A New Method for Total Fat Detection in Raw Milk Based on Dual Low-Coherence Interferometer
Abstract
1. Introduction
2. Materials and Methods
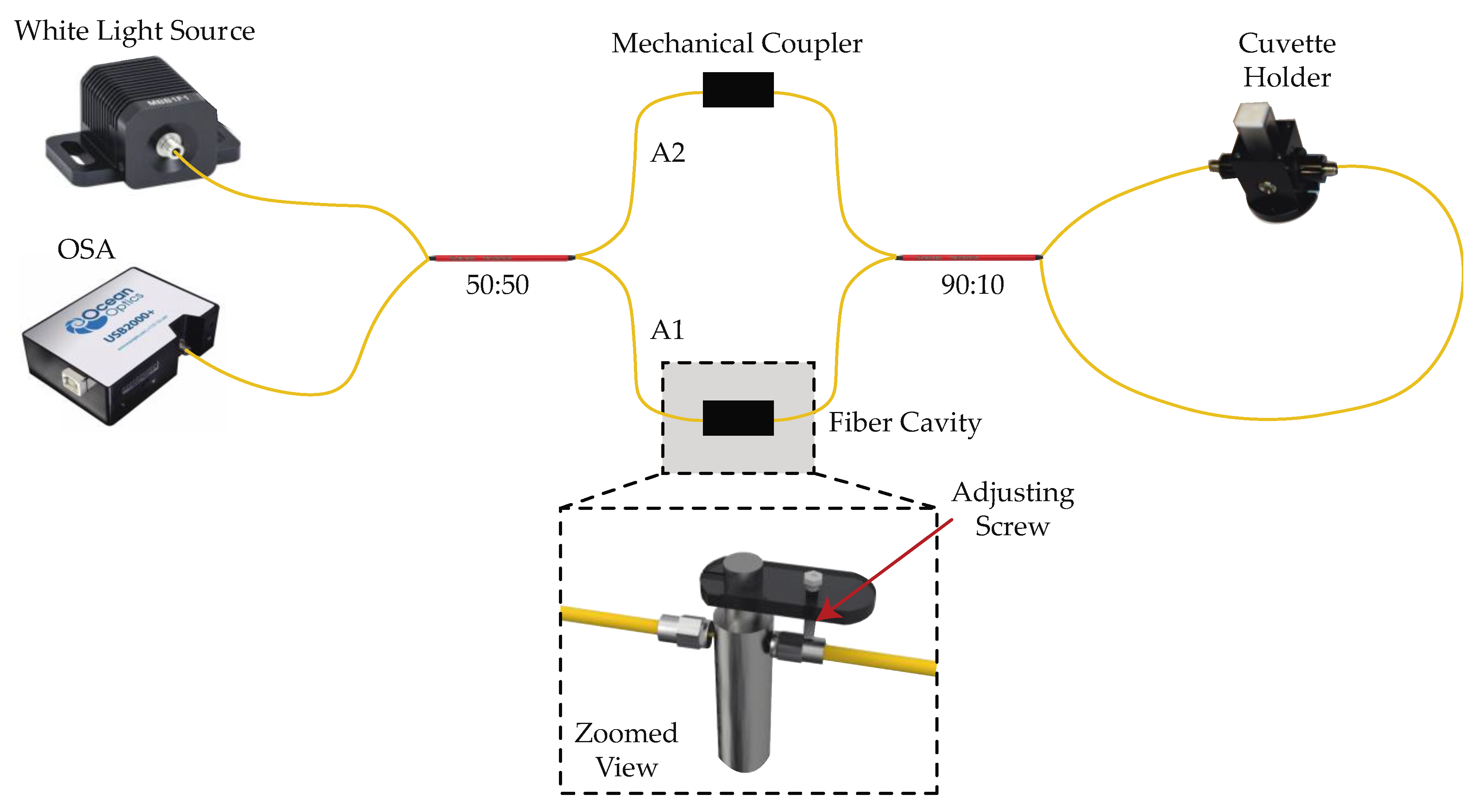
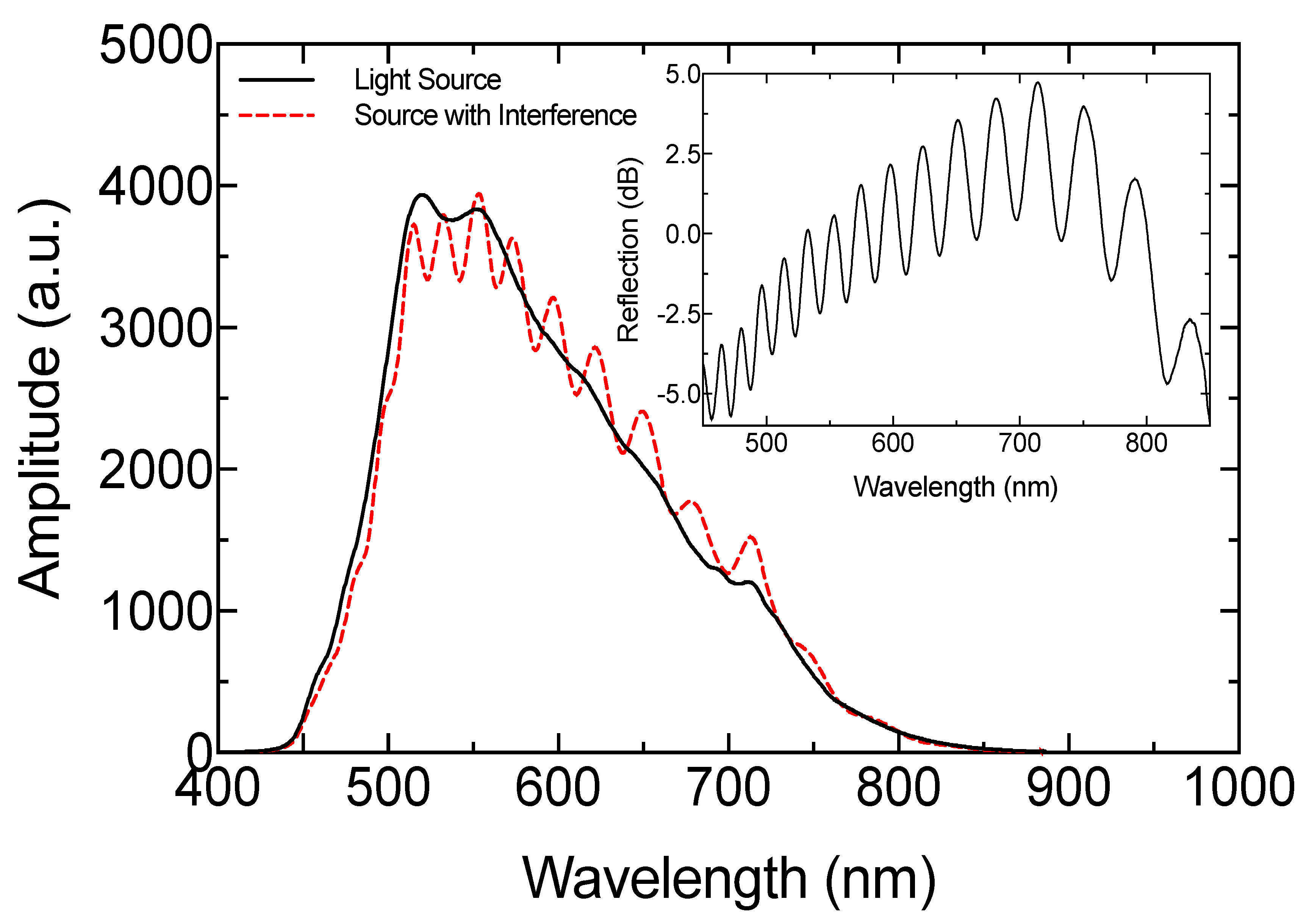
2.1. Experimental Setup
2.2. Sample Preparation
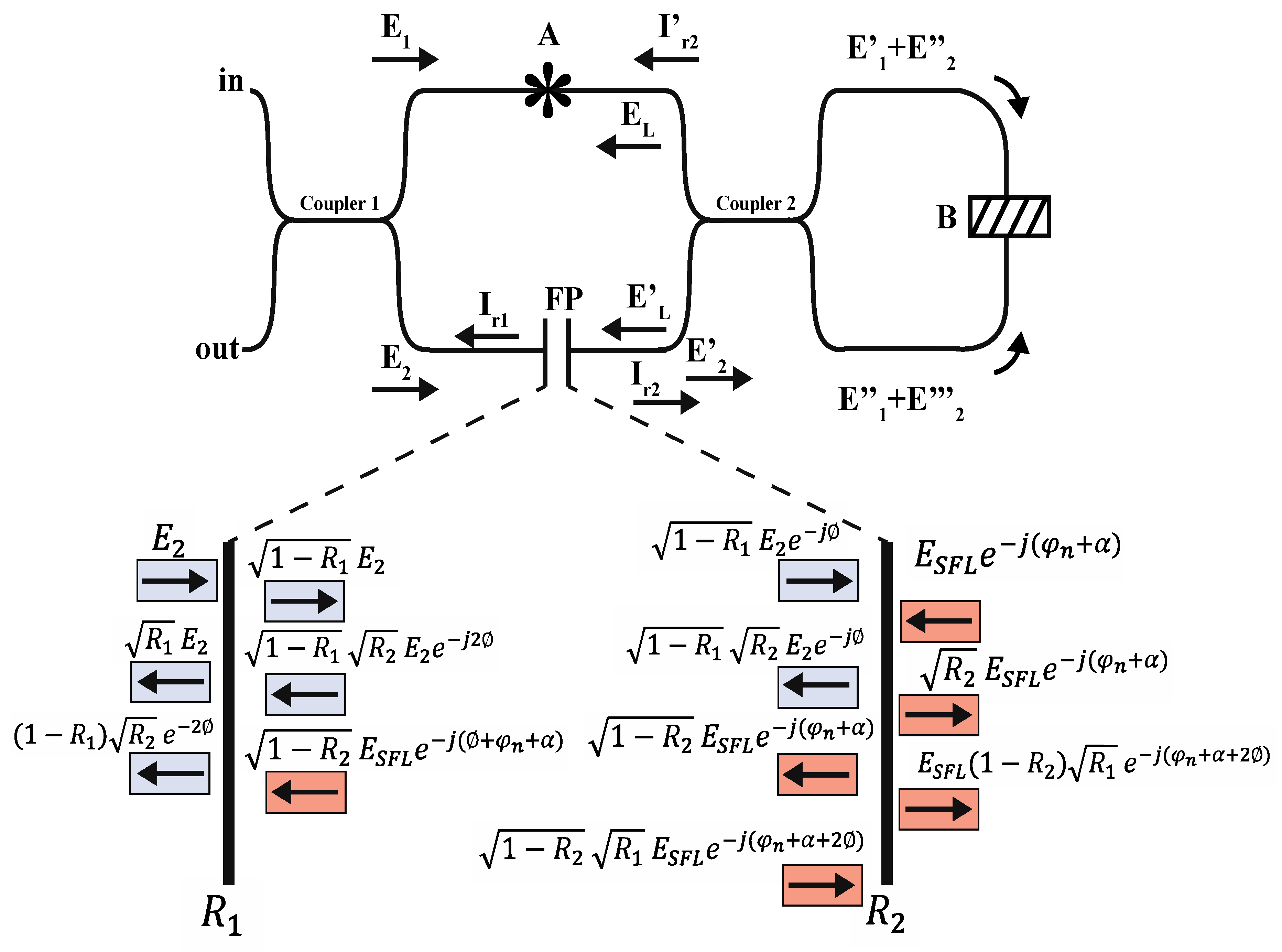
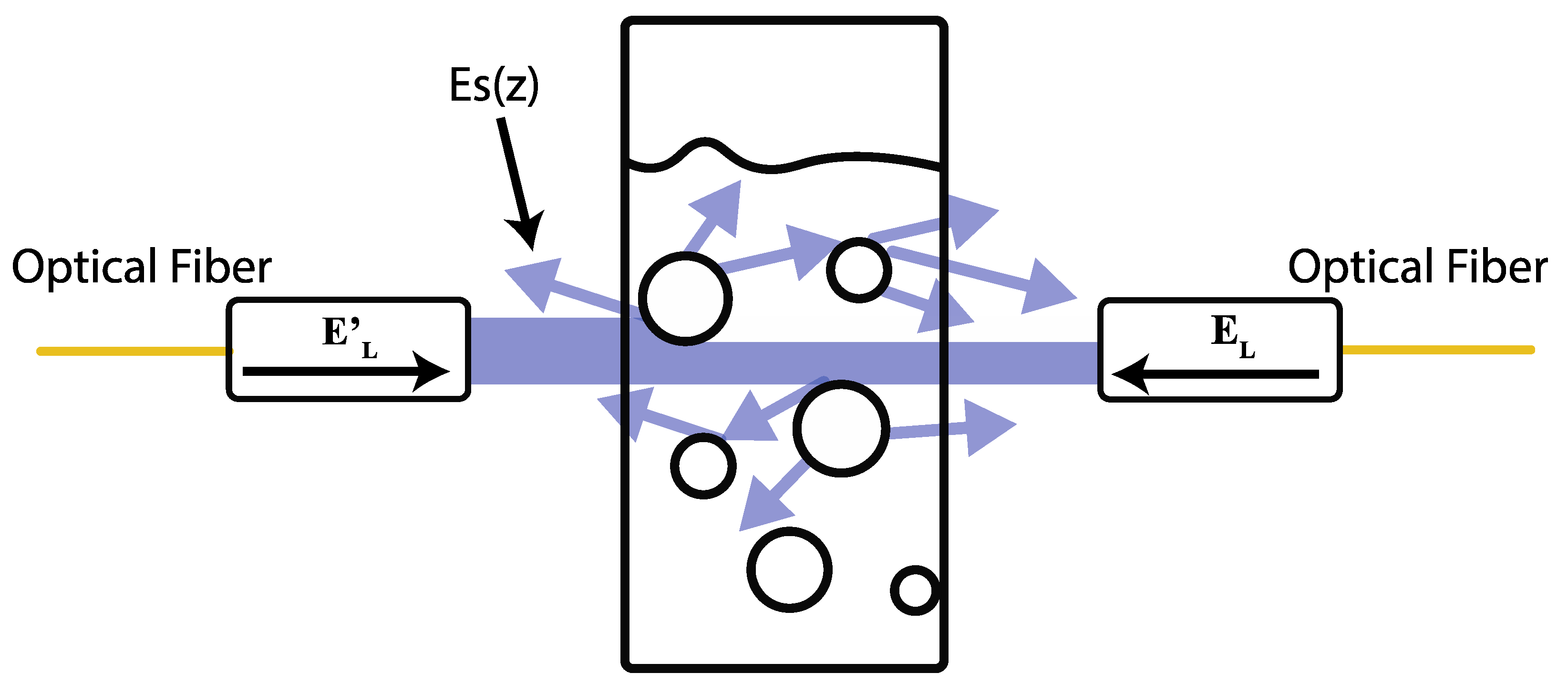
2.3. Theoretical Model
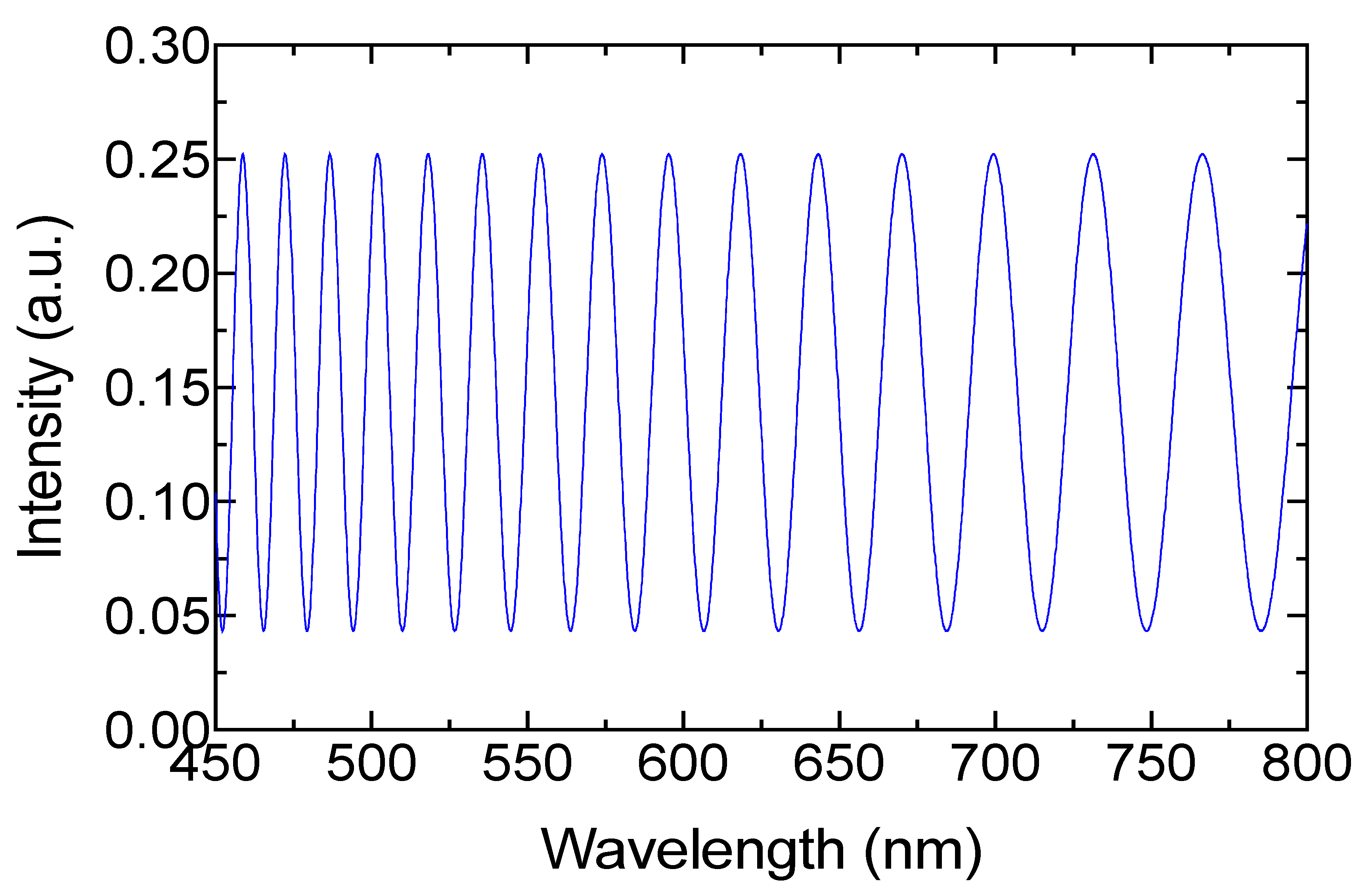
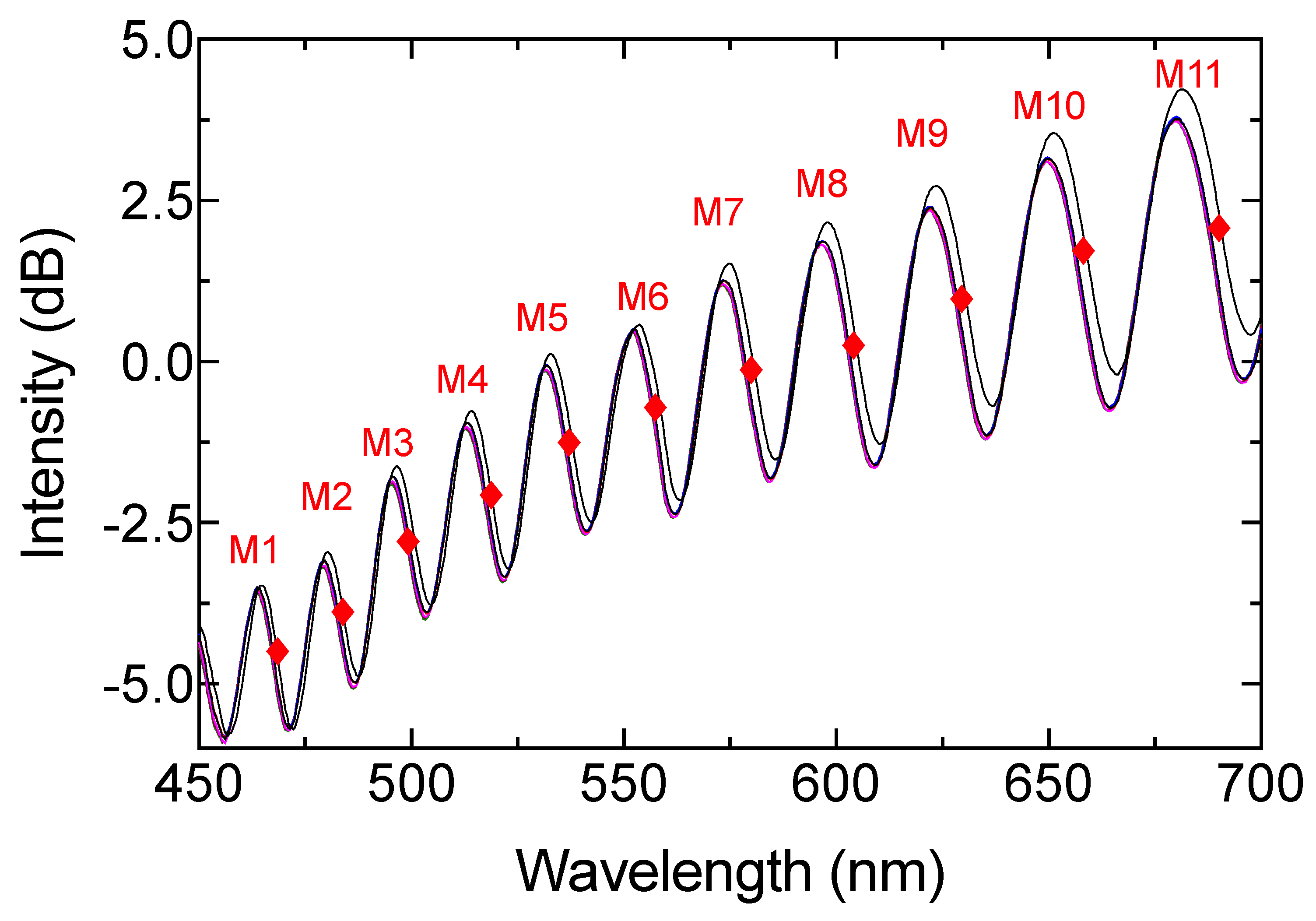
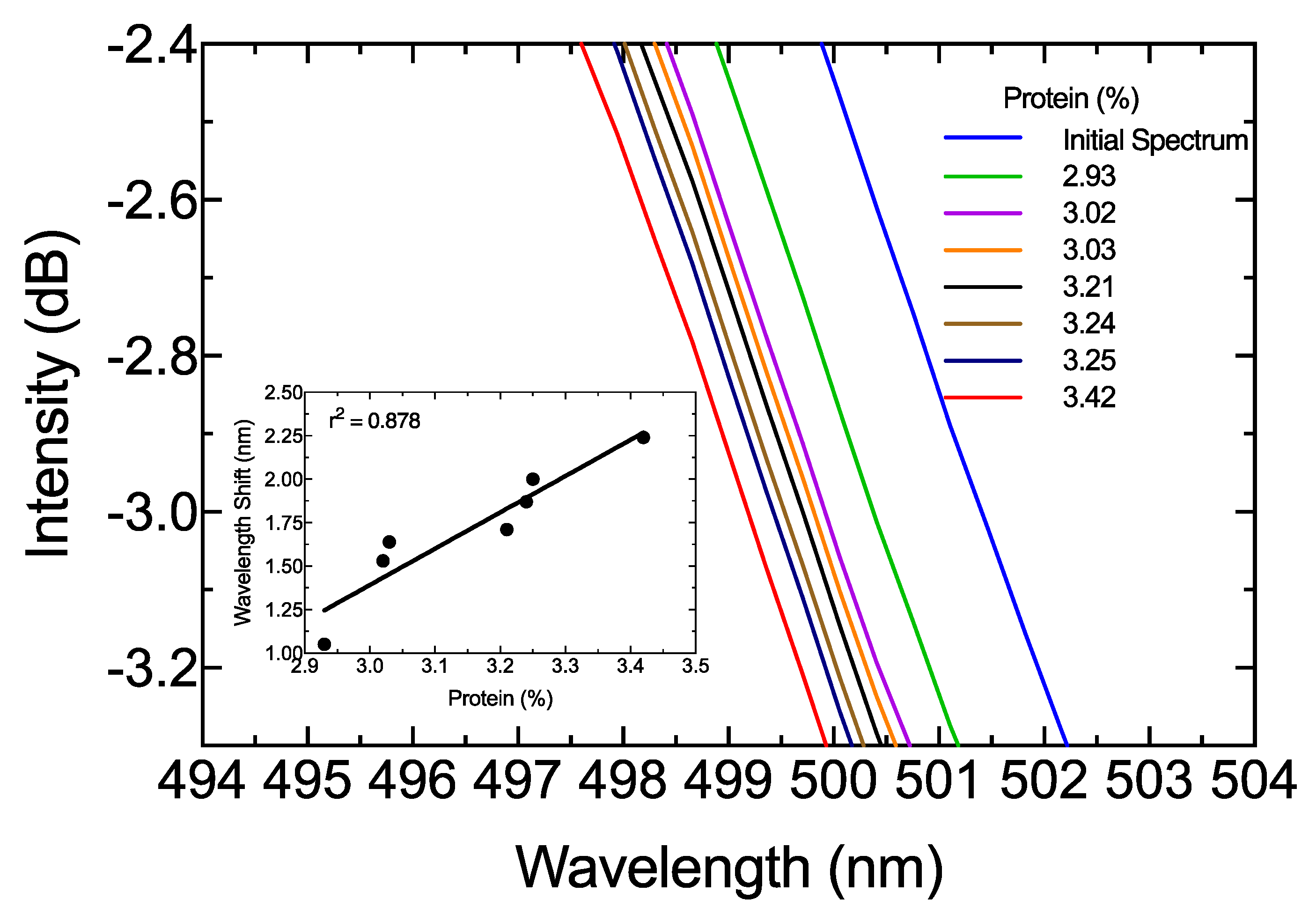
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ohtani, S.; Wang, T.; Nishimura, K.; Irie, M. Milk fat analysis by fiber-optic spectroscopy. Asian-Australas. J. Anim. Sci. 2005, 18, 580–583. [Google Scholar] [CrossRef]
- Li, X.; Huo, G.; Wang, Y.; Sun, H.; Kong, Q. Research on Rapid Detection Method of Protein and Fat in Raw Milk Based on Mid-infrared Spectrum. Int. J. Multimed. Ubiquitous Eng. 2016, 11, 131–142. [Google Scholar] [CrossRef]
- Niero, G.; Penasa, M.; Gottardo, P.; Cassandro, M.; De Marchi, M. Short communication: Selecting the most informative mid-infrared spectra wavenumbers to improve the accuracy of prediction models for detailed milk protein content. J. Dairy Sci. 2016, 99, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Aernouts, B.; Van Beers, R.; Watté, R.; Huybrechts, T.; Lammertyn, J.; Saeys, W. Visible and near-infrared bulk optical properties of raw milk. J. Dairy Sci. 2015, 98, 6727–6738. [Google Scholar] [CrossRef]
- Kucheryavskiy, S.; Melenteva, A.; Bogomolov, A. Determination of fat and total protein content in milk using conventional digital imaging. Talanta 2014, 121, 144–152. [Google Scholar] [CrossRef]
- Bogomolov, A.; Melenteva, A. Scatter-based quantitative spectroscopic analysis of milk fat and total protein in the region 400–1100 nm in the presence of fat globule size variability. Chemom. Intell. Lab. Syst. 2013, 126, 129–139. [Google Scholar] [CrossRef]
- Feng, X.; Su, R.; Xu, N.; Wang, X.; Yu, A.; Zhang, H.; Cao, Y. Portable analyzer for rapid analysis of total protein, fat and lactose contents in raw milk measured by non-dispersive short-wave near-infrared spectrometry. Chem. Res. Chin. Univ. 2013, 29, 15–19. [Google Scholar] [CrossRef]
- Burns, D.A.; Ciurczak, E.W. Handbook of Near-Infrared Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Regnima, G.-O.; Koffi, T.; Bagui, O.; Kouacou, A.; Kristensson, E.; Zoueu, J.; Berrocal, E. Quantitative measurements of turbid liquids via structured laser illumination planar imaging where absorption spectrophotometry fails. Appl. Opt. 2017, 56, 3929–3938. [Google Scholar] [CrossRef]
- Gowri, A.; Rajamani, A.S.; Ramakrishna, B.; Sai, V.V.R. U-bent plastic optical fiber probes as refractive index based fat sensor for milk quality monitoring. Opt. Fiber Technol. 2019, 47, 15–20. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Z.; Qian, K.; Wang, L.; Lan, X. A rapid method for measuring fat content in milk based on W-type optical fibre sensor system. Trans. Inst. Meas. Control 2016, 38, 1471–1479. [Google Scholar] [CrossRef]
- Kawasaki, M.; Kawamura, S.; Tsukahara, M.; Morita, S.; Komiya, M.; Natsuga, M. Near-infrared spectroscopic sensing system for on-line milk quality assessment in a milking robot. Comput. Electron. Agric. 2008, 63, 22–27. [Google Scholar] [CrossRef]
- Bogomolov, A.; Melenteva, A.; Dahm, D.J. Fat globule size effect on visible and shortwave near infrared spectra of milk. J. Near Infrared Spectrosc. 2013, 21, 435–440. [Google Scholar] [CrossRef]
- Lee, C.E.; Taylor, H.F. Fiber-optic Fabry-Perot temperature sensor using a low-coherence light source. J. Light. Technol. 1991, 9, 129–134. [Google Scholar] [CrossRef]
- Wang, S.; Lv, R.; Zhao, Y.; Qian, J. A Mach-Zehnder interferometer-based High Sensitivity Temperature sensor for human body monitoring. Opt. Fiber Technol. 2018, 45, 93–97. [Google Scholar] [CrossRef]
- Tian, Z.; Yam, S.S.H.; Loock, H.P. Single-mode fiber refractive index sensor based on core-offset attenuators. IEEE Photonics Technol. Lett. 2008, 20, 1387–1389. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, Y.H.; Park, K.S.; Eom, J.B.; Kim, M.J.; Rho, B.S.; Choi, H.Y. Interferometric fiber optic sensors. Sensors 2012, 12, 2467–2486. [Google Scholar] [CrossRef]
- Bylund, G. Dairy Processing Handbook; Tetra Pak Processing Systems AB: Lund, Sweden, 1995. [Google Scholar]
- Deutsch, B.; Beams, R.; Novotny, L. Nanoparticle detection using dual-phase interferometry. Appl. Opt. 2010, 49, 4921–4925. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, L.; Jin, W. Improving the reliability of multiplexed fiber optic low-coherence interferometric sensors by use of novel twin-loop network topologies. Rev. Sci. Instrum. 2007, 78, 055106. [Google Scholar] [CrossRef]
- Crofcheck, C.L.; Payne, F.A.; Mengüç, M.P. Characterization of milk properties with a radiative transfer model. Appl. Opt. 2002, 41, 2028. [Google Scholar] [CrossRef]
- Crofcheck, C.; Wade, J.; Swamy, J.N.; Aslan, M.M.; Mengüç, M.P. Effect of fat and casein particles in milk on the scattering of elliptically polarized light. Trans. Am. Soc. Agric. Eng. 2005, 48, 1147–1155. [Google Scholar] [CrossRef]
- Stocker, S.; Foschum, F.; Krauter, P.; Bergmann, F.; Hohmann, A.; Happ, C.S.; Kienle, A. Broadband Optical Properties of Milk. Appl. Spectrosc. 2017, 71, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Sarma, S.E. Light scattering and transmission measurement using digital imaging for online analysis of constituents in milk. Proc. SPIE 2015, 9525, 95254A. [Google Scholar]
- Shapiro, D.B.; Hull, P.G.; Hunt, A.J.; Hearst, J.E. Calculations of the Mueller scattering matrix for a DNA plectonemic helix. J. Chem. Phys. 1994, 101, 4214–4221. [Google Scholar] [CrossRef]
- Xin, Q.; Ling, H.Z.; Long, T.J.; Zhu, Y. The rapid determination of fat and protein content in fresh raw milk using the laser light scattering technology. Opt. Laser Eng. 2006, 44, 858–869. [Google Scholar] [CrossRef]
- Medjadba, H.; Lecler, S.; Mokhtar Simohamed, L.; Fontaine, J.; Meyrueis, P. Investigation of mode coupling effects on sensitivity and bias of a multimode fiber loop interferometer: Application to an optimal design of a multimode fiber gyroscope. Opt. Fiber Technol. 2011, 17, 50–58. [Google Scholar] [CrossRef]
- McCarthy, O.J. Physical and Physico-Chemical Properties of Milk. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2002; Volume 3, pp. 467–477. [Google Scholar]
- Bogomolov, A.; Dietrich, S.; Boldrini, B.; Kessler, R.W. Quantitative determination of fat and total protein in milk based on visible light scatter. Food Chem. 2012, 134, 412–418. [Google Scholar] [CrossRef]
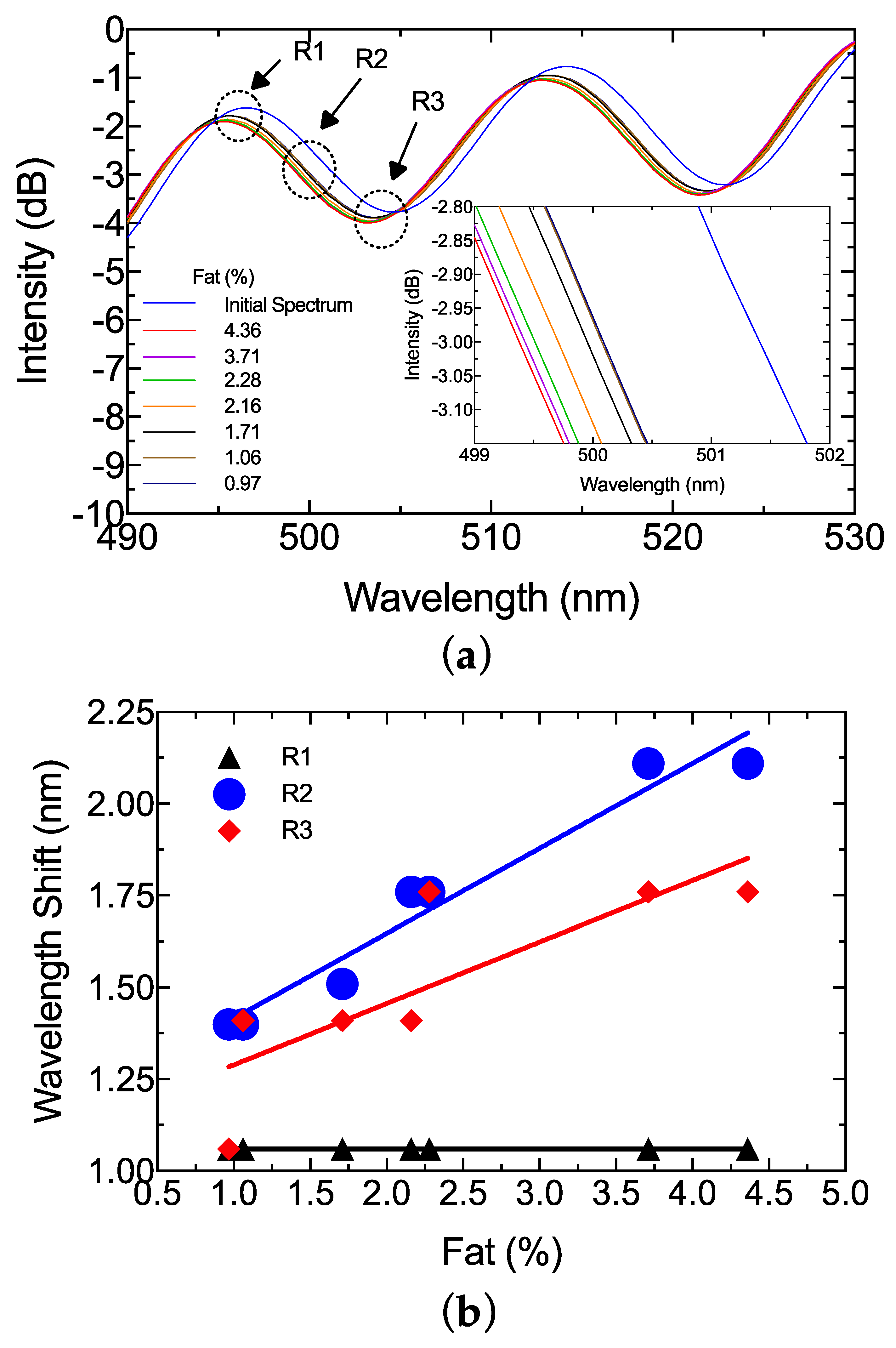

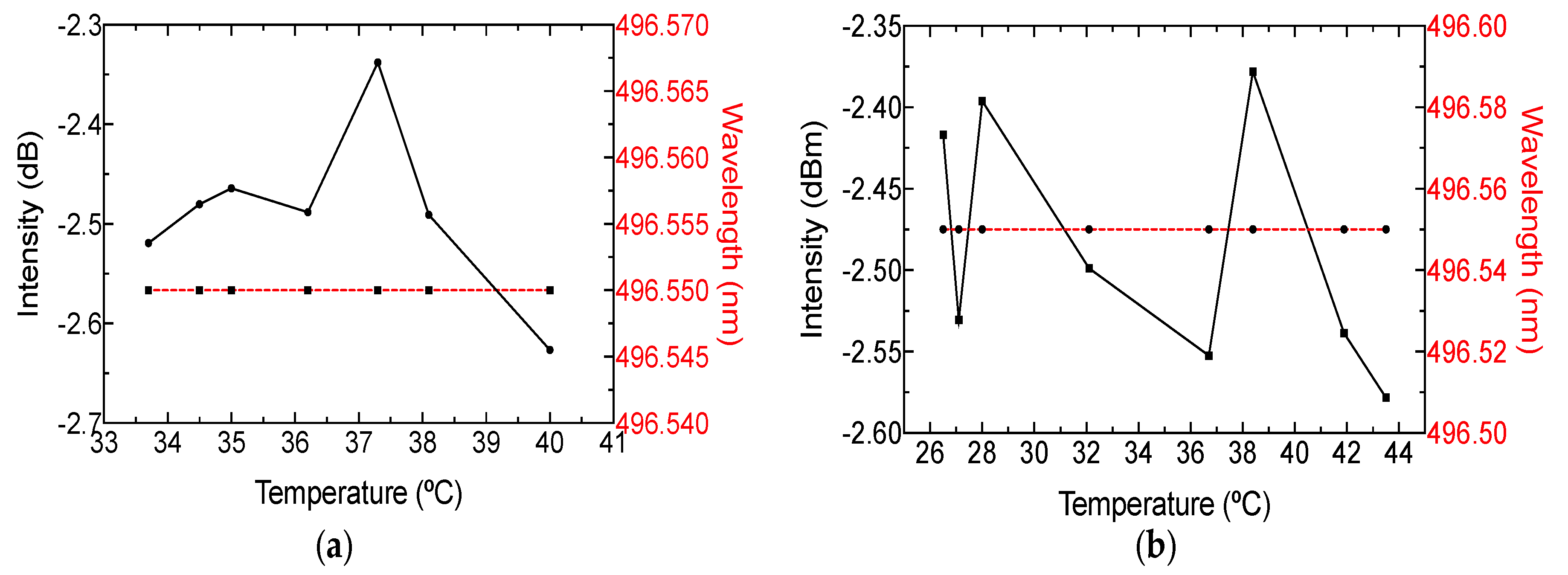
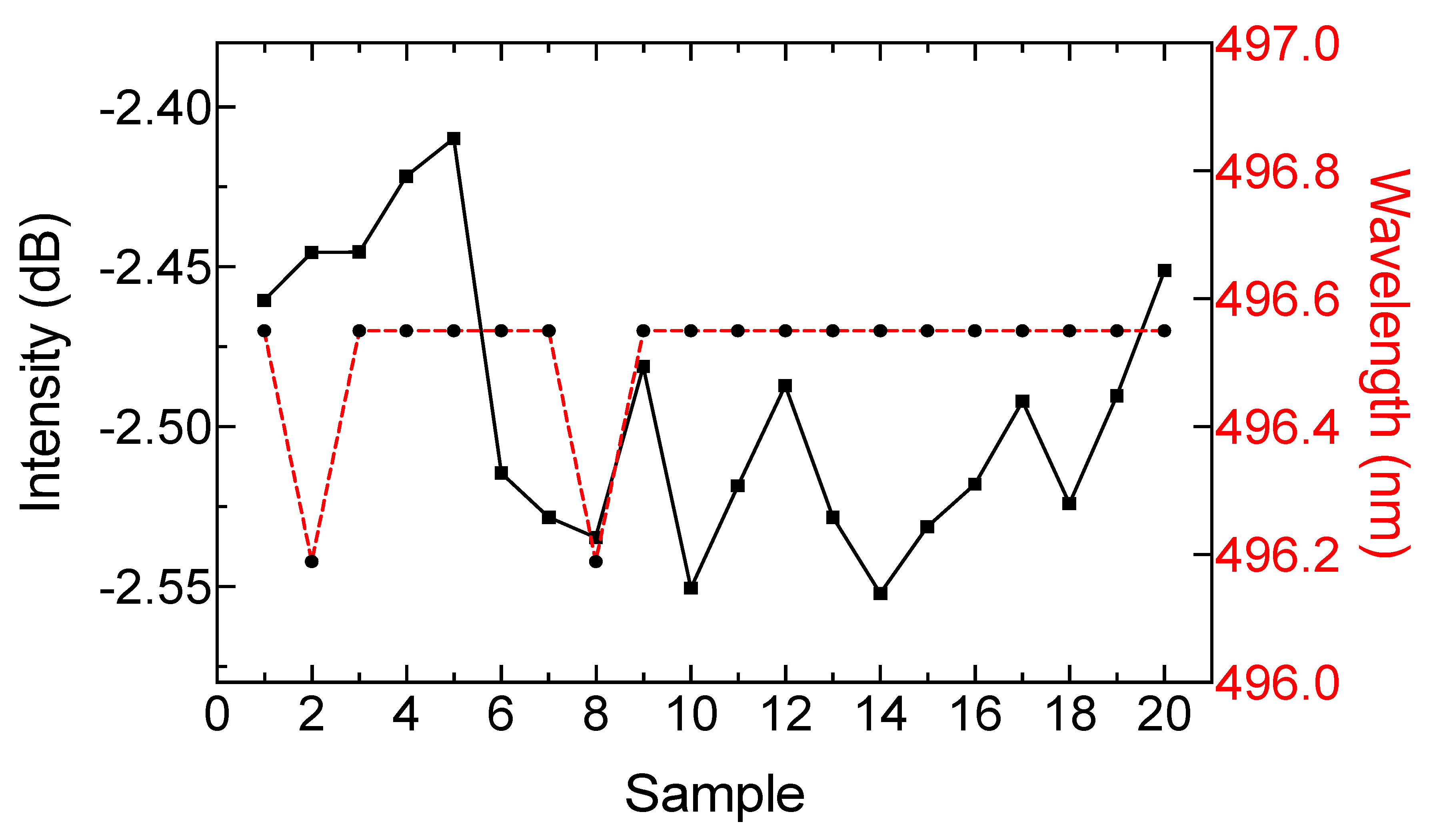
| Sample | Fat (%) | Protein (%) |
|---|---|---|
| F1 | 0.97 | 3.16 |
| F2 | 1.06 | 3.17 |
| F3 | 1.71 | 3.05 |
| F4 | 2.16 | 3.21 |
| F5 | 2.28 | 3.20 |
| F6 | 3.71 | 3.08 |
| F7 | 4.36 | 3.23 |
| Region | R Square |
|---|---|
| M1 | 0.9558 |
| M2 | * |
| M3 | 0.9739 |
| M4 | 0.8118 |
| M5 | 0.9157 |
| M6 | 0.8118 |
| M7 | 0.8484 |
| M8 | 0.7077 |
| M9 | 0.8935 |
| M10 | 0.9135 |
| M11 | 0.9158 |
| Milk Constituent | Acquisition Method | Spectral Range (nm) | Sensitivity | Validation | Reference | |
|---|---|---|---|---|---|---|
| R2 | RMSEP * | |||||
| Fat | Light Scatter Digital Imaging | 400–700 | - | 0.973 | - | [5] |
| Protein | - | 0.974 | - | |||
| Fat | Absorbance Refractive Index | 400–800 | 0.15 Abs/%fat | - | - | [10] |
| Fat | Light Scatter | 1300–1400 | - | 0.975 | - | [4] |
| Fat | Transmission | 2500–25,000 | - | 0.91 | 0.045 | [2] |
| Protein | - | 0.801 | 0.02 | |||
| Fat | Reflectance | 200–2000 | 0.984 | 0.00265 | [11] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastélum-Barrios, A.; Soto-Zarazúa, G.M.; García-Trejo, J.F.; Sierra-Hernandez, J.M.; Jauregui-Vazquez, D. A New Method for Total Fat Detection in Raw Milk Based on Dual Low-Coherence Interferometer. Sensors 2019, 19, 4562. https://doi.org/10.3390/s19204562
Gastélum-Barrios A, Soto-Zarazúa GM, García-Trejo JF, Sierra-Hernandez JM, Jauregui-Vazquez D. A New Method for Total Fat Detection in Raw Milk Based on Dual Low-Coherence Interferometer. Sensors. 2019; 19(20):4562. https://doi.org/10.3390/s19204562
Chicago/Turabian StyleGastélum-Barrios, Abraham, Genaro M. Soto-Zarazúa, Juan F. García-Trejo, Juan M. Sierra-Hernandez, and Daniel Jauregui-Vazquez. 2019. "A New Method for Total Fat Detection in Raw Milk Based on Dual Low-Coherence Interferometer" Sensors 19, no. 20: 4562. https://doi.org/10.3390/s19204562
APA StyleGastélum-Barrios, A., Soto-Zarazúa, G. M., García-Trejo, J. F., Sierra-Hernandez, J. M., & Jauregui-Vazquez, D. (2019). A New Method for Total Fat Detection in Raw Milk Based on Dual Low-Coherence Interferometer. Sensors, 19(20), 4562. https://doi.org/10.3390/s19204562





