A Colorimetric pH Sensor Based on Clitoria sp and Brassica sp for Monitoring of Food Spoilage Using Chromametry
Abstract
1. Introduction
2. Material and Methods
2.1. Materials and Reagents
2.2. Extraction of Anthocyanins from Clitoria sp and Brassica sp
2.3. Fabrication of Colorimetric pH Sensor Film
2.4. Film Characterization for Colorimetric pH Sensor
2.4.1. Spectroscopy Analysis
2.4.2. Color Measurements
2.4.3. Repeatability and Reproducibility Study
2.5. Food Application
2.5.1. Sample Preparation
2.5.2. Total Volatile Base Nitrogen (TVB-N) Analysis
2.5.3. Microbiological Test
2.6. Statistical Analysis
3. Results and Discussion
3.1. Determination of Anthocyanin Content in Samples of Clitoria sp and Brassica sp.
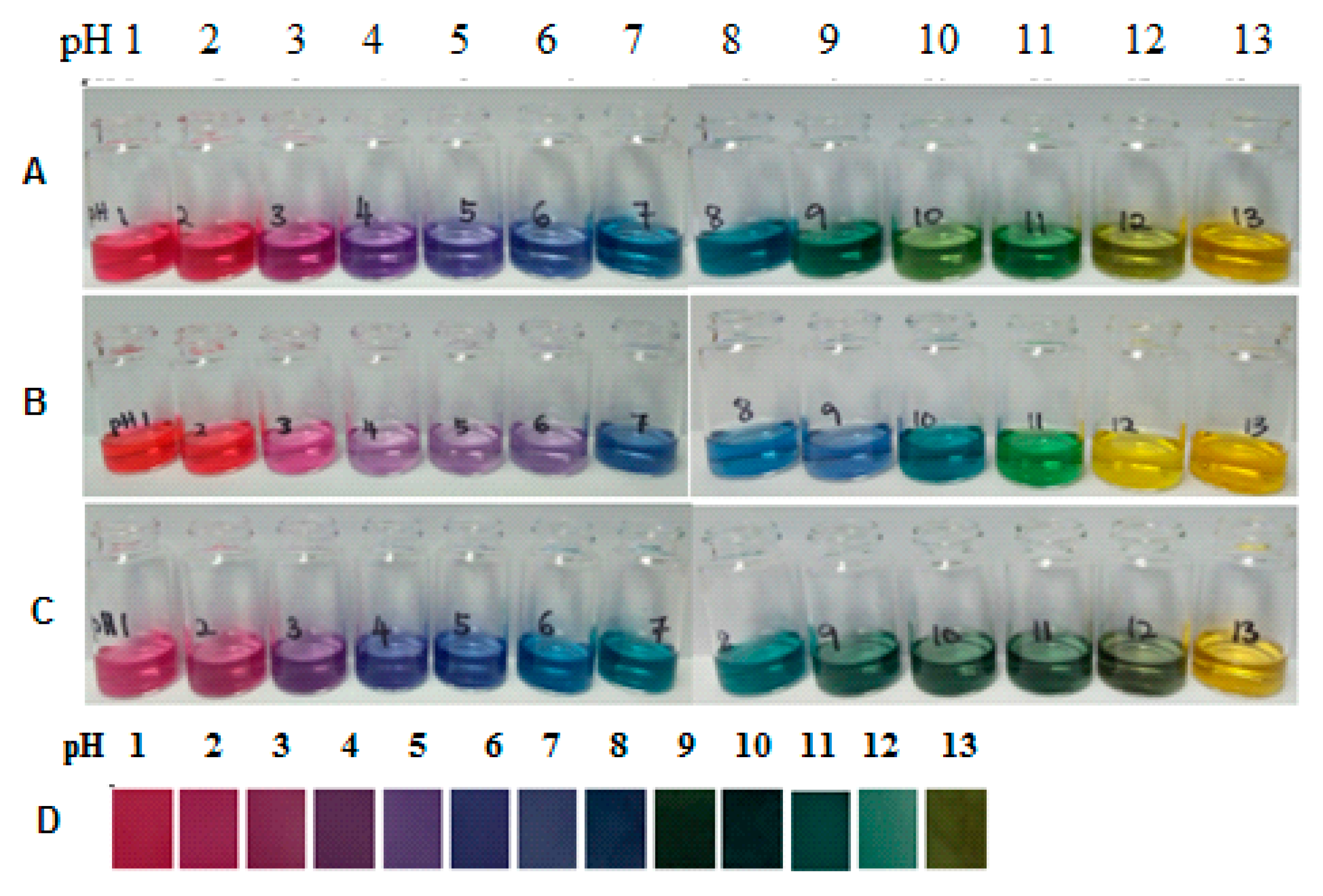
3.2. Colour of Mixed and Single Natural Dyes Containing Anthocyanin in Buffer Solution (pH 1–13)
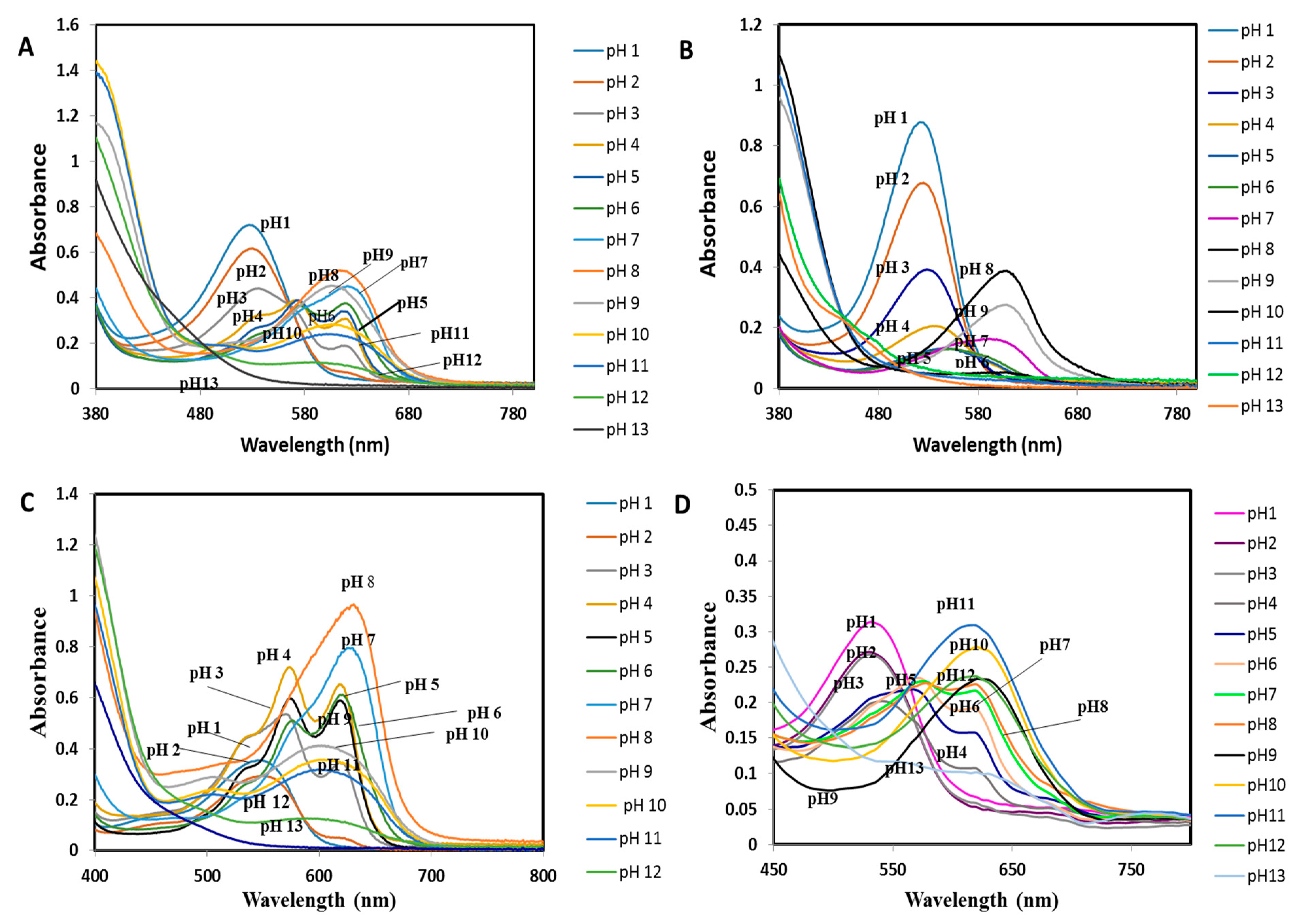
3.3. UV-vis Spectra of the Mixture and Single Extracts of Natural Dye in Various pH Ranges
3.4. Chroma Colour Parameter Values
3.5. Repeatability and Reproducibility Studies
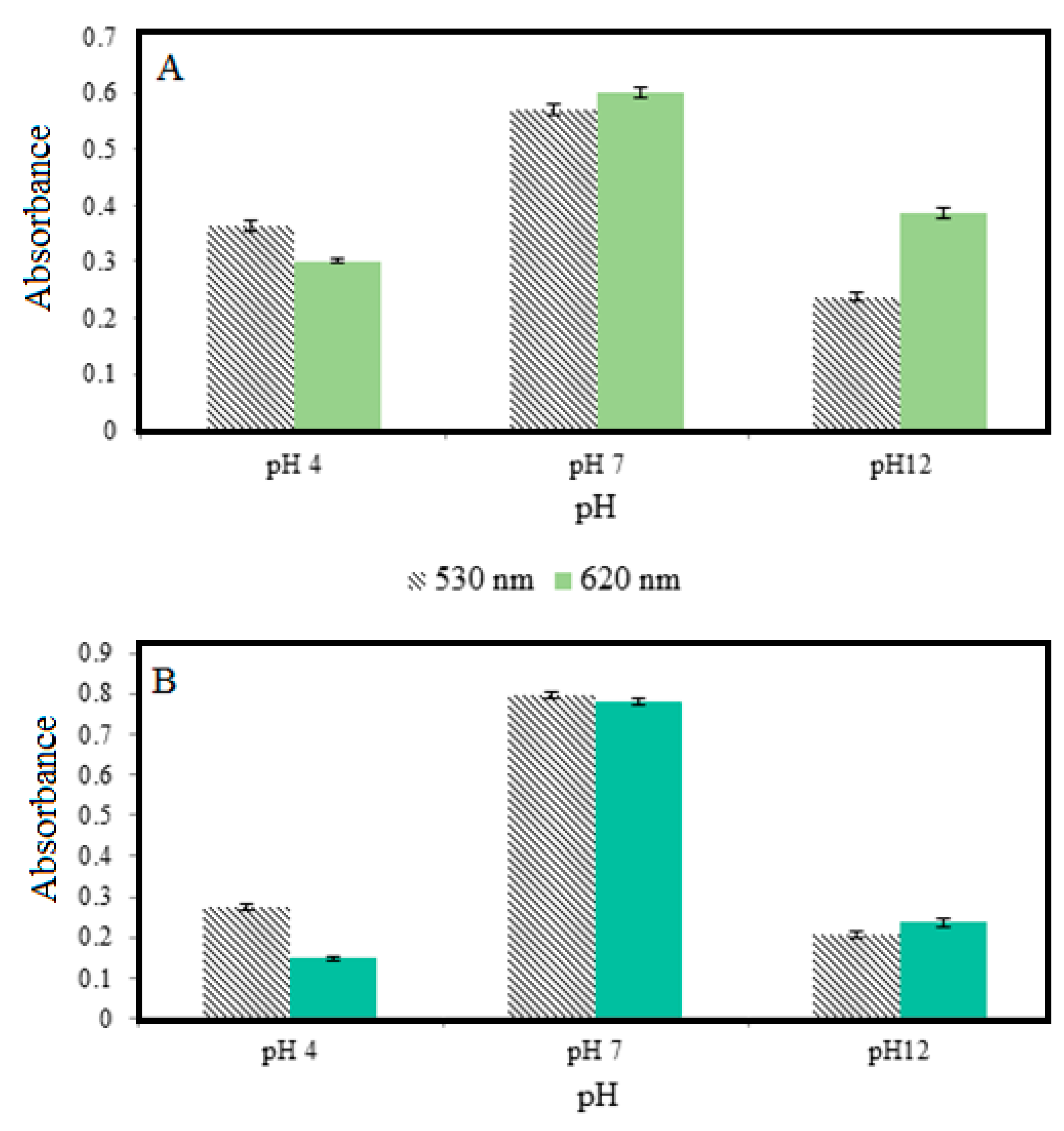
3.6. Sensor Response of Food Samples
3.6.1. Sensor Response of Food Samples during Storage
3.6.2. Correlation between pH Samples with Colorimetric Parameters for Shrimp and Durian Samples
3.6.3. Correlation Study of the Colorimetric pH Sensor Film on the Freshness of Package Shrimps and Durians Stored at Ambient Temperature (28 ± 1 °C)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Nopwinyuwong, A.; Trevanich, S.; Suppakul, P. Development of a novel colorimetric indicator label for monitoring freshness of intermediate-moisture dessert spoilage. Talanta 2010, 81, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Fung, F.; Wang, H.S.; Menone, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J.; Hajslova, J. Application of gas chromatography in food analysis. Trends Anal. Chem. 2002, 21, 686–697. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Cai, Z. The latest developments and applications of mass spectrometry in food-safety and quality analysis. Trends Anal. Chem. 2013, 52, 170–185. [Google Scholar] [CrossRef]
- Qu, J.H.; Liu, D.; Cheng, J.H.; Sun, D.W.; Ma, J.; Pu, H.; Zeng, X.A. Applications of near-infrared spectroscopy in food safety evaluation and control: A review of recent research advances. Crit. Rev. Food Sci. Nutr. 2015, 55, 1939–1954. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Sun, D.W. Application of hyperspectral imaging in food safety inspection and control: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 1039–1058. [Google Scholar] [CrossRef]
- Dainty, R. Chemical/biochemical detection of spoilage. Int. J. Food Microbiol. 1996, 33, 19–33. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.W. Colour measurements by computer vision for food quality control—A Review. Trends Food Sci. Technol. 2012, 29, 5–20. [Google Scholar] [CrossRef]
- Kerry, M.N.; O’Grady, S.A. Hogan, Past, current and potential utilization of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef]
- Papadakis, S.E.; Abdul-Malek, S.; Kamdem, R.E.; Yam, K.L. A versatile and inexpensive technique for measuring color of foods. Food Tech. 2000, 54, 48–51. [Google Scholar]
- Ibrahim, U.K.; Muhammad, I.I.; Salleh, R.M. The Effect of pH on Color Behavior of Brassica oleracea Anthocyanin. J. Appl. Sci. 2011, 11, 2406–2410. [Google Scholar]
- Smolander, M. The use of freshness indicators in packaging. In Novel Food Packaging Techniques; Ahvenainen, R., Ed.; Wood head Publishing Ltd.: Cambridge, UK, 2003; pp. 128–143. [Google Scholar]
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Saranraj, P.; Geetha, M. Microbial Spoilage of Bakery Products and Its Control by Preservatives. J. Pharm. Biol. Arch. 2012, 3, 38–48. [Google Scholar]
- Halagarda, M.; Kędzior, W.; Ewa, P. Nutritional Value and Potential Chemical Food Safety Hazards of Selected Traditional and Conventional Pork Hams from Poland. J. Food Qual. 2017, 2017, 9037016. [Google Scholar] [CrossRef]
- El-Ashgar, N.M.; El-Basioni, A.I.; El-Nahhal, I.M.; Zourob, S.M.; El-Agez, T.M.; Taya, S.A. Sol-Gel Thin Films Immobilized with Bromocresol Purple pH-Sensitive Indicator in Presence of Surfactants. Anal. Chem. 2012, 60438911. [Google Scholar] [CrossRef]
- Kuswandi, B.; Oktaviana, J.R.; Abdullah, A.; Heng, L.Y. A novel on-package sticker sensor based on methyl red for real-time monitoring of broiler chicken cut freshness. Packag. Technol. Sci. 2014, 27, 69–81. [Google Scholar] [CrossRef]
- Shukla, V.; Kandeepan, G.; Vishnuraj, M.R. Development of on package indicator sensor for real-time monitoring of meat quality. Vet. World 2015, 8, 393–397. [Google Scholar] [CrossRef]
- Zaggout, F.R. Entrapment of phenol red pH indicator into a sol-gel matrix. Mater. Lett. 2006, 60, 1026–1030. [Google Scholar] [CrossRef]
- Turturica, M.; Oancea, A.M.; Rapeanu, G. Anthocyanins: Naturally occurring fruit pigments with functional properties. Ann. Univ. Dunarea Galati Fascicle VI Food Technol. 2015, 39, 9–24. [Google Scholar]
- Ferreira, A.R.V.; Alves, V.D.; Coelhoso, I.M. Polysaccharide-Based Membranes in Food Packaging Applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Shamina, A.; Shiva, K.N.; Parthasarathy, V.A. Food colours of plant origin; review, CAB Reviews: Perspectives in Agriculture, Veterinary Science. Nutr. Nat. Resour. 2007, 2, 87. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from ediblesources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Rizk, E.M.; Azouz, A.; Lobna, A.M.H. Evaluation of red cabbage anthocyanin pigments and its potential uses as antioxidant and natural food colorants. Arab Univ. J. Agric. Sci. 2009, 17, 361–372. [Google Scholar]
- Kazuma, K.; Noda, N.; Suzuki, M. Flavonoid composition related to petal color in different lines of Clitoria ternatea. Phytochemistry 2003, 64, 1133–1139. [Google Scholar] [CrossRef]
- Santos, P.V.; Ditchfield, C.; Tadini, C.C. Development and evaluation of a novel pH indicator biodegradable film based on cassava starch. J. Appl. Polym. Sci. 2011, 120, 1069–1079. [Google Scholar] [CrossRef]
- Majdinasab, M.; Hosseini, S.M.H.; Sepidname, M.; Negahdarifar, M.; Li, P. Development of a novel colorimetric sensor based on alginate beads for monitoring rainbow trout spoilage. J. Food Sci. Technol. 2018, 55, 1695–1704. [Google Scholar] [CrossRef]
- Hsieh, S.H.; Shen, M.Y.; Huang, Y.S.; He, Q.Q.; Chen, H.C. Mechanical Properties of Glass Bead-Modified Polymer composite. Polym. Polym. Compos. 2018, 26, 35–44. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. Recent Advances in Edible Polymer Based Hydrogels as a sustainable Alternative to Conventional Polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, S.; Chen, X. A visual pH sensing film using natural dyes from Bauhinia blakeana Dunn. Sens. Actuators B Chem. 2014, 198, 268–273. [Google Scholar] [CrossRef]
- El-Nahhal, I.M. Entrapment of phenol red (PR) pH indicator into sol–gel matrix in presence of some surfactants. J. Sol-Gel Sci. Technol. 2015, 75, 313–322. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Saxena, A. Bacterial carrageenases: An overview of production and biotechnological applications. 3 Biotech 2016, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Kungsuwan, K.; Sing, K.; Phetkao, S.; Utama-ang, N. Effect of pH and anthocyanin concentration on colour and antioxidant activity of Clitoria ternatea extract. Food Appl. Biol. J. 2014, 2, 31–46. [Google Scholar]
- Devarayan, K.; Kim, B.S. Reversible and universal pH sensing cellulose nanofibers for health monitor. Sens. Actuators B Chem. 2015, 209, 281–286. [Google Scholar] [CrossRef]
- Schanda, J. Colorimetry: Understanding the CIE System; John Wiley and Sons: Hoboken, NJ, USA, 2007; p. 467. [Google Scholar]
- Anshika, A.; Anant, R.; Natarajan, T.S.; Chandra, T.S. Development of universal pH sensing electrospun nanofibers. Sens. Actuators B Chem. 2012, 161, 1097–1101. [Google Scholar]
- Sutee, W.; Chaluntorn, V.; Tanachai, P.; Parichart, T.; Yuthana, P. Kinetics and nondestructive measurement of total volatile basic nitrogen and thiobarbituric acid-relative substances in chilled Tabtim fish fillets using Near Infrared Spectroscopy (NIRS). J. Int. Food Eng. 2016, 2, 16–20. [Google Scholar]
- International Commission on Microbiological Specification for Foods (ICMSF). Microorganisms in Food. 4. Application of Hazard Analysis Critical Control Points (HACCP) System to Ensure Microbiological Safety and Quality; Block-well Scientific Publication: Oxford/London, UK, 1978. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; Wiley: New York, NY, USA, 1984. [Google Scholar]
- Baublis, A.; Spomer, A.; Berber-Jimenez, M.D. Anthocyanin pigments: Comparison of extract stability. J. Food Sci. 1994, 59, 1219–1221. [Google Scholar] [CrossRef]
- Kazuma, K.; Kogawa, K.; Noda, N.; Kato, N.; Suzuki, M. Identification of delphinidin 3-O-(6″-O-malonyl)-beta-glucoside-3′-O-beta-glucoside, a postulated intermediate in the biosynthesis of ternatin C5 in the blue petals of Clitoria ternatea (butterfly pea). Chem. Biodivers. 2004, 1, 1762–1770. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage antocyanins: Profile, isolation, identification and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Dyrby, M.; Westergaard, N.; Stapelfeldt, H. Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chem. 2001, 72, 431–437. [Google Scholar] [CrossRef]
- Asen, S.; Stewart, R.N.; Norris, K.H. Co-pigmentation of anthocyanins in plant tissues and its effect on color. Phytochemistry 1972, 11, 1139–1144. [Google Scholar] [CrossRef]
- Bkowska, A.; Kucharska, A.Z.; Oszmianski, J. The effects of heating, UV irradiation, and storage on stability of the anthocyanin–polyphenol copigment complex. Food Chem. 2003, 81, 349–355. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L. Preparation of a visual pH-sensing film based on tara gum incorporating cellulose and extracts from grape skins. Sens. Actuators B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Montilla, C.; Arzaba, M.R.; Hillebrand, S.; Winterhalter, P. Anthocyanin composition of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) cultivars Antonina, Beta Sweet, Deep Purple and Purple Haze. J. Agric. Food Chem. 2001, 59, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Rakic, V.P.; Skrt, M.A.; Miljkovic, M.N.; Kostic, D.A.; Sokolovic, D.T.; Ulri, N.E.P. Effects of pH on the stability of Cyanidin and Cyanidin 3-O-β-glucopyranoside in aqueous solution. Chem. Ind. 2015, 69, 511–522. [Google Scholar] [CrossRef]
- Oliveira, J.; Freitas, V.D.; Silva, A.M.S.; Mateus, N. Reaction between Hydroxycinnamic Acids and Anthocyanin−Pyruvic Acid Adducts Yielding New Portisins. J. Agric. Food Chem. 2007, 55, 6349–6356. [Google Scholar] [CrossRef]
- Voon, Y.Y.; Sheikh Abdul Hamid, N.; Rusul, G.; Osman, A.; Quek, S.Y. Physicochemical, microbial and sensory changes of minimally processed durian (Durio zibethinus cv. D24) during storage at 4 and 28 °C. Postharvest Biol. Technol. 2006, 42, 168–175. [Google Scholar] [CrossRef]


| Compound Detected | Retention Time (Min) | [M]+ (m/z) | Formula | |
|---|---|---|---|---|
| (A) | Clitoriasp | |||
| 1 | Ternatin B1 | 29.45 | 1945.5063 | C90 H97 O48 |
| 2 | Ternatin B2 | 29.37 | 1637.4229 | C75 H81 O41 |
| 3 | Ternatin C1 | 19.43 | 1329.3364 | C60 H65 O34 |
| 4 | Ternatin D1 | 35.67 | 1783.4616 | C84 H87 O43 |
| 5 | Ternatin D2 | 32.41 | 1475.3745 | C69 H71 O36 |
| 6 | Delphinidin-3-glucoside | 21.21 | 627.1696 | C27 H31 O17 |
| 7 | Delfinidin | 26.08 | 303.0492 | C15 H11 O7 |
| 8 | Peonidin-3-O-glucoside | 31.01 | 625.1806 | C28 H33 O16 |
| 9 | Peonidin 3-sambubioside | 20.19 | 595.1663 | C27 H31 O15 |
| 10 | 3-O-beta-d-glucoside 5-O-(6-coumaroyl-beta-d-glucoside | 34.92 | 757.1982 | C36 H37 O18 |
| 11 | Cyanidin 3-(6″-caffeyl-2‴-sinapylsambubioside)-5-(6-malonylglucosid) | 24.23 | 1197.2949 | C55 H57 O30 |
| 12 | Cyanidin 3-O- [ 2-O- (6-O-E-coumaroyl-beta-d-glucopyanosyl) ] -{6-O- [ 4-O- (6-O-E-coumaroyl-beta-d-glucopyranosyl) -E-caffeoyl ] -beta-d-glucopyranosyl}-5-O-beta-d-glucopyranoside | 32.04 | 1197.2949 | C66 H69 O33 |
| (B) | Brassicasp | |||
| 1 | Cyanidin 3,3′,5-tri-O-glucoside | 13.88 | 773.2151 | C33 H41O21 |
| 2 | Cyanidin 3-(6″-sinapylsophoroside)-5-glucoside | 21.13 | 979.2714 | C44H51O25 |
| 3 | Cyanidin 3-(diferuloylsophoroside) 5-glucoside | 34.39 | 1125.3103 | C53H57O27 |
| 4 | Cyanidin 3-O-[beta-d-glucopyranoside]-7,3′-di-O-[6-O-(sinapyl)-beta-d-glucopyranoside | 34.82 | 1185.3305 | C55H61O29 |
| pH | L* | a* | b* | C* | ΔE* |
|---|---|---|---|---|---|
| 1.0 | 62.23 ± 0.03 B | 47.23 ± 0.06 A | 5.24 ± 0.04 B | 47.51 ± 0.06 A | 57.74 ± 0.06 A |
| 2.0 | 64.30 ± 0.04 A | 43.79 ± 0.04 B | 0.50 ± 0.04 C | 43.79 ± 0.04 B | 53.78 ± 0.05 C |
| 3.0 | 61.89 ± 0.01 C | 35.87 ± 0.04 C | −4.41 ± 0.03 E | 36.14 ± 0.04 C | 49.60 ± 0.04 E |
| 4.0 | 60.75 ± 0.05 D | 24.74 ± 0.05 D | −12.32 ± 0.02 I | 27.64 ± 0.05 D | 45.16 ± 0.02 G |
| 5.0 | 59.24 ± 0.20 E | 19.09 ± 0.14 E | −17.12 ± 0.17 J | 25.64 ± 0.21 E | 45.47 ± 0.23 G |
| 6.0 | 57.91 ± 0.24 G | 11.02 ± 0.05 F | −20.01 ± 0.02 L | 22.84 ± 0.04 F | 45.18 ± 0.22 G |
| 7.0 | 59.44 ± 0.48 E | 7.49 ± 0.26 G | −18.36 ± 0.29 K | 19.83 ± 0.37 G | 42.28 ± 0.67 I |
| 8.0 | 54.08 ± 0.03 I | 6.92 ± 0.02 H | −18.25 ± 0.01 K | 19.52 ± 0.02 H | 46.71 ± 0.04 F |
| 9.0 | 43.20 ± 0.03 J | −9.41 ± 0.02 K | −5.82 ± 0.01 F | 11.06 ± 0.01 M | 53.18 ± 0.03 D |
| 10.0 | 40.86 ± 0.03 K | −8.66 ± 0.02 J | −9.85 ± 0.02 H | 13.12 ± 0.01 L | 56.15 ± 0.03 B |
| 11.0 | 56.07 ± 0.56 H | −14.72 ± 0.27 M | −7.98 ± 0.18 G | 16.75 ± 0.31 I | 42.83 ± 0.71 H |
| Correlation Parameter (y) | pH Range (x) | Linear Correlation Relationship (n = 5 Data Points) | R2 |
|---|---|---|---|
| L* | 1–10 | y = −2.3293x + 69.201 | 0.76366 |
| a* | 1–10 | y = −6.5453x + 53.807 | 0.97391 |
| b* | 1–8 | y = −3.6944x + 6.0336 | 0.86177 |
| C* | 1–9 | y = −4.2672x + 49.555 | 0.94694 |
| ∆E* | 1–5 | y = −3.316x + 60.298 | 0.93431 |
| ∆E* | 6–10 | y = 3.284x + 22.428 | 0.81002 |
| Storage Time (h/day) | L* | a* | b* | C* | ΔE* |
|---|---|---|---|---|---|
| Shrimp samples | |||||
| 1.0 h | 36.81 ± 0.62 | 2.20 ± 0.22 | −5.09 ± 0.17 | 5.55 ± 0.20 | 58.66 ± 0.62 |
| 2.0 h | 28.47 ± 0.69 | 0.04 ± 0.08 | −5.13 ± 0.26 | 5.13 ± 0.26 | 66.87 ± 0.76 |
| 2.5 h | 26.91 ± 0.65 | −0.09 ± 0.22 | −4.72 ± 0.38 | 4.72 ± 0.38 | 68.37 ± 0.63 |
| 3.0 h | 26.31 ± 0.73 | −0.64 ± 0.10 | −4.44 ± 0.41 | 4.49 ± 0.40 | 68.93 ± 0.70 |
| 4.0 h | 25.73 ± 0.20 | −1.18 ± 0.10 | −3.57 ± 0.79 | 3.77 ± 0.74 | 69.43 ± 0.25 |
| Durian samples | |||||
| 0 day | 39.18 ± 0.77 | 3.62 ± 0.22 | −4.13 ± 0.31 | 5.49 ± 0.40 | 56.27 ± 0.84 |
| 2 day | 26.38 ± 0.61 | −1.63 ± 0.10 | −3.82 ± 0.20 | 4.15 ± 0.23 | 68.82 ± 0.62 |
| 4 day | 24.50 ± 0.28 | 3.12 ± 0.08 | −2.93 ± 0.04 | 4.28 ± 0.08 | 70.67 ± 0.27 |
| 6 day | 25.58 ± 0.11 | 3.04 ± 0.04 | −2.88 ± 0.14 | 4.19 ± 0.13 | 69.59 ± 0.12 |
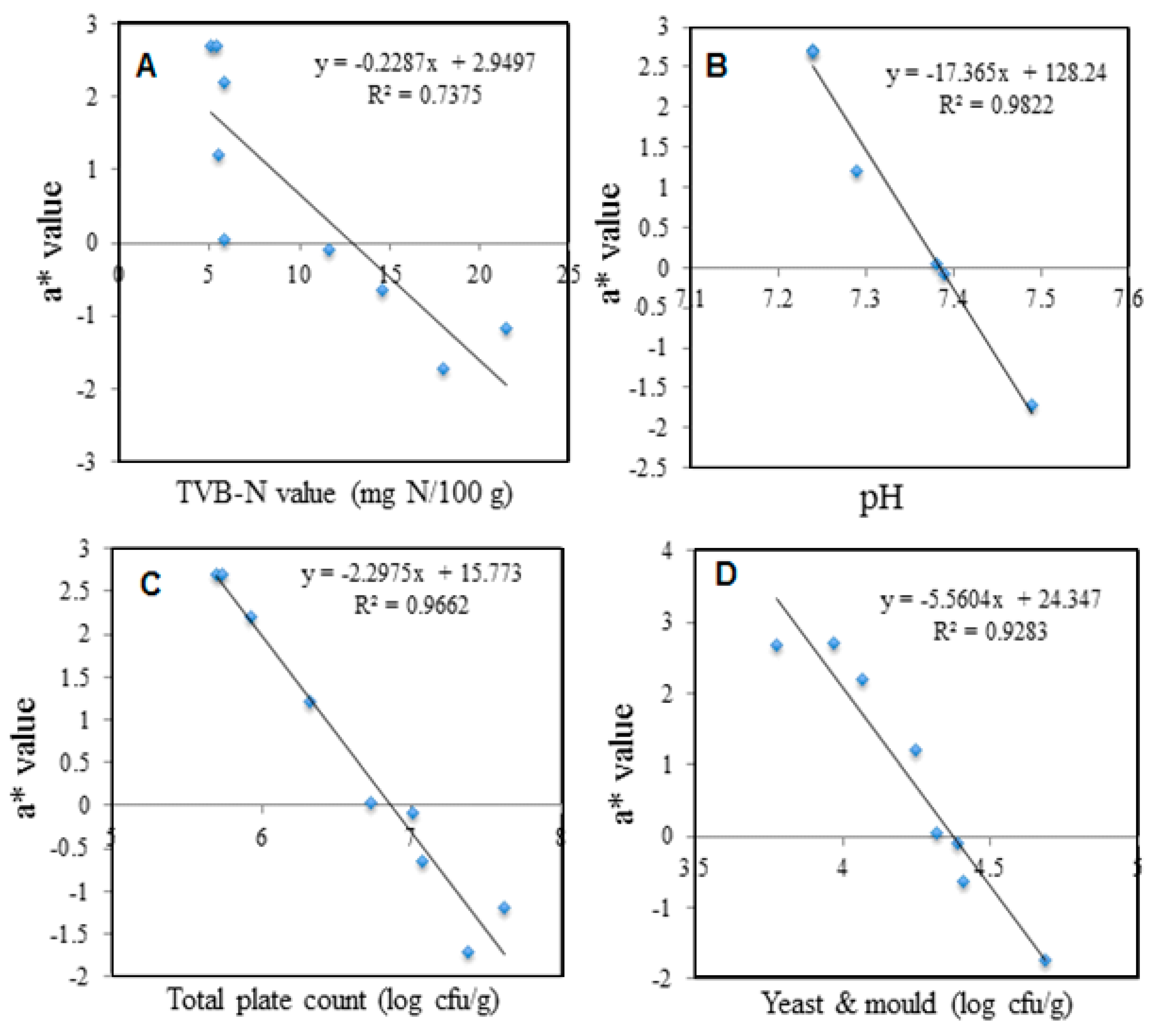
| Spoilage Parameters | L* | a* | b* | C* | ΔE* |
|---|---|---|---|---|---|
| Shrimp samples Correlation (n = 5) with | |||||
| TVBN | R2 = 0.5300 | R2 = 0.7375 | R2 = 0.7025 | R2 = 0.6127 | R2 = 0.5271 |
| (y = −0.7604x +39.259) | (y = −0.2287x +2.9497) | (y = 0.0887x −5.886) | (y = 0.1025x + 6.2913) | (y = 0.7393x + 56.35) | |
| pH | R2 = 0.8217 | R2 = 0.9822 | R2 = 0.8213 | R2 = 0.9236 | R2 = 0.7255 |
| (y = −29.529x + 247.01) | (y = −17.365x + 128.24) | (y = 5.4259x −44.683) | (y = −8.0921x + 64.669) | (y = 46.231x – 277.03) | |
| TPC | R2 = 0.8872 | R2 = 0.9662 | R2 = 0.6887 | R2 = 0.7752 | R2 = 0.8856 |
| (y = −8.636x + 88.487) | (y = −2.2975x + 15.773) | (y = 0.7707x – 10.063) | (y = −1.0117x + 11.92) | (y = 8.4114x + 8.3878) | |
| Yeast & mold | R2 = 0.6648 | R2 = 0.9283 | R2 = 0.8842 | R2 = 0.8693 | R2 = 0.8544 |
| (y = −11.452x + 80.281) | (y = −5.5604x + 24.347) | (y = 1.7352x – 12.432) | (y = −2.0813x + 14.14) | (y = 21.044x – 25.782) | |
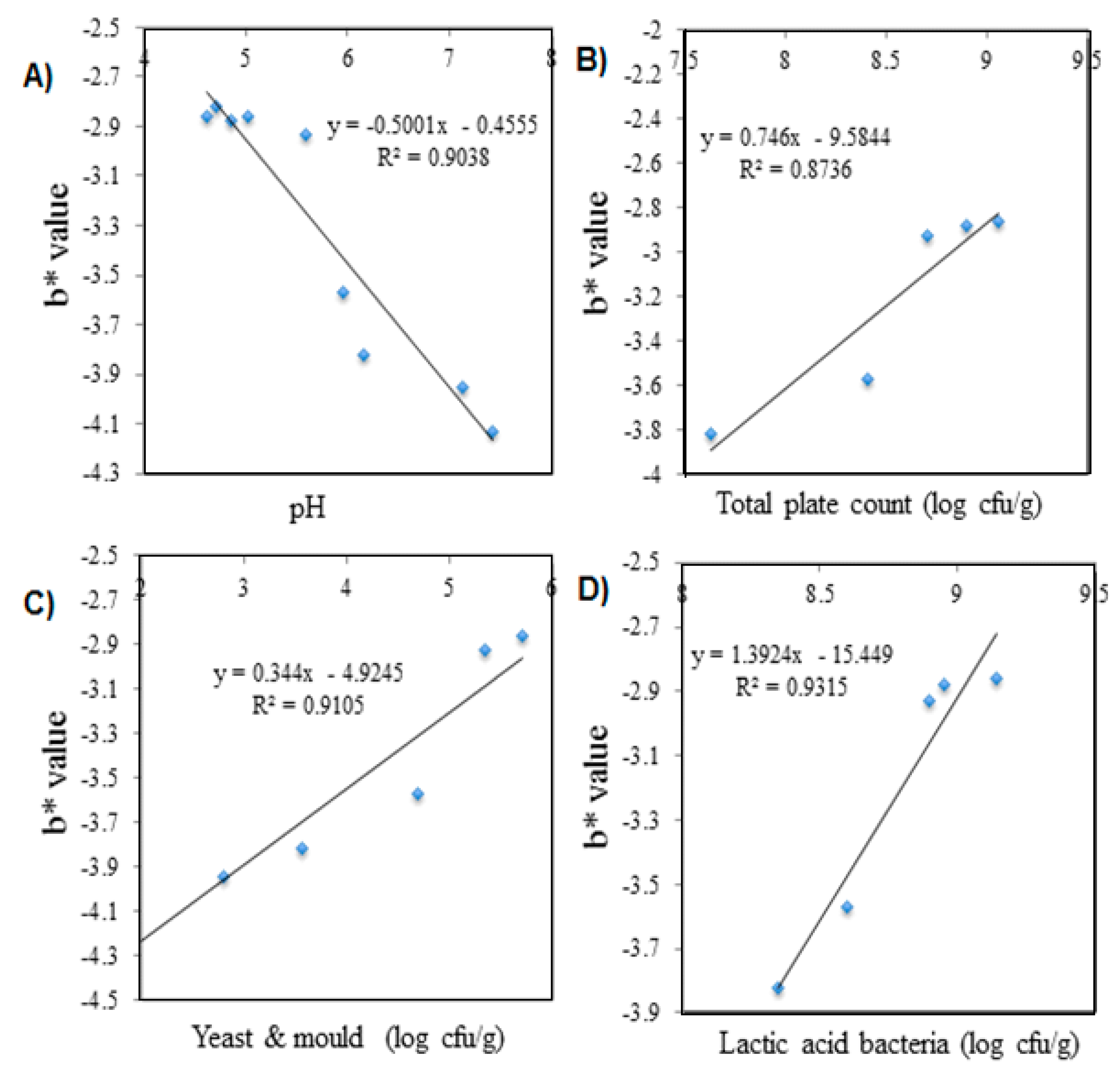
| Durian samples | |||||
| Correlation (n = 5) with | |||||
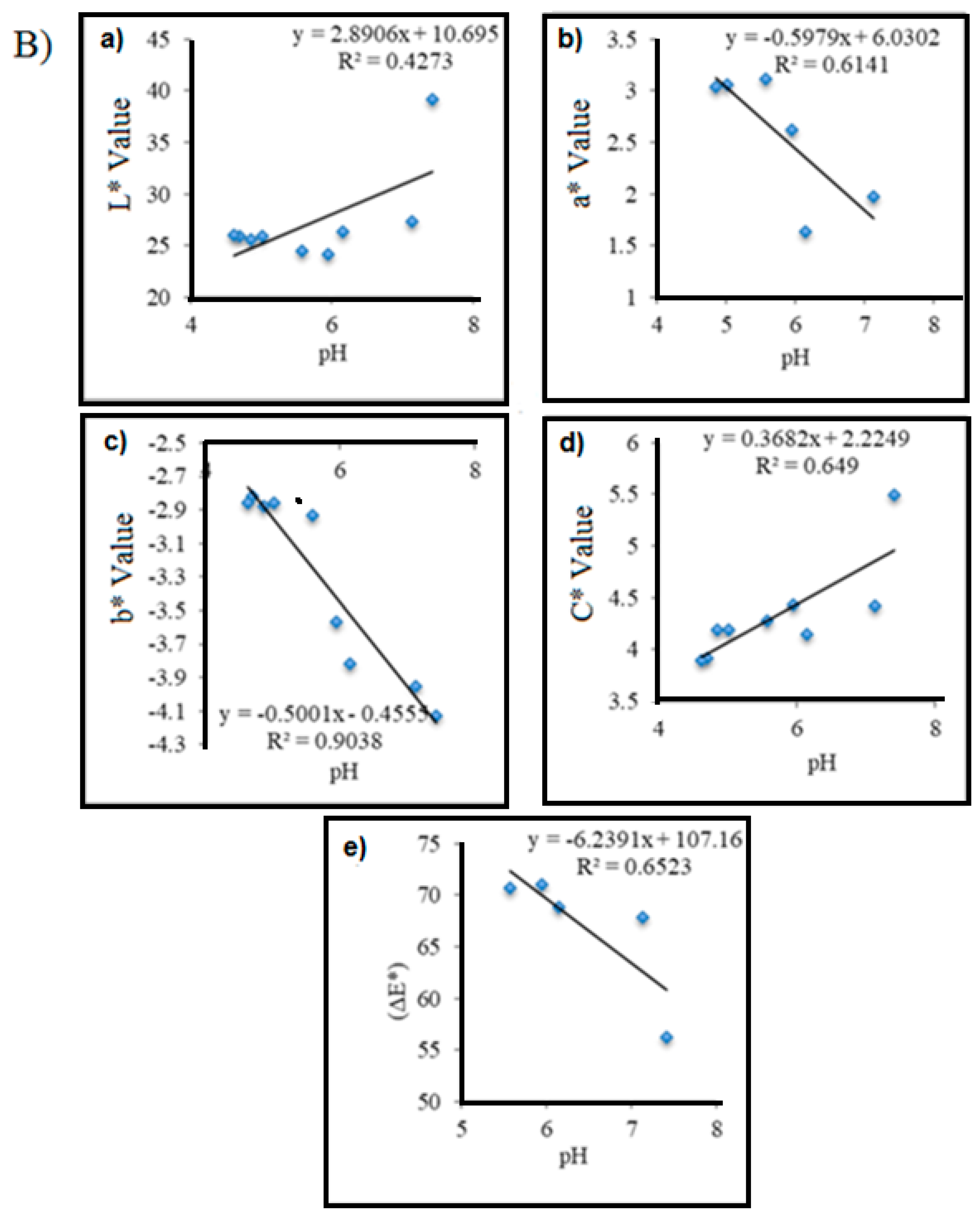
| pH | R2 = 0.4273 | R2 = 0.6141 | R2 = 0.9038 | R2 = 0.649 | R2 = 0.6523 |
| (y = 2.8906x + 10.695) | (y = −0.5979x + 6.0302) | (y = −0.5001x − 0.4555) | (y = 0.3682x + 2.2249) | (y = −6.2391x + 107.16) | |
| TPC | R2 = 0.6485 | R2 = 0.642 | R2 = 0.8736 | R2 = 0.6582 | R2 = 0.6446 |
| (y = −1.5199x + 38.567) | (y = 0.7261x − 3.609) | (y = 0.746x − 9.5844) | (y = −0.1583x + 5.5111) | (y = 1.4857x + 56.899) | |
| Yeast & mold | R2 = 0.5995 | R2 = 0.8216 | R2 = 0.9105 | R2 = 0.5706 | R2 = 0.5955 |
| (y = 2.7226x + 39.181) | (y = 0.4882x + 0.3074) | (y = 0.344x −4.9245) | (y = −0.2745x + 5.5356) | (y = 2.6603x + 56.303) | |
| LAB | R2 = 0.6473 | R2 = 0.828 | R2 = 0.9315 | R2 = 0.6606 | R2 = 0.6438 |
| (y = −1.2407x + 36.329) | (y = 1.827x – 13.367) | (y = 1.3924x – 15.449) | (y = −0.1226x + 5.2682) | (y = 1.2132x + 59.084) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, N.A.; Yook Heng, L.; Salam, F.; Mat Zaid, M.H.; Abu Hanifah, S. A Colorimetric pH Sensor Based on Clitoria sp and Brassica sp for Monitoring of Food Spoilage Using Chromametry. Sensors 2019, 19, 4813. https://doi.org/10.3390/s19214813
Ahmad NA, Yook Heng L, Salam F, Mat Zaid MH, Abu Hanifah S. A Colorimetric pH Sensor Based on Clitoria sp and Brassica sp for Monitoring of Food Spoilage Using Chromametry. Sensors. 2019; 19(21):4813. https://doi.org/10.3390/s19214813
Chicago/Turabian StyleAhmad, Noor Azizah, Lee Yook Heng, Faridah Salam, Mohd Hazani Mat Zaid, and Sharina Abu Hanifah. 2019. "A Colorimetric pH Sensor Based on Clitoria sp and Brassica sp for Monitoring of Food Spoilage Using Chromametry" Sensors 19, no. 21: 4813. https://doi.org/10.3390/s19214813
APA StyleAhmad, N. A., Yook Heng, L., Salam, F., Mat Zaid, M. H., & Abu Hanifah, S. (2019). A Colorimetric pH Sensor Based on Clitoria sp and Brassica sp for Monitoring of Food Spoilage Using Chromametry. Sensors, 19(21), 4813. https://doi.org/10.3390/s19214813




