; Winkin, N.; Kraft, M.; Vogt, H. Integrated multi-sensor system for parallel in-situ monitoring of cell nutrients, metabolites, cell density and pH in biotechnological processes. Sens. Actuators B Chem. 2016, 236, 937–946. [Google Scholar] [CrossRef]
- Obeidat, Y.; Catandi, G.; Carnevale, E.; Chicco, A.J.; DeMann, A.; Field, S.; Chen, T. A multi-sensor system for measuring bovine embryo metabolism. Biosens. Bioelectron. 2019, 126, 615–623. [Google Scholar] [CrossRef]
- Ges, I.A.; Currie, K.P.M.; Baudenbacher, F. Electrochemical detection of catecholamine release using planar iridium oxide electrodes in nanoliter microfluidic cell culture volumes. Biosens. Bioelectron. 2012, 34, 30–36. [Google Scholar] [CrossRef]
- Ges, I.A.; Brindley, R.L.; Currie, K.P.M.; Baudenbacher, F.J. A microfluidic platform for chemical stimulation and real time analysis of catecholamine secretion from neuroendocrine cells. Lab A Chip 2013, 13, 4663–4673. [Google Scholar] [CrossRef]
- Bellin, D.L.; Sakhtah, H.; Zhang, Y.; Price-Whelan, A.; Dietrich, L.E.P.; Shepard, K.L. Electrochemical camera chip for simultaneous imaging of multiple metabolites in biofilms. Nat. Commun. 2016, 7, 10535. [Google Scholar] [CrossRef] [PubMed]
- Sanger, K.; Zór, K.; Bille Jendresen, C.; Heiskanen, A.; Amato, L.; Toftgaard Nielsen, A.; Boisen, A. Lab-on-a-disc platform for screening of genetically modified E. coli cells via cell-free electrochemical detection of p-Coumaric acid. Sens. Actuators B Chem. 2017, 253, 999–1005. [Google Scholar] [CrossRef]
- Ino, K.; Kitagawa, Y.; Watanabe, T.; Shiku, H.; Koide, M.; Itayama, T.; Yasukawa, T.; Matsue, T. Detection of hormone active chemicals using genetically engineered yeast cells and microfluidic devices with interdigitated array electrodes. Electrophoresis 2009, 30, 3406–3412. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.L.; Formisano, N.; Estrela, P.; Carrara, S.; Tkac, J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016, 60, 69–80. [Google Scholar]
- Wei, W.; Zhang, L.; Ni, Q.; Pu, Y.; Yin, L.; Liu, S. Fabricating a reversible and regenerable electrochemical biosensor for quantitative detection of antibody by using “triplex-stem” DNA molecular switch. Anal. Chim. Acta 2014, 845, 38–44. [Google Scholar] [CrossRef]
- Dinh, T.; Phan, H.-P.; Kashaninejad, N.; Nguyen, T.-K.; Dao, D.V.; Nguyen, N.-T. An on-Chip SiC MEMS Device with Integrated Heating, Sensing, and Microfluidic Cooling Systems. Adv. Mater. Interfaces 2018, 5, 1800764. [Google Scholar] [CrossRef]
- Chon, C.H.; Li, D. Temperature Control in Microfluidic Systems. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: Boston, MA, USA, 2008; pp. 1976–1980. [Google Scholar]
- Ges, I.A.; Baudenbacher, F. Enzyme-coated microelectrodes to monitor lactate production in a nanoliter microfluidic cell culture device. Biosens. Bioelectron. 2010, 26, 828–833. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, P.; Hao, J.; Wang, Y.; Ohsaka, T.; Mao, L. Continuous and Simultaneous Electrochemical Measurements of Glucose, Lactate, and Ascorbate in Rat Brain Following Brain Ischemia. Anal. Chem. 2014, 86, 3895–3901. [Google Scholar] [CrossRef]
- Frey, O.; Misun, P.M.; Fluri, D.A.; Hengstler, J.G.; Hierlemann, A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 2014, 5, 4250. [Google Scholar] [CrossRef]
- Misun, P.M.; Birchler, A.K.; Lang, M.; Hierlemann, A.; Frey, O. Fabrication and Operation of Microfluidic Hanging-Drop Networks. In Cell-Based Microarrays: Methods and Protocols; Ertl, P., Rothbauer, M., Eds.; Springer: New York, NY, USA, 2018; pp. 183–202. [Google Scholar]
- McInnes, I.B. Chapter 26—Cytokines. In Kelley and Firestein’s Textbook of Rheumatology, 10th ed.; Firestein, G.S., Gabriel, S.E., O’Dell, J.R., Budd, R.C., McInnes, I.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 396–407. [Google Scholar]
- Pricop, L.; Gokhale, J.; Redecha, P.; Ng, S.C.; Salmon, J.E. Reactive oxygen intermediates enhance Fc gamma receptor signaling and amplify phagocytic capacity. J. Immunol. 1999, 162, 7041–7048. [Google Scholar]
- Zhou, Q.; Patel, D.; Kwa, T.; Haque, A.; Matharu, Z.; Stybayeva, G.; Gao, Y.; Diehl, A.M.; Revzin, A. Liver injury-on-a-chip: Microfluidic co-cultures with integrated biosensors for monitoring liver cell signaling during injury. Lab A Chip 2015, 15, 4467–4478. [Google Scholar] [CrossRef] [PubMed]
- Son, K.J.; Rahimian, A.; Shin, D.-S.; Siltanen, C.; Patel, T.; Revzin, A. Microfluidic compartments with sensing microbeads for dynamic monitoring of cytokine and exosome release from single cells. Analyst 2016, 141, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Lauschke, V.M.; Hendriks, D.F.G.; Bell, C.C.; Andersson, T.B.; Ingelman-Sundberg, M. Novel 3D Culture Systems for Studies of Human Liver Function and Assessments of the Hepatotoxicity of Drugs and Drug Candidates. Chem. Res. Toxicol. 2016, 29, 1936–1955. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Deng, R.; Hao Tong, W.; Huan, L.; Chan Way, N.; IslamBadhan, A.; Iliescu, C.; Yu, H. A perfusion incubator liver chip for 3D cell culture with application on chronic hepatotoxicity testing. Sci. Rep. 2017, 7, 14528. [Google Scholar] [CrossRef]
- Delalat, B.; Cozzi, C.; Rasi Ghaemi, S.; Polito, G.; Kriel, F.H.; Michl, T.D.; Harding, F.J.; Priest, C.; Barillaro, G.; Voelcker, N.H. Microengineered Bioartificial Liver Chip for Drug Toxicity Screening. Adv. Funct. Mater. 2018, 28, 1801825. [Google Scholar] [CrossRef]
- Booth, M.A.; Harbison, S.; Travas-Sejdic, J. Effects of Redox Couple on the Response of Polypyrrole-Based Electrochemical DNA Sensors. Electroanalysis 2012, 24, 1311–1317. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.-R.; Harrison, D.G.; Bhatnagar, A. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement From the American Heart Association. Circ. Res. 2016, 119, e39–e75. [Google Scholar] [CrossRef]
- Li, Y.; Sella, C.; Lemaître, F.; Guille-Collignon, M.; Thouin, L.; Amatore, C. Electrochemical Detection of Nitric Oxide and Peroxynitrite Anion in Microchannels at Highly Sensitive Platinum-Black Coated Electrodes. Application to ROS and RNS Mixtures prior to Biological Investigations. Electrochim. Acta 2014, 144, 111–118. [Google Scholar] [CrossRef]
- Li, Y.; Sella, C.; Lemaître, F.; Guille Collignon, M.; Thouin, L.; Amatore, C. Highly Sensitive Platinum-Black Coated Platinum Electrodes for Electrochemical Detection of Hydrogen Peroxide and Nitrite in Microchannel. Electroanalysis 2013, 25, 895–902. [Google Scholar] [CrossRef]
- Li, Y.; Meunier, A.; Fulcrand, R.; Sella, C.; Amatore, C.; Thouin, L.; Lemaître, F.; Guille-Collignon, M. Multi-chambers Microsystem for Simultaneous and Direct Electrochemical Detection of Reactive Oxygen and Nitrogen Species Released by Cell Populations. Electroanalysis 2016, 28, 1865–1872. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Abdoli, A.; Rahmanian, M.; Bardania, H.; Bayandori, M.; Moosavi Basri, S.M.; Kalbasi, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Microfluidic Brain-on-a-Chip: Perspectives for Mimicking Neural System Disorders. Mol. Neurobiol. 2019, 56, 8489–8512. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, C.; Lischinsky, J.; Jing, M.; Zhou, J.; Wang, H.; Zhang, Y.; Dong, A.; Wu, Z.; Wu, H.; et al. A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. bioRxiv 2018, 449546. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K. Microscale microbial culture. Future Microbiol. 2015, 10, 143–146. [Google Scholar] [CrossRef]
- Liu, X.; Kokare, C. Chapter 11—Microbial Enzymes of Use in Industry. In Biotechnology of Microbial Enzymes; Brahmachari, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 267–298. [Google Scholar]
- Calero, P.; Jensen, S.I.; Nielsen, A.T. Broad-Host-Range ProUSER Vectors Enable Fast Characterization of Inducible Promoters and Optimization of p-Coumaric Acid Production in Pseudomonas putida KT2440. ACS Synth. Biol. 2016, 5, 741–753. [Google Scholar] [CrossRef]
- Michael, I.J.; Kim, T.-H.; Sunkara, V.; Cho, Y.-K. Challenges and Opportunities of Centrifugal Microfluidics for Extreme Point-of-Care Testing. Micromachines 2016, 7, 32. [Google Scholar] [CrossRef]
- Coluccio, M.L.; Perozziello, G.; Malara, N.; Parrotta, E.; Zhang, P.; Gentile, F.; Limongi, T.; Raj, P.M.; Cuda, G.; Candeloro, P.; et al. Microfluidic platforms for cell cultures and investigations. Microelectron. Eng. 2019, 208, 14–28. [Google Scholar] [CrossRef]
- Moshksayan, K.; Kashaninejad, N.; Warkiani, M.E.; Lock, J.G.; Moghadas, H.; Firoozabadi, B.; Saidi, M.S.; Nguyen, N.-T. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens. Actuators B Chem. 2018, 263, 151–176. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, P.; Bilal, M.; Wang, W.; Hu, H.; Zhang, X. Enhanced biosynthesis of phenazine-1-carboxamide by engineered Pseudomonas chlororaphis HT66. Microb. Cell Factories 2018, 17, 117. [Google Scholar] [CrossRef]
- Ray, A.; Rentas, C.; Caldwell, G.A.; Caldwell, K.A. Phenazine derivatives cause proteotoxicity and stress in C. elegans. Neurosci. Lett. 2015, 584, 23–27. [Google Scholar] [CrossRef]
- Othman, M.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Extractive Fermentation of Lactic Acid in Lactic Acid Bacteria Cultivation: A Review. Front. Microbiol. 2017, 8, 2285. [Google Scholar] [CrossRef] [PubMed]
- Kilic, T.; Navaee, F.; Stradolini, F.; Renaud, P.; Carrara, S. Organs-on-chip monitoring: Sensors and other strategies. Microphysiol. Syst. 2018, 2. [Google Scholar] [CrossRef]
- Tuyiringire, N.; Tusubira, D.; Munyampundu, J.-P.; Tolo, C.U.; Muvunyi, C.M.; Ogwang, P.E. Application of metabolomics to drug discovery and understanding the mechanisms of action of medicinal plants with anti-tuberculosis activity. Clin. Transl. Med. 2018, 7, 29. [Google Scholar] [CrossRef]
- Finch, G.; Yilmaz, A.; Utz, M. An optimised detector for in-situ high-resolution NMR in microfluidic devices. J. Magn. Reson. 2016, 262, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Smits, J.; Damron, J.T.; Kehayias, P.; McDowell, A.F.; Mosavian, N.; Fescenko, I.; Ristoff, N.; Laraoui, A.; Jarmola, A.; Acosta, V.M. Two-dimensional nuclear magnetic resonance spectroscopy with a microfluidic diamond quantum sensor. Sci. Adv. 2019, 5, eaaw7895. [Google Scholar] [CrossRef] [PubMed]
- Oosthoek-de Vries, A.J.; Bart, J.; Tiggelaar, R.M.; Janssen, J.W.G.; van Bentum, P.J.M.; Gardeniers, H.J.G.E.; Kentgens, A.P.M. Continuous Flow 1H and 13C NMR Spectroscopy in Microfluidic Stripline NMR Chips. Anal. Chem. 2017, 89, 2296–2303. [Google Scholar] [CrossRef]
- Mompeán, M.; Sánchez-Donoso, R.M.; de la Hoz, A.; Saggiomo, V.; Velders, A.H.; Gomez, M.V. Pushing nuclear magnetic resonance sensitivity limits with microfluidics and photo-chemically induced dynamic nuclear polarization. Nat. Commun. 2018, 9, 108. [Google Scholar] [CrossRef]
- Mensack, M.M.; Holcomb, R.E.; Henry, C.S. Potential of Microfluidics and Single Cell Analysis in Metabolomics (Micrometabolomics). Metab. Pract. 2013, 239–259. [Google Scholar] [CrossRef]
- Issa, N.T.; Wathieu, H.; Ojo, A.; Byers, S.W.; Dakshanamurthy, S. Drug Metabolism in Preclinical Drug Development: A Survey of the Discovery Process, Toxicology, and Computational Tools. Curr. Drug Metab. 2017, 18, 556–565. [Google Scholar] [CrossRef]
- Kim, S.-Y. Cancer Energy Metabolism: Shutting Power off Cancer Factory. Biomol. Ther. 2018, 26, 39–44. [Google Scholar] [CrossRef]
- Amoedo, N.D.; Obre, E.; Rossignol, R. Drug discovery strategies in the field of tumor energy metabolism: Limitations by metabolic flexibility and metabolic resistance to chemotherapy. Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1858, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Uetrecht, J.P. Mechanism of idiosyncratic drug reactions: Reactive metabolite formation, protein binding and the regulation of the immune system. Curr. Drug Metab. 2002, 3, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Larasati, Y.A.; Yoneda-Kato, N.; Nakamae, I.; Yokoyama, T.; Meiyanto, E.; Kato, J.-Y. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci. Rep. 2018, 8, 2039. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931. [Google Scholar] [CrossRef] [PubMed]
- Moussavi-Harami, S.F.; Mladinich, K.M.; Sackmann, E.K.; Shelef, M.A.; Starnes, T.W.; Guckenberger, D.J.; Huttenlocher, A.; Beebe, D.J. Microfluidic device for simultaneous analysis of neutrophil extracellular traps and production of reactive oxygen species. Integr. Biol. (Camb) 2016, 8, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Beale, D.J.; Paten, A.M.; Kouremenos, K.; Swarup, S.; Schirra, H.J.; Wishart, D. Systems Biology and Multi-Omics Integration: Viewpoints from the Metabolomics Research Community. Metabolites 2019, 9, 76. [Google Scholar] [CrossRef]
- Kitano, H. Systems Biology: A Brief Overview. Science 2002, 295, 1662–1664. [Google Scholar] [CrossRef]
- Feng, X.; Du, W.; Luo, Q.; Liu, B.-F. Microfluidic chip: Next-generation platform for systems biology. Anal. Chim. Acta 2009, 650, 83–97. [Google Scholar] [CrossRef]
- Spirov, A.V. Microfluidics Approaches in Modern Developmental Biology. Russ. J. Dev. Biol. 2018, 49, 146–158. [Google Scholar] [CrossRef]
- Urbanski, J.P.; Johnson, M.T.; Craig, D.D.; Potter, D.L.; Gardner, D.K.; Thorsen, T. Noninvasive Metabolic Profiling Using Microfluidics for Analysis of Single Preimplantation Embryos. Anal. Chem. 2008, 80, 6500–6507. [Google Scholar] [CrossRef]
- Heo, Y.S.; Cabrera, L.M.; Bormann, C.L.; Smith, G.D.; Takayama, S. Real time culture and analysis of embryo metabolism using a microfluidic device with deformation based actuation. Lab A Chip 2012, 12, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Rohde, C.B.; Yanik, M.F. Sub-cellular precision on-chip small-animal immobilization, multi-photon imaging and femtosecond-laser manipulation. Lab A Chip 2008, 8, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Levario, T.J.; Lim, B.; Shvartsman, S.Y.; Lu, H. Microfluidics for High-Throughput Quantitative Studies of Early Development. Annu. Rev. Biomed. Eng. 2016, 18, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Krajniak, J.; Lu, H. Long-term high-resolution imaging and culture of C. elegans in chip-gel hybrid microfluidic device for developmental studies. Lab A Chip 2010, 10, 1862–1868. [Google Scholar] [CrossRef]
- Letizia, M.C.; Cornaglia, M.; Trouillon, R.; Sorrentino, V.; Mouchiroud, L.; Bou Sleiman, M.S.; Auwerx, J.; Gijs, M.A.M. Microfluidics-enabled phenotyping of a whole population of C. elegans worms over their embryonic and post-embryonic development at single-organism resolution. Microsyst. Nanoeng. 2018, 4, 6. [Google Scholar] [CrossRef]
- Banse, S.A.; Blue, B.W.; Robinson, K.J.; Jarrett, C.M.; Phillips, P.C. The Stress-Chip: A microfluidic platform for stress analysis in Caenorhabditis elegans. PLoS ONE 2019, 14, e0216283. [Google Scholar] [CrossRef]
- Khalili, A.; Rezai, P. Microfluidic devices for embryonic and larval zebrafish studies. Brief. Funct. Genom. 2019, elz006. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.; Nolan, T.M.; Yin, Y.; Aluru, M.R.; Dong, L. Automated microfluidic plant chips-based plant phenotyping system. In Proceedings of the 2017 IEEE 12th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Los Angeles, CA, USA, 9–12 April 2017. [Google Scholar]
- Sharei, A.; Zoldan, J.; Adamo, A.; Sim, W.Y.; Cho, N.; Jackson, E.; Mao, S.; Schneider, S.; Han, M.J.; Lytton-Jean, A.; et al. A vector-free microfluidic platform for intracellular delivery. Proc. Natl. Acad. Sci. USA 2013, 110, 2082–2087. [Google Scholar] [CrossRef]
- Kim, Y.; Lee Chung, B.; Ma, M.; Mulder, W.J.; Fayad, Z.A.; Farokhzad, O.C.; Langer, R. Mass production and size control of lipid-polymer hybrid nanoparticles through controlled microvortices. Nano Lett. 2012, 12, 3587–3591. [Google Scholar] [CrossRef]
- Lim, J.M.; Swami, A.; Gilson, L.M.; Chopra, S.; Choi, S.; Wu, J.; Langer, R.; Karnik, R.; Farokhzad, O.C. Ultra-high throughput synthesis of nanoparticles with homogeneous size distribution using a coaxial turbulent jet mixer. ACS Nano 2014, 8, 6056–6065. [Google Scholar] [CrossRef]
- Zhang, Q.; Austin, R.H. Applications of Microfluidics in Stem Cell Biology. BioNanoScience 2012, 2, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, Z.; Jo, M.C.; Zhang, K.; Li, Y.; Zeng, Z.; Li, N.; Zu, Y.; Qin, L. CRISPR-Cas9 delivery to hard-to-transfect cells via membrane deformation. Sci. Adv. 2015, 1, e1500454. [Google Scholar] [CrossRef] [PubMed]
- Gorgannezhad, L.; Stratton, H.; Nguyen, N.-T. Microfluidic-Based Nucleic Acid Amplification Systems in Microbiology. Micromachines 2019, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Tufenkji, N.; Moraes, C. Microfluidics in microbiology: Putting a magnifying glass on microbes. Integr. Biol. 2016, 8, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, P.; Boss, D.; Rappaz, B.; Moratal, C.; Hernandez, M.-C.; Depeursinge, C.; Magistretti, P.J.; Marquet, P. Simultaneous Optical Recording in Multiple Cells by Digital Holographic Microscopy of Chloride Current Associated to Activation of the Ligand-Gated Chloride Channel GABAA Receptor. PLoS ONE 2012, 7, e51041. [Google Scholar] [CrossRef]
- Märk, J.; Dortay, H.; Wagener, A.; Zhang, E.; Buchmann, J.; Grötzinger, C.; Friedrich, T.; Laufer, J. Dual-wavelength 3D photoacoustic imaging of mammalian cells using a photoswitchable phytochrome reporter protein. Commun. Phys. 2018, 1, 3. [Google Scholar] [CrossRef]
- Maceiczyk, R.M.; Hess, D.; Chiu, F.W.Y.; Stavrakis, S.; deMello, A.J. Differential detection photothermal spectroscopy: Towards ultra-fast and sensitive label-free detection in picoliter & femtoliter droplets. Lab A Chip 2017, 17, 3654–3663. [Google Scholar]
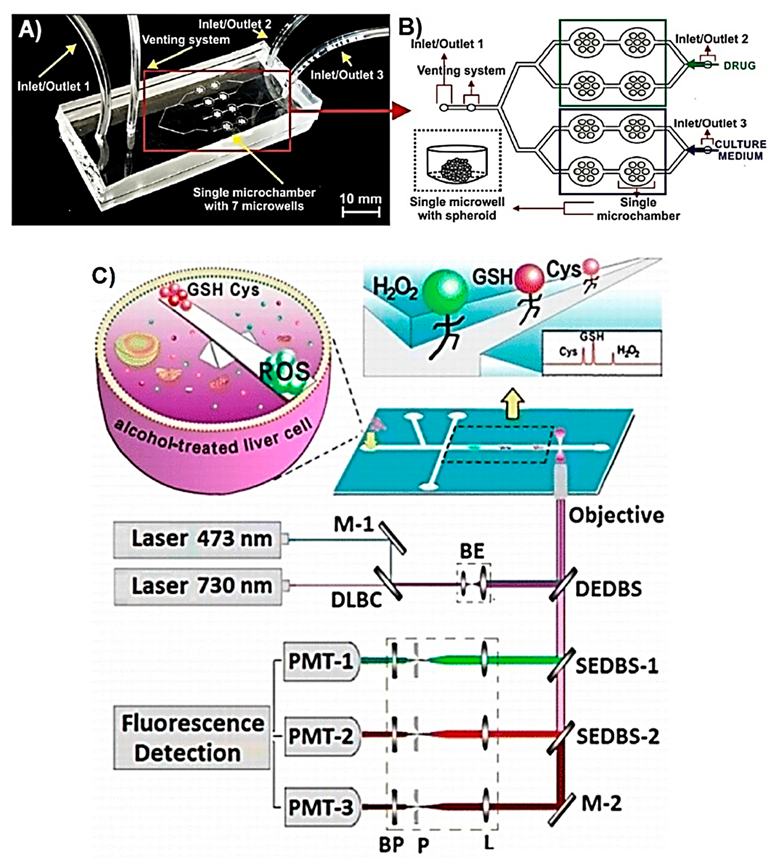
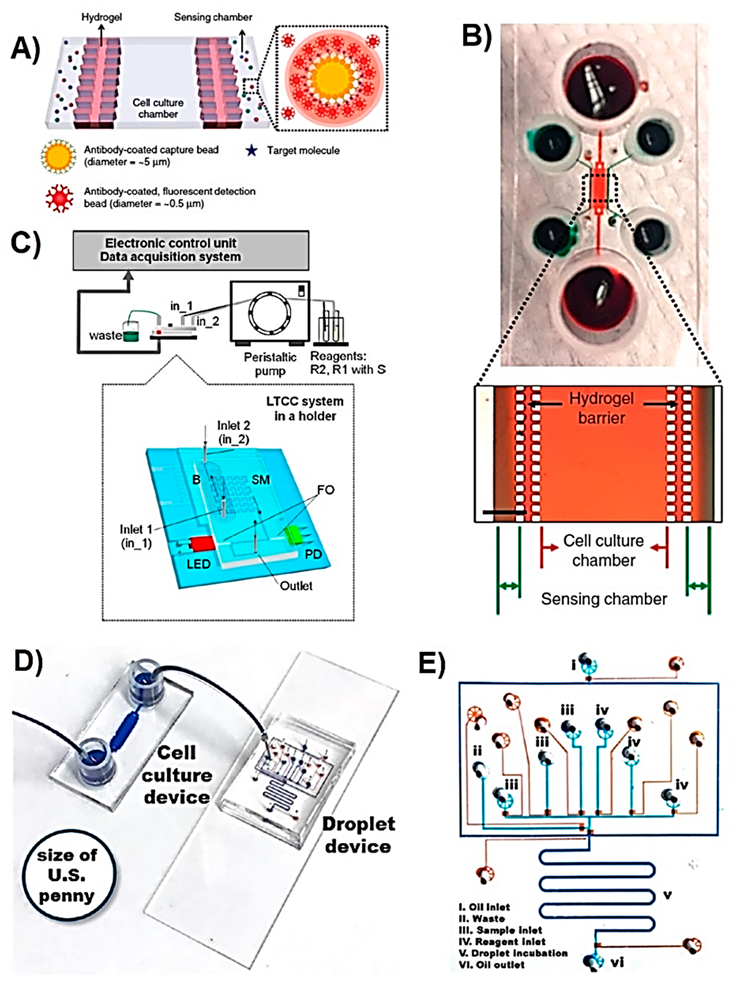
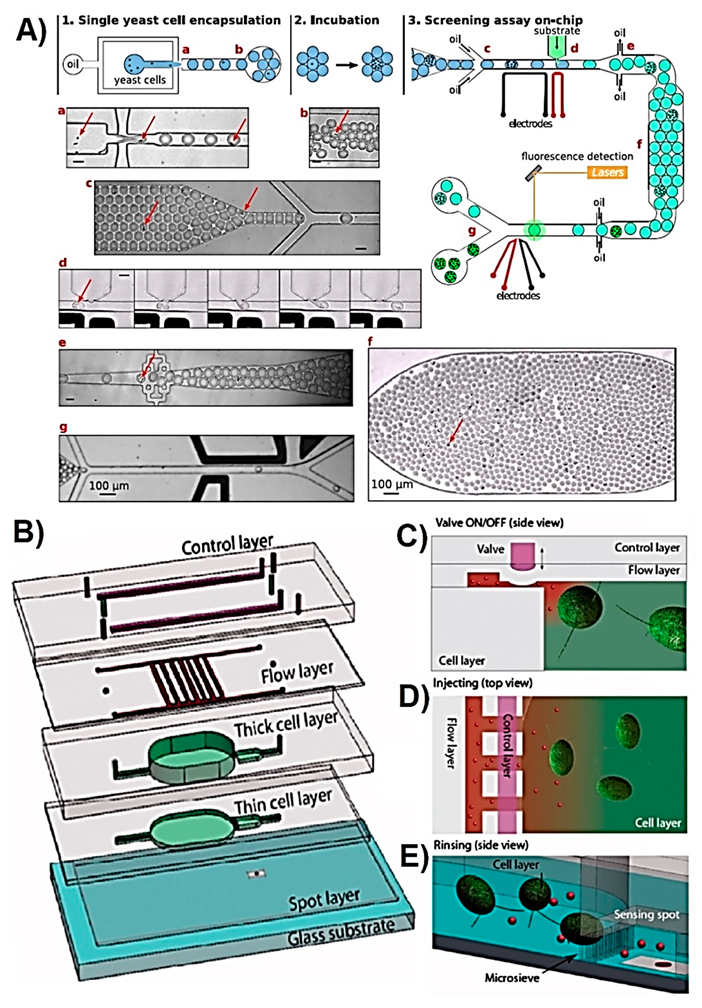
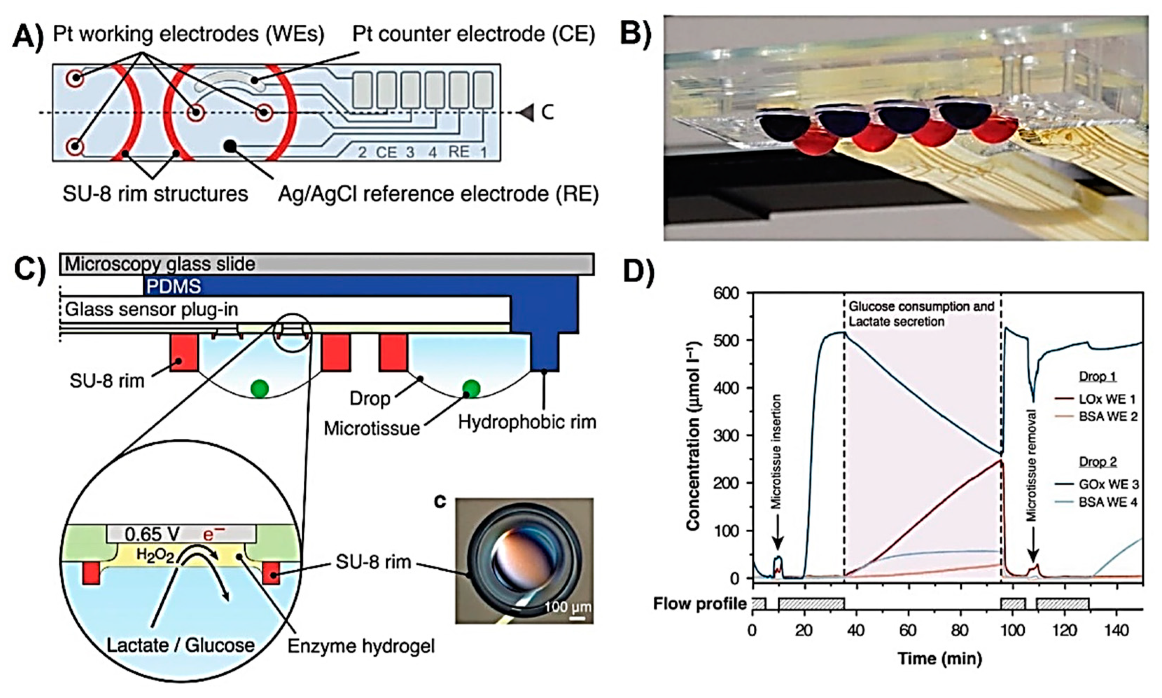
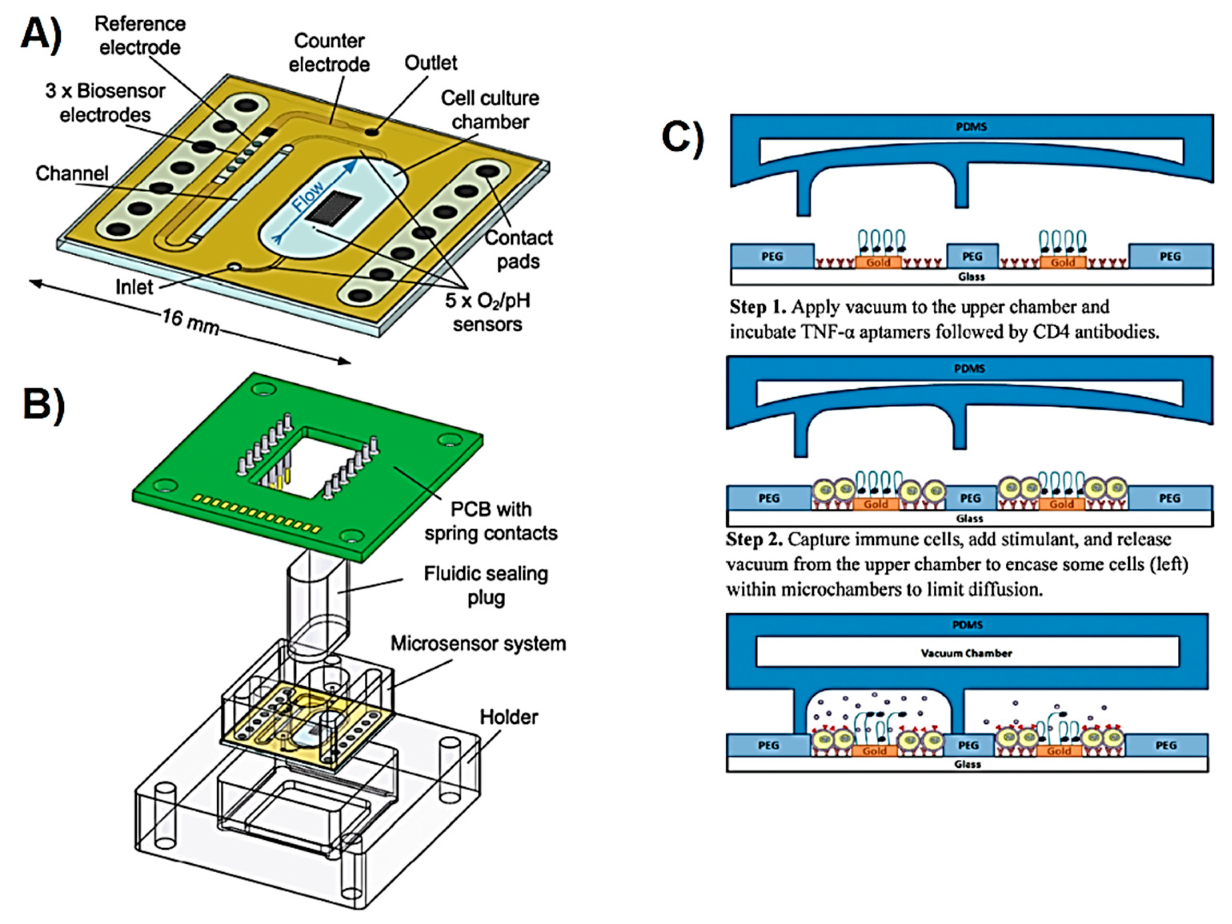
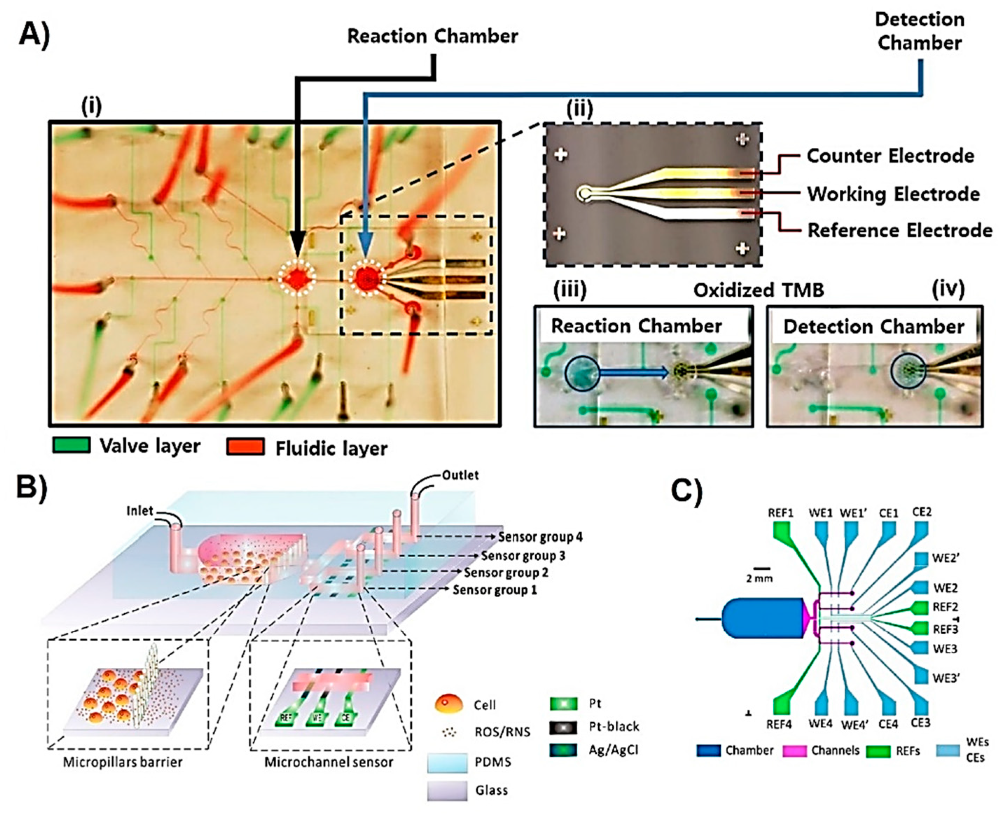
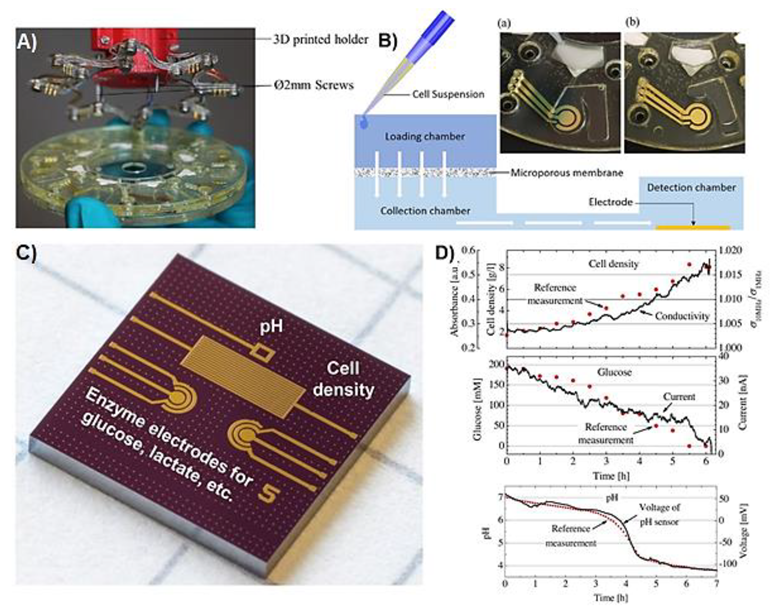
| Metabolite | Method | DL | Linear Range | Cell Type | Stimulant | Location | Purpose | FC | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| H2O2 | Fluor. | 5.6 nM | 0.02–5 µM | Macrophage | PMA | Intra. | Oxidative stress | Electrophoresis | [40] |
| H2O2 | Fluor. | 90 nM | 0.0072–1 µM | Hepatocyte | No | Intra. | Single cell analysis | Cell lysis Electrophoresis | [41] |
| H2O2 | Fluor. | 0.1 nM | 0.055–11.6 amol | Hepatocyte | Ethanol | Intra. | Single cell analysis | Electrophoresis | [42] |
| H2O2 | Colour. | 40 nM | NR | Microalgae | Cd2+, QD | Extra. | Ecotoxicology | Valve Microsieve Continuous sensing | [43] |
| H2O2 | Fluor. | NR | NR | Glyoblastoma HUVEC | α-lipoic acid catechin ascorbic acid | Intra. | Antioxidant screening | Cell co-culture Tumour microvascular structure | [44] |
| O2*− | Fluor. | 10 nM | 0.08–5 µM | Macrophage | PMA | Intra. | Oxidative stress | Electrophoresis | [40] |
| O2*− | Fluor. | 4.8 nM | 0.01–2 µM | Rat adrenal medulla | No | Intra. | Single cell analysis | Cell lysis Electrophoresis | [45] |
| NO | Fluor. | 5.3 nM | 0.0075–5 µM | Rat adrenal medulla | No | Intra. | Single cell analysis | Cell lysis Electrophoresis | [45] |
| ROS | Fluor. | 6.9 amol | NR | Erythrocyte | H2O2 | Intra. | Oxidative stress | Cell lysis Electrophoresis | [46] |
| ROS | Fluor. | NR | NR | Endothelial | Glucose Shear stress | Intra. | Oxidative stress | Cell culture | [47] |
| ROS | Fluor. | NR | NR | Fibroblast adenocarcinoma spheroid | Nano-TPP | Intra. | PDT analysis | Cell-co culture Spheroid formation | [48] |
| Cysteine | Fluor. | 0.02 µM | 60.5–7260 amol | Hepatocyte | Ethanol | Intra. | Single cell analysis | Electrophoresis | [42] |
| Glutathione | Fluor. | 0.01 µM | 38.5–17600 amol | Hepatocyte | Ethanol | Intra. | Single cell analysis | Electrophoresis | [42] |
| Glutathione | Fluor. | NR | NR | Glyoblastoma HUVEC | α-lipoic acid catechin ascorbic acid | Intra. | Antioxidant screening | Cell co-culture Tumour microvascular structure | [44] |
| Glutathione | Fluor. | 0.5 amol | NR | Erythrocyte | H2O2 | Intra. | Oxidative stress | Cell lysis Electrophoresis | [46] |
| L-tryptophan | Fluor. | NR | <1 g/L | E. coli | No | Intra. | Synthetic biology | Valve Droplet formation High throughput screening | [49] |
| Tyrosine | Fluor. | NR | NR | S. cerevisiae | No | Extra. | Protein engineering Synthetic biology | Droplet formation High throughput screening | [50] |
| Urea | Colour. | 2 µM | 0–1 mM | Hepatocyte | No | Intra. | Hepatocyte culture monitoring | Mixer Waveguide | [51] |
| ATP | Biol. | 0.2 µM | 0.2–50 µM | E. coli | No | Intra. | Single cell analysis | Electrophoresis | [52] |
| IL-2 | Fluor. | NR | 0–400 ng/mL | T lymphocyte | PMA, Ionomycin | Extra. | Immunology | Cell capture | [53] |
| IL-6 | Fluor. | 143 pg/mL | NR | Monocyte | LPS, LTA, CpG-B, Flagellin, poly I:C | Extra. | Immunology | Cell capture Cell culture Reconfigurable barriers | [54] |
| IL-10 | Fluor. | 177 pg/mL | NR | Monocyte | LPS, LTA, CpG-B, Flagellin, poly I:C | Extra. | Immunology | Cell capture Cell culture Reconfigurable barriers | [54] |
| TNF-α | Fluor. | 109 pg/mL | NR | Monocyte | LPS, LTA, CpG-B, Flagellin, poly I:C | Extra. | Immunology | Cell capture Cell culture Reconfigurable barriers | [54] |
| IFN-γ | Fluor. | NR | 0–100 ng/mL | T lymphocyte | PMA, Ionomycin | Extra. | Immunology | Cell capture | [53] |
| TGF-β1 | Fluor. | 21 pM | 0–300 pM | Hepatocyte | No | Extra. | GF secretion monitoring | Cell culture Hydrogel barrier | [55] |
| HGF | Fluor. | 6 pM | 0–40 pM | Hepatocyte | No | Extra. | GF secretion monitoring | Cell culture Hydrogel barrier | [55] |
| VEGF165 | Lum. | 0.17 pM | 0.52–52 pM | Epidermoid carcinoma | Paclitaxel | Extra. | Protein-DNA interaction | Hydrodynamic focusing | [56] |
| Bile acid | Colour. | 2.1 µM | 0–150 µM | Hepatocyte spheroid | Ethanol | Extra. | Toxicology | Droplet formation Spheroid culture | [57] |
| Streptavidin | Fluor. | NR | 1–40 mg/L | S. cerevisiae | No | Extra. | Protein engineering Synthetic biology | Droplet formation High throughput screening | [50] |
| Lactate dehydrogenase | Fluor. | 0.5 U/L | 0–80 U/L | Hepatocyte spheroid | Ethanol | Extra. | Toxicology | Droplet formation Spheroid culture | [57] |
| Recombinant enzymes | Fluor. | NR | NR | Yarrowia lipolytic | No | Extra. | Protein engineering Library screening | Droplet formation Yeast culture High throughput screening | [58] |
| Metalloproteinase 9 | Fluor. | 2.3 nM | 0–80 nM | Lymphoma | PMA | Extra. | Single cell analysis | Cell capture Hydrogel islands Reconfigurable barriers | [59] |
| B[a]P | Electro-chemilum. | NR | NR | N/A DNA oligonucleotide | No | N/A | Genotoxicity | High throughput screening | [60] |
| Metabolite | Method | DL | Linear Range | Cell Type | Stimulant | Purpose | Functionality | Ref |
|---|---|---|---|---|---|---|---|---|
| H2O2 | CV, SWV | NR | 1–800 µM | Rat heart tissue | No | Heart pathophysiology | Tissue culture Electrical stimulation | [83] |
| H2O2 | Amp. | 0.2 µM | 0–100 µM | Hepatocyte | Ethanol | Toxicology | Cell culture | [84] |
| H2O2 | Amp. | NR | NR | Macrophage | Calcium ionophore | Oxidative stress | Cell culture | [85] |
| O2*− | Amp. | 38 nM | 0.75–3.5 µM | Breast carcinoma | PMA | Oxidative stress | Integrated into culture flask | [86] |
| NO* | Amp. | NR | NR | Macrophage | Calcium ionophore | Oxidative stress | Cell culture | [85] |
| NO2− | Amp. | NR | NR | Macrophage | Calcium ionophore | Oxidative stress | Cell culture | [85] |
| ONOO− | Amp. | NR | NR | Macrophage | Calcium ionophore | Oxidative stress | Cell culture | [85] |
| Total ROS and RNS | Amp. | NR | NR | Macrophage | Calcium ionophore | Oxidative stress | Cell culture Continuous sensing | [87] |
| TNF-α | SWV | 5 ng/mL | 5–100 ng/mL | Monocyte | PMA, Ionomycin | Intercellular communication | Cell culture Reconfigurable barrier formation Analyte concentration | [88] |
| TNF-α | SWV | 5.46 ng/mL | 9–88 ng/mL | T lymphocyte Monocyte | PMA, Ionomycin | Cell secretion | Cell capture Multiplex sensing | [89] |
| GST-α | EIS | 0.01 ng/mL | 0.1–100 ng/mL | Hepatocyte Cardiomyocyte | Acetaminophen, Doxorubicin | Drug screening | Valve Automated Multi-functional sensing platform | [90] |
| TGF-β1 | SWV | 1 ng/mL | 0–250 ng/mL | Hepatic stellate | PDGF | Fibrosis | Cell culture Reconfigurable barrier formation | [91] |
| IFN-γ | SWV | 5 ng/mL | 5–100 ng/mL | T lymphocyte | PMA, Ionomycin | Cell secretion | Cell capture Reconfigurable microcup formation | [92] |
| IFN-γ | SWV | 6.35 ng/mL | 9–130 ng/mL | T lymphocyte Monocyte | PMA, Ionomycin | Cell secretion | Cell capture Multiplex sensing | [89] |
| Transferrin | Amp. | 0.03 ng/mL | 10–4000 ng/mL | Hepatocyte | Acetaminophen | Toxicology | Valve Automated Multi-functional sensing platform | [93] |
| Albumin | Amp. | 0.03 ng/mL | 15–4000 ng/mL | Hepatocyte | Acetaminophen | Toxicology | Valve Automated Multi-functional sensing platform | [93] |
| Albumin | EIS | 0.09 ng/mL | 0.1–100 ng/mL | Hepatocyte Cardiomyocyte | Acetaminophen, Doxorubicin | Drug screening | Valve Automated Multi-functional sensing platform | [90] |
| CK-MB | EIS | 0.0024 ng/mL | 0.01–10 ng/mL | Hepatocyte Cardiomyocyte | Acetaminophen, Doxorubicin | Drug screening | Valve Automated Multi-functional sensing platform | [90] |
| Lactate | Amp. | 65 fmol | 65–266 fmol | Cardiac myocyte | FCCP, Saponin | Single cell analysis | Cell culture Working volume of pL | [94] |
| Lactate | Amp. | 7.4 µM | 0–101.5 µM | Rabbit myocyte | Electric stimulation | Single cell analysis | Cell culture Working volume of pL | [95] |
| Lactate | Amp. | 0.16 mM | 0.2–10 mM | Rat cardiomyocyte | FCCP | Energy metabolism | Continuous monitoring | [96] |
| Lactate | Amp. | 90 µM | 0–3 mM | Brain cancer | Cytochalasin B | Drug screening | Cell culture Continuous real-time multiparameter monitoring | [97] |
| Lactate | Amp. | NR | 0.5–10 mM | Hepatocyte | Rotenone | Toxicology | Continuous monitoring | [98] |
| Lactate | Amp. | NR | 0.06–0.3 mM | Colorectal adenocarcinoma | Triton-X100, CuCl2, Acetaminophen | Toxicology | Enzyme µ-bioreactor | [99] |
| Lactate | Amp. | 7 µM | 0–1 mM | Colon carcinoma spheroid | No | Energy metabolism | Hanging drop Spheroid culture | [100] |
| Lactate | Amp. | NR | 0–900 mM | Saccharomyces cerevisiae, Lactobacillus acidophilus | No | Bioprocess monitoring | Multiplex real-time sensing | [101] |
| Lactate | Amp. | 0.4 µM | 0–6 mM | Bovine embryo | No | Energy metabolism | Embryo culture Multiplex real-time sensing | [102] |
| Norepinephrine | CV | NR | 0–400 µM | Chromaffin | Tyrode’s solution with 50 mM K+ | Exocytotic transmitter release profiling | Real-time sensing | [103] |
| Norepinephrine | CV | NR | 10–500 µM | Chromaffin | Carbachol, KCl, PACAP | Exocytotic transmitter release profiling | Cell trap Real-time sensing | [104] |
| Epinephrine | CV | NR | 10–500 µM | Chromaffin | Carbachol, KCl, PACAP | Exocytotic transmitter release profiling | Cell trap Real-time sensing | [104] |
| Dopamine | CV | NR | 10–500 µM | Chromaffin | Carbachol, KCl, PACAP | Exocytotic transmitter release profiling | Cell trap Real-time sensing | [104] |
| PCA, 5-MCA, PYO | SWV | NR | NR | P. aeruginosa | No | Metabolite profiling in biofilm | Cell culture High-throughput screening | [105] |
| p-coumaric acid | SWV | NR | 0.125–2 mM | E. coli | Tyrosine | Bioprocess monitoring | Filtration | [106] |
| β-gal | Amp. | NR | NR | S. cerevisiae | 17β-estradiol, Tamoxifen, Vitamin K3 | Hormone active chemical screening | Electrophoresis Single cell analysis | [107] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dervisevic, E.; Tuck, K.L.; Voelcker, N.H.; Cadarso, V.J. Recent Progress in Lab-On-a-Chip Systems for the Monitoring of Metabolites for Mammalian and Microbial Cell Research. Sensors 2019, 19, 5027. https://doi.org/10.3390/s19225027
Dervisevic E, Tuck KL, Voelcker NH, Cadarso VJ. Recent Progress in Lab-On-a-Chip Systems for the Monitoring of Metabolites for Mammalian and Microbial Cell Research. Sensors. 2019; 19(22):5027. https://doi.org/10.3390/s19225027
Chicago/Turabian StyleDervisevic, Esma, Kellie L. Tuck, Nicolas H. Voelcker, and Victor J. Cadarso. 2019. "Recent Progress in Lab-On-a-Chip Systems for the Monitoring of Metabolites for Mammalian and Microbial Cell Research" Sensors 19, no. 22: 5027. https://doi.org/10.3390/s19225027
APA StyleDervisevic, E., Tuck, K. L., Voelcker, N. H., & Cadarso, V. J. (2019). Recent Progress in Lab-On-a-Chip Systems for the Monitoring of Metabolites for Mammalian and Microbial Cell Research. Sensors, 19(22), 5027. https://doi.org/10.3390/s19225027







