Ultrasensitive Electrical Detection of Hemagglutinin for Point-of-Care Detection of Influenza Virus Based on a CMP-NANA Probe and Top-Down Processed Silicon Nanowire Field-Effect Transistors
Abstract
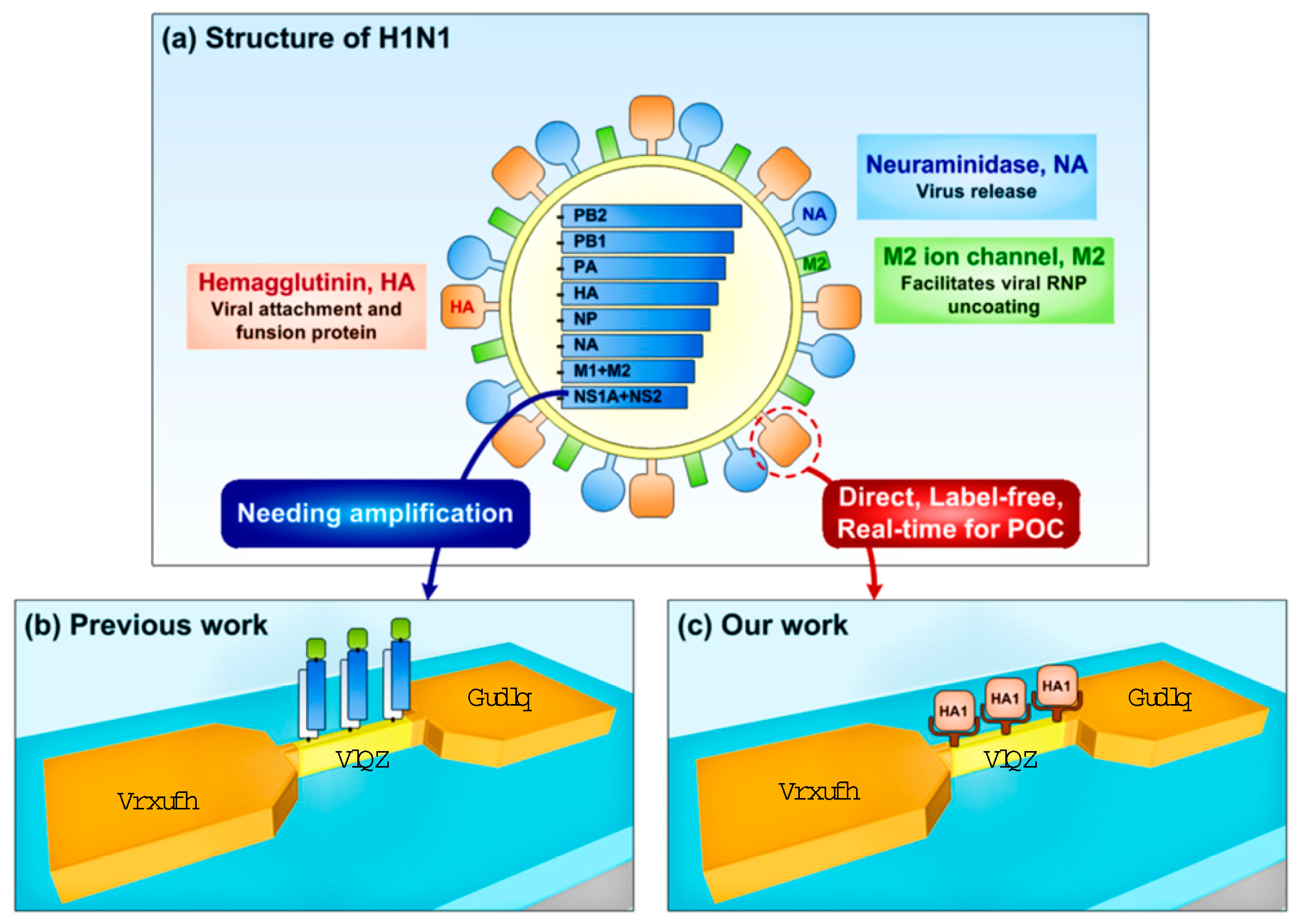
1. Introduction
2. Experimental Section
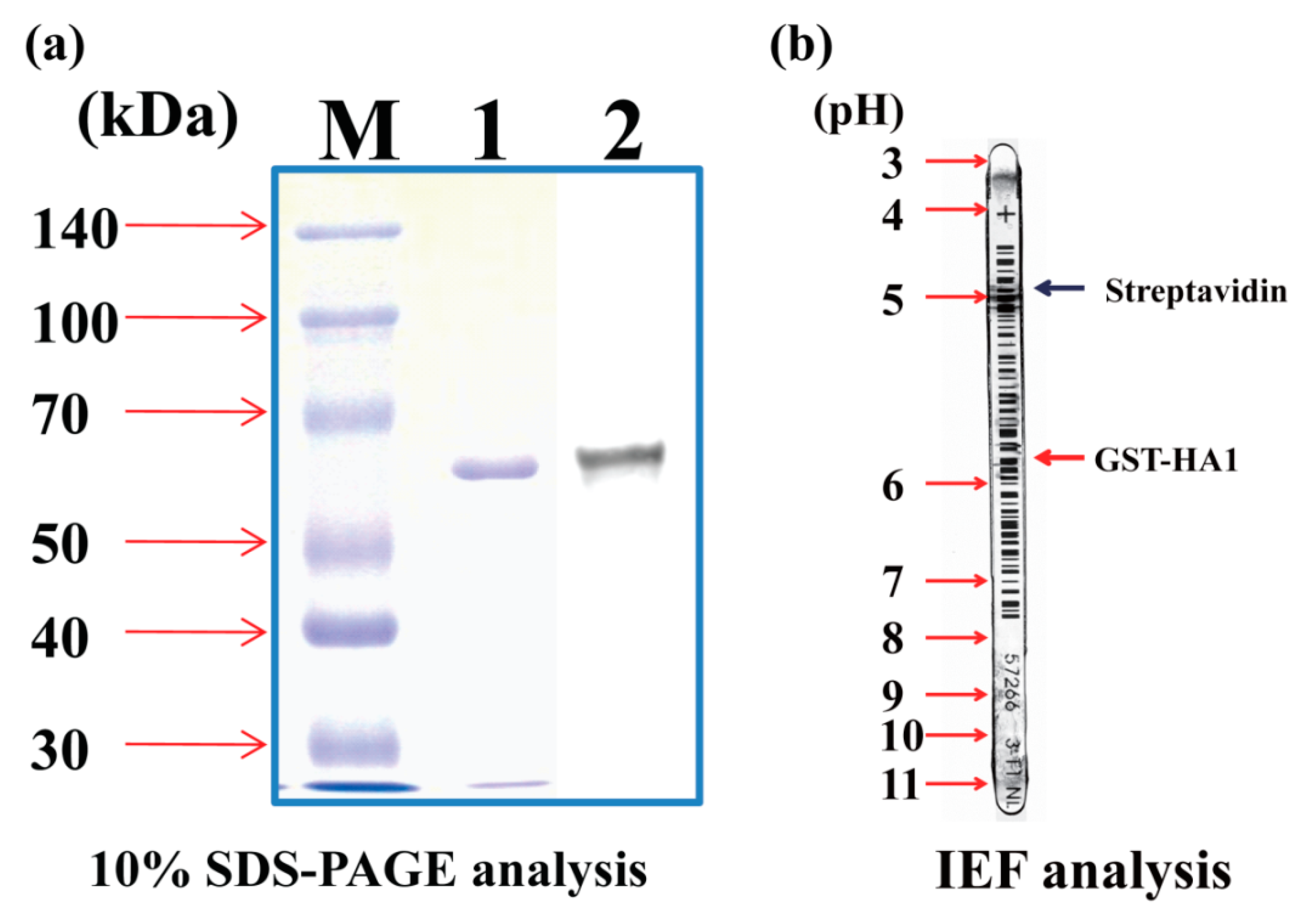
2.1. Purification of the GST-Tagged HA1 (HA1-GST)
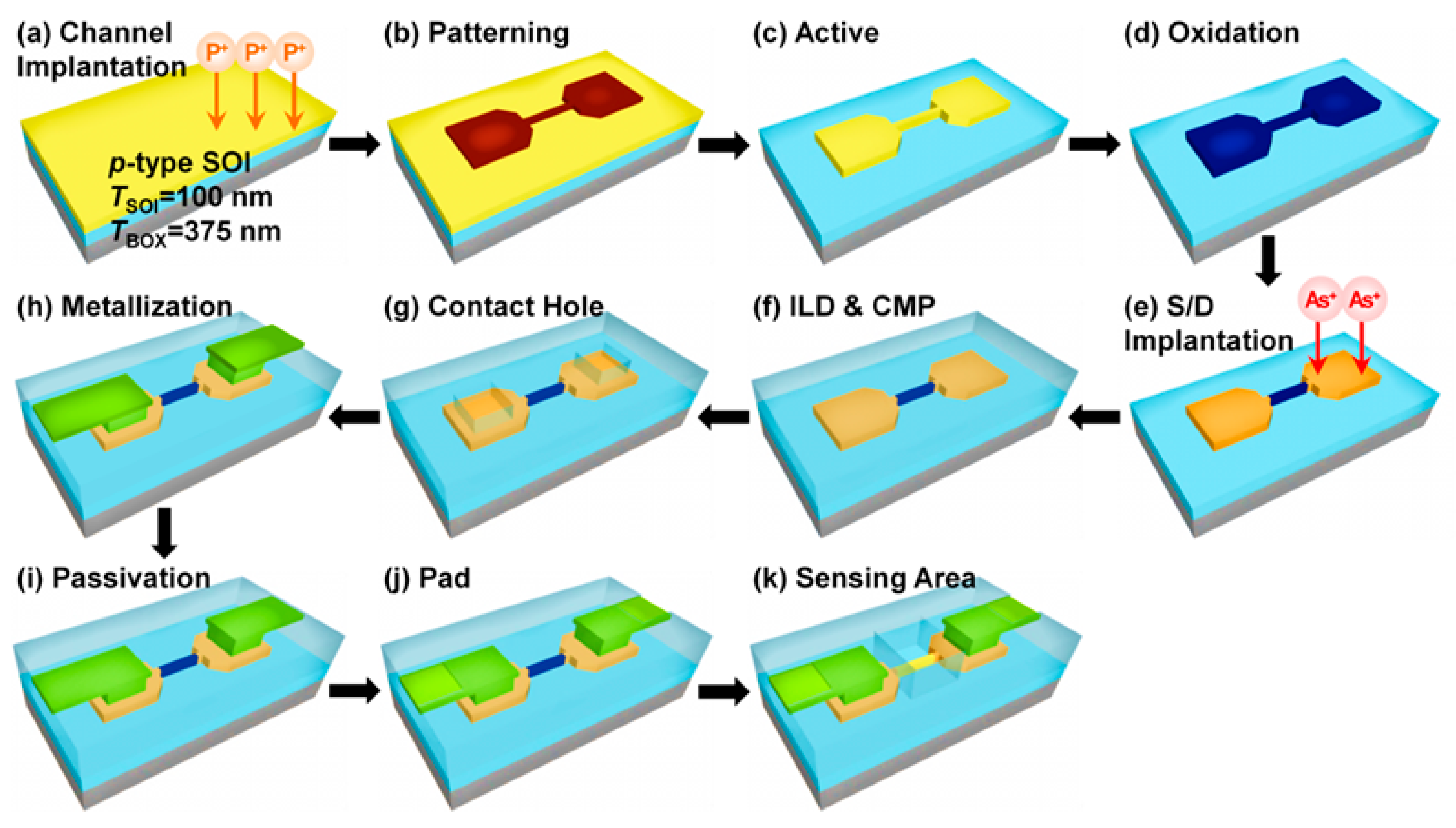
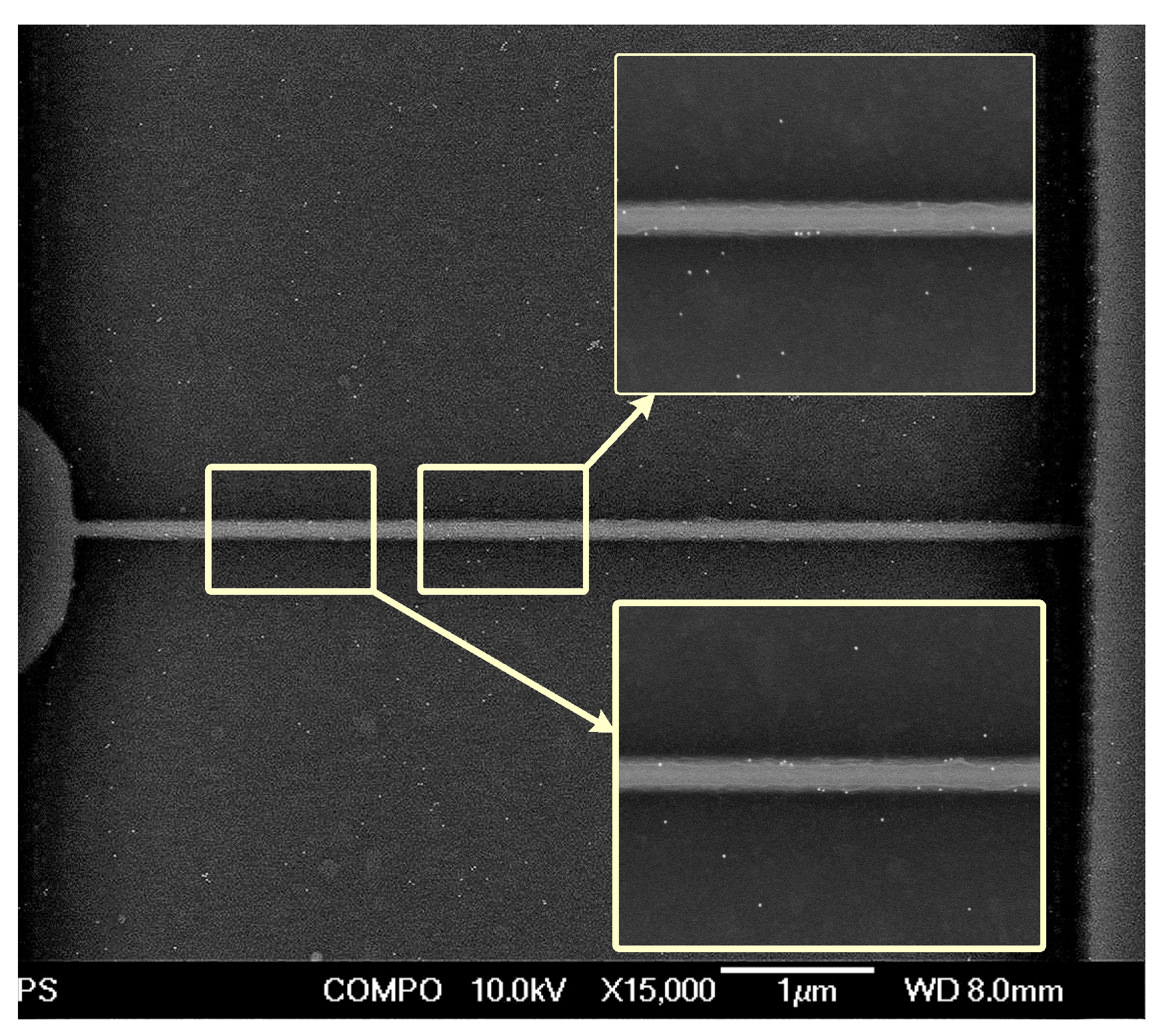
2.2. Fabrication and Electrical Characterization of the SiNW FETs
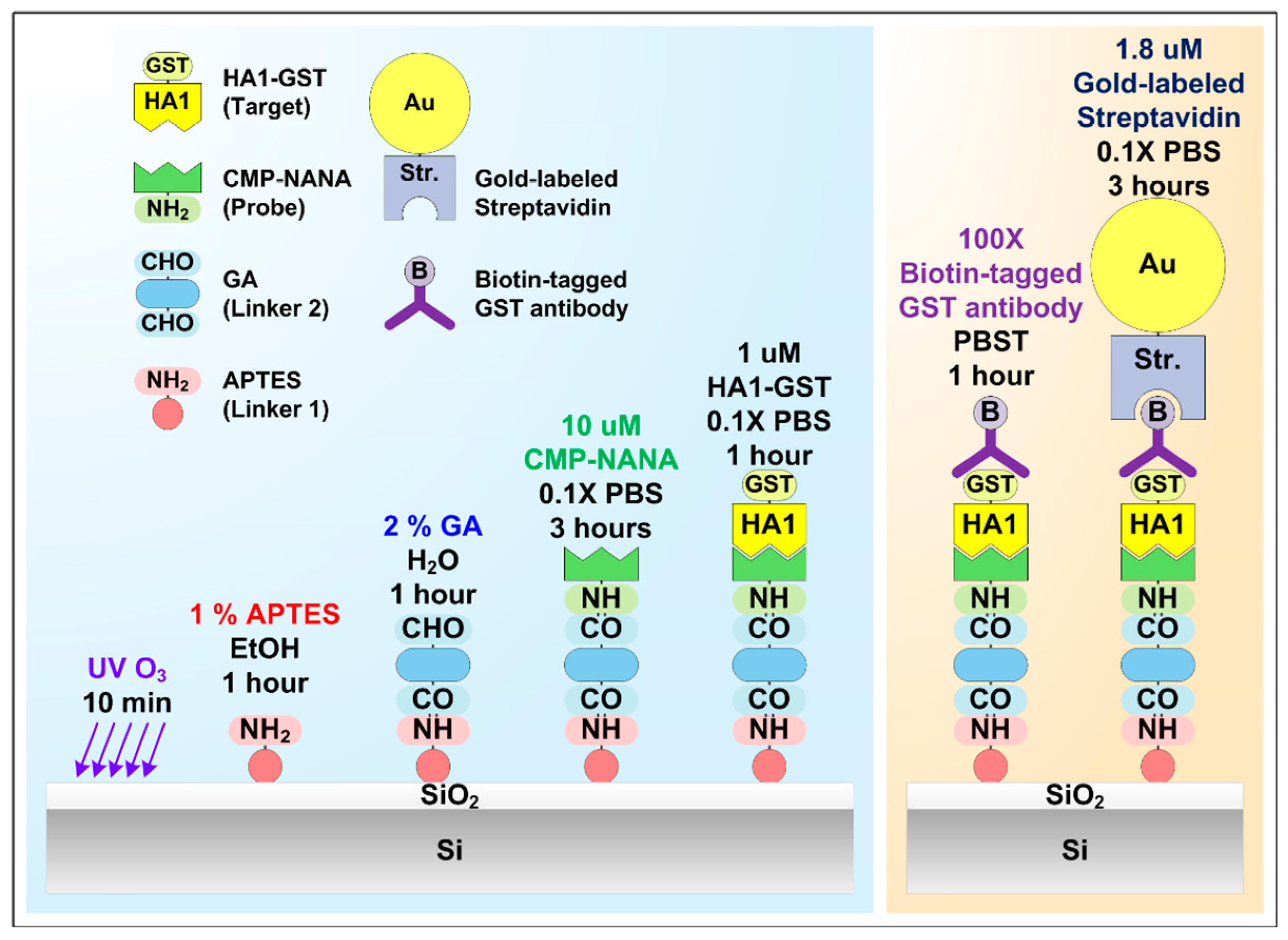
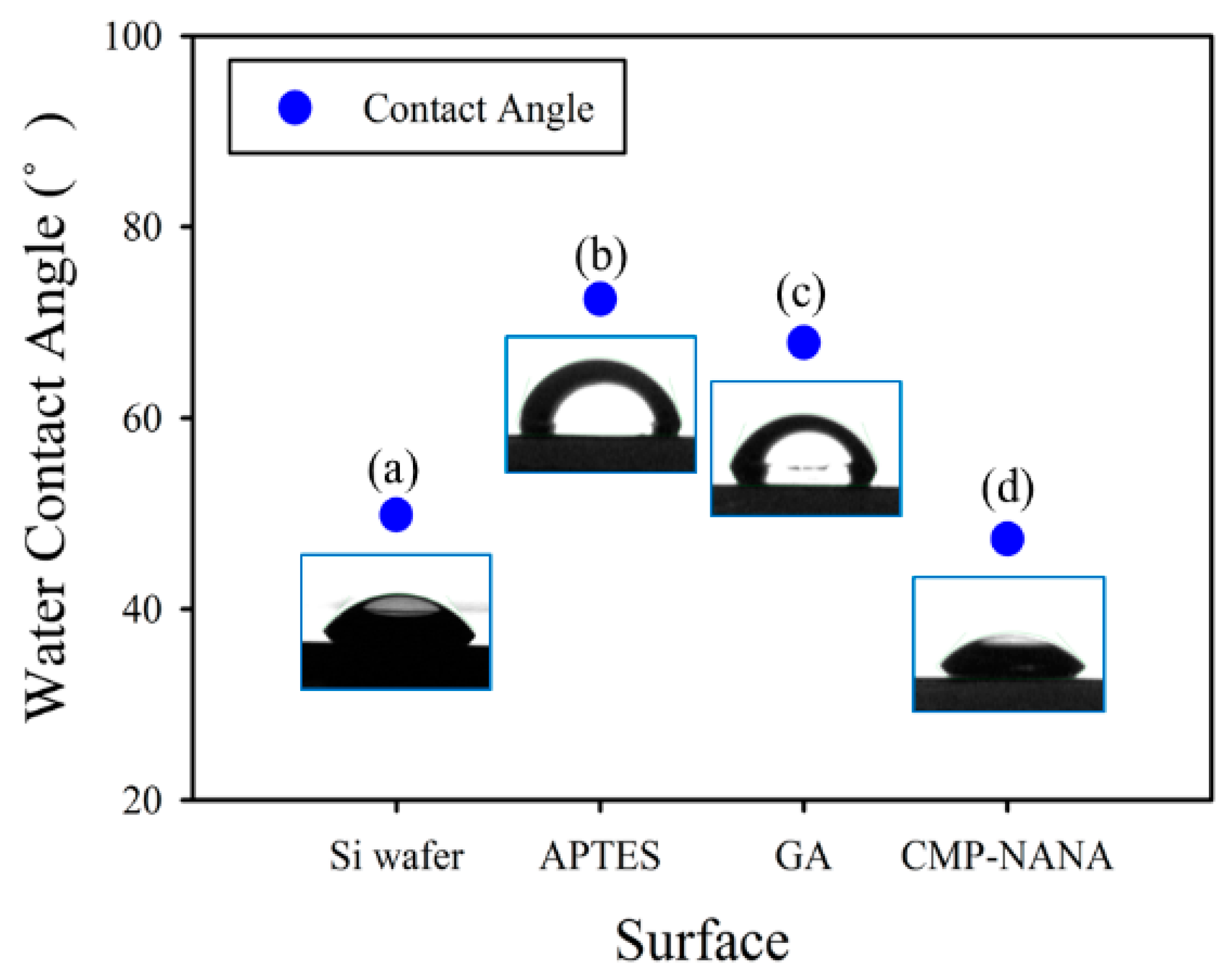
2.3. Surface Functionalization of the SiNWs
3. Result and Discussion
3.1. pH Sensing in the SiNW FET
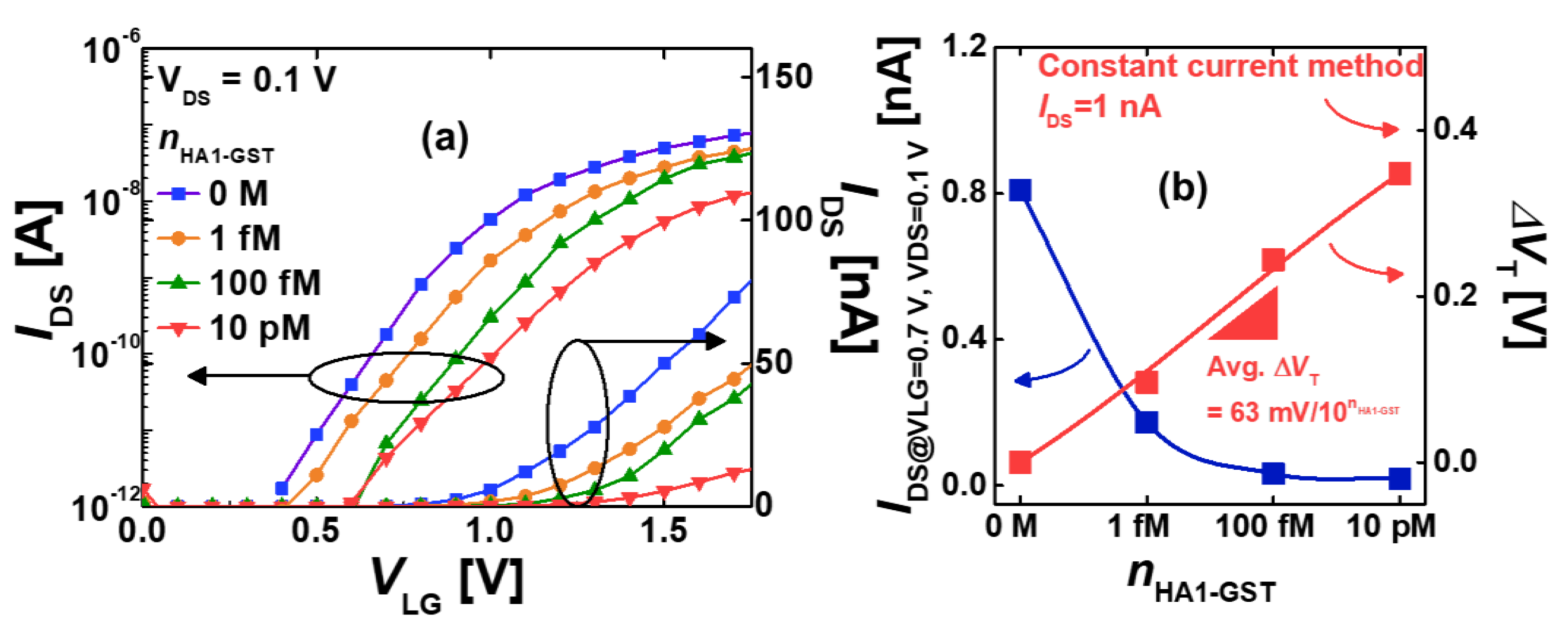
3.2. Electrical Detection of HA1-GST
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Committee, E.; Committee, T.; Zealand, N. Media Centre H1N1 in Post-Pandemic Period. Available online: https://www.who.int/mediacentre/news/statements/2010/h1n1_vpc_20100810/en/ (accessed on 16 October 2019).
- He, Q.; Velumani, S.; Du, Q.; Lim, C.W.; Ng, F.K.; Donis, R.; Kwang, J. Detection of H5 Avian Influenza Viruses by Antigen-Capture Enzyme-Linked Immunosorbent Assay Using H5-Specific Monoclonal Antibody. Clin. Vaccine Immunol. 2007, 14, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Bey, R.F.; Baarsch, M.J.; Simonson, R.R. ELISA Method for Detection of Influenza A Infection in Swine. J. Vet. Diagnostic Investig. 1993, 5, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Velumani, S.; Ho, H.-T.; He, F.; Musthaq, S.; Prabakaran, M.; Kwang, J. A Novel Peptide ELISA for Universal Detection of Antibodies to Human H5N1 Influenza Viruses. PLoS ONE 2011, 6, e20737. [Google Scholar] [CrossRef] [PubMed]
- Watcharatanyatip, K.; Boonmoh, S.; Chaichoun, K.; Songserm, T.; Woratanti, M.; Dharakul, T. Multispecies detection of antibodies to influenza A viruses by a double-antigen sandwich ELISA. J. Virol. Methods 2010, 163, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Dziąbowska, K.; Czaczyk, E.; Nidzworski, D. Detection Methods of Human and Animal Influenza Virus—Current Trends. Biosensors 2018, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Use of PCR-enzyme immunoassay for identification of influenza A virus matrix RNA in clinical samples negative for cultivable virus. J. Clin. Microbiol. 1994, 32, 623–628.
- Poon, L.L.M.; Chan, K.H.; Smith, G.J.; Leung, C.S.W.; Guan, Y.; Yuen, K.Y.; Peiris, J.S.M. Molecular Detection of a Novel Human Influenza (H1N1) of Pandemic Potential by Conventional and Real-Time Quantitative RT-PCR Assays. Clin. Chem. 2009, 55, 1555–1558. [Google Scholar] [CrossRef]
- Stone, B.; Burrows, J.; Schepetiuk, S.; Higgins, G.; Hampson, A.; Shaw, R.; Kok, T. Rapid detection and simultaneous subtype differentiation of influenza A viruses by real time PCR. J. Virol. Methods 2004, 117, 103–112. [Google Scholar] [CrossRef]
- Martinez-Sobrido, L.; Cadagan, R.; Steel, J.; Basler, C.F.; Palese, P.; Moran, T.M.; Garcia-Sastre, A. Hemagglutinin-Pseudotyped Green Fluorescent Protein-Expressing Influenza Viruses for the Detection of Influenza Virus Neutralizing Antibodies. J. Virol. 2010, 84, 2157–2163. [Google Scholar] [CrossRef]
- Munasinghe, V.R.N.; Corrie, J.E.T.; Kelly, G.; Martin, S.R. Fluorescent ligands for the hemagglutinin of influenza A: Synthesis and ligand binding assays. Bioconjug. Chem. 2007, 18, 231–237. [Google Scholar] [CrossRef]
- Bahgat, M.M.; Kutkat, M.A.; Nasraa, M.H.; Mostafa, A.; Webby, R.; Bahgat, I.M.; Ali, M.A.A. Characterization of an avian influenza virus H5N1 Egyptian isolate. J. Virol. Methods 2009, 159, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Y.; Lassiter, K.; Li, Y.; Hargis, B.; Tung, S.; Berghman, L.; Bottje, W. Interdigitated array microelectrode based impedance immunosensor for detection of avian influenza virus H5N1. Talanta 2009, 79, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.C.B.; Awazu, K.; Tominaga, J.; Kumar, P.K.R. Monitoring biomolecular interactions on a digital versatile disk: A BioDVD platform technology. ACS Nano 2008, 2, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Payungporn, S.; Chutinimitkul, S.; Chaisingh, A.; Damrongwantanapokin, S.; Buranathai, C.; Amonsin, A.; Theamboonlers, A.; Poovorawan, Y. Single step multiplex real-time RT-PCR for H5N1 influenza A virus detection. J. Virol. Methods 2006, 131, 143–147. [Google Scholar] [CrossRef]
- Chen, L.; Sheng, Z.; Zhang, A.; Guo, X.; Li, J.; Han, H.; Jin, M. Quantum-dots-based fluoroimmunoassay for the rapid and sensitive detection of avian influenza virus subtype H5N1. Luminescence 2010, 25, 419–423. [Google Scholar] [CrossRef]
- Gopinath, S.C.B.; Misono, T.S.; Kawasaki, K.; Mizuno, T.; Imai, M.; Odagiri, T.; Kumar, P.K.R. An RNA aptamer that distinguishes between closely related human influenza viruses and inhibits haemagglutinin-mediated membrane fusion. J. Gen. Virol. 2006, 87, 479–487. [Google Scholar] [CrossRef]
- Watanabe, M.; Nakagawa, N.; Ito, M.; Ihara, T. Sensitivity of rapid immunoassay for influenza A and B in the early phase of the disease. Pediatr. Int. 2009, 51, 211–215. [Google Scholar] [CrossRef]
- Akaishi, Y.; Matsumoto, T.; Harada, Y.; Hirayama, Y. Evaluation of the rapid influenza detection tests GOLD SIGN FLU and Quick Navi-Flu for the detection of influenza A and B virus antigens in adults during the influenza season. Int. J. Infect. Dis. 2016, 52, 55–58. [Google Scholar] [CrossRef]
- Ryu, S.W.; Suh, I.B.; Ryu, S.M.; Shin, K.S.; Kim, H.S.; Kim, J.; Uh, Y.; Yoon, K.J.; Lee, J.H. Comparison of three rapid influenza diagnostic tests with digital readout systems and one conventional rapid influenza diagnostic test. J. Clin. Lab. Anal. 2018, 32, 1–7. [Google Scholar] [CrossRef]
- Shao, Q.; Que, R.; Shao, M.; Zhou, Q.; Ma, D.D.D.; Lee, S.-T. Shape controlled flower-like silicon oxide nanowires and their pH response. Appl. Surf. Sci. 2011, 257, 5559–5562. [Google Scholar] [CrossRef]
- Kim, W.; Javey, A.; Vermesh, O.; Wang, Q.; Li, Y.; Dai, H. Hysteresis Caused by Water Molecules in Carbon Nanotube Field-Effect Transistors. Nano Lett. 2003, 3, 193–198. [Google Scholar] [CrossRef]
- Wang, L.; Bu, Y.; Li, L.; Ao, J.-P. Effect of thermal oxidation treatment on pH sensitivity of AlGaN/GaN heterostructure ion-sensitive field-effect transistors. Appl. Surf. Sci. 2017, 411, 144–148. [Google Scholar] [CrossRef]
- Liu, L.; Shao, J.; Li, X.; Zhao, Q.; Nie, B.; Xu, C.; Ding, H. High performance flexible pH sensor based on carboxyl-functionalized and DEP aligned SWNTs. Appl. Surf. Sci. 2016, 386, 405–411. [Google Scholar] [CrossRef]
- Kathiravan, D.; Huang, B.-R.; Saravanan, A.; Yeh, C.-J.; Leou, K.-C.; Lin, I.-N. Interfacial effects in ZnO nanotubes/needle-structured graphitic diamond nanohybrid for detecting dissolved acetone at room temperature. Appl. Surf. Sci. 2017, 426, 630–638. [Google Scholar] [CrossRef]
- Kharitonov, A.B.; Shipway, A.N.; Willner, I. An Au Nanoparticle/Bisbipyridinium Cyclophane-Functionalized Ion-Sensitive Field-Effect Transistor for the Sensing of Adrenaline. Anal. Chem. 1999, 71, 5441–5443. [Google Scholar] [CrossRef] [PubMed]
- Poghossian, A.; Schöning, M.J. Label-Free Sensing of Biomolecules with Field-Effect Devices for Clinical Applications. Electroanalysis 2014, 26, 1197–1213. [Google Scholar] [CrossRef]
- Hai, W.; Goda, T.; Takeuchi, H.; Yamaoka, S.; Horiguchi, Y.; Matsumoto, A.; Miyahara, Y. Specific Recognition of Human Influenza Virus with PEDOT Bearing Sialic Acid-Terminated Trisaccharides. ACS Appl. Mater. Interfaces 2017, 9, 14162–14170. [Google Scholar] [CrossRef]
- Shen, F.; Wang, J.; Xu, Z.; Wu, Y.; Chen, Q.; Li, X.; Jie, X.; Li, L.; Yao, M.; Guo, X.; et al. Rapid Flu Diagnosis Using Silicon Nanowire Sensor. Nano Lett. 2012, 12, 3722–3730. [Google Scholar] [CrossRef]
- Kao, C.H.; Chen, H.; Huang, C.-Y. Effects of Ti addition and annealing on high-k Gd2O3 sensing membranes on polycrystalline silicon for extended-gate field-effect transistor applications. Appl. Surf. Sci. 2013, 286, 328–333. [Google Scholar] [CrossRef]
- Bunjongpru, W.; Sungthong, A.; Porntheeraphat, S.; Rayanasukha, Y.; Pankiew, A.; Jeamsaksiri, W.; Srisuwan, A.; Chaisriratanakul, W.; Chaowicharat, E.; Klunngien, N.; et al. Very low drift and high sensitivity of nanocrystal-TiO2 sensing membrane on pH-ISFET fabricated by CMOS compatible process. Appl. Surf. Sci. 2013, 267, 206–211. [Google Scholar] [CrossRef]
- Khanna, V.K.; Oelßner, W.; Guth, U. Interfacial and adhesional aspects in polyurethane (PUR) membrane coating on Si3N4 surface of ISFET gate for REFET fabrication. Appl. Surf. Sci. 2009, 255, 7798–7804. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Choi, S.-J.; Han, J.-W.; Park, T.J.; Lee, S.Y.; Choi, Y.-K. Double-Gate Nanowire Field Effect Transistor for a Biosensor. Nano Lett. 2010, 10, 2934–2938. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Zheng, G.; Hayden, O.; Lakadamyali, M.; Zhuang, X.; Lieber, C.M. Electrical detection of single viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017–14022. [Google Scholar] [CrossRef] [PubMed]
- Kao, L.T.-H.; Shankar, L.; Kang, T.G.; Zhang, G.; Tay, G.K.I.; Rafei, S.R.M.; Lee, C.W.H. Multiplexed detection and differentiation of the DNA strains for influenza A (H1N1 2009) using a silicon-based microfluidic system. Biosens. Bioelectron. 2011, 26, 2006–2011. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Hung, C.-H.; Hsiao, C.-Y.; Lin, H.-C.; Ko, F.-H.; Yang, Y.-S. Poly-silicon nanowire field-effect transistor for ultrasensitive and label-free detection of pathogenic avian influenza DNA. Biosens. Bioelectron. 2009, 24, 3019–3024. [Google Scholar] [CrossRef]
- Gao, A.; Lu, N.; Dai, P.; Li, T.; Pei, H.; Gao, X.; Gong, Y.; Wang, Y.; Fan, C. Silicon-Nanowire-Based CMOS-Compatible Field-Effect Transistor Nanosensors for Ultrasensitive Electrical Detection of Nucleic Acids. Nano Lett. 2011, 11, 3974–3978. [Google Scholar] [CrossRef]
- Takeda, S.; Sbagyo, A.; Sakoda, Y.; Ishii, A.; Sawamura, M.; Sueoka, K.; Kida, H.; Mukasa, K.; Matsumoto, K. Application of carbon nanotubes for detecting anti-hemagglutinins based on antigen–antibody interaction. Biosens. Bioelectron. 2005, 21, 201–205. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Hong, S.; Lee, D.; Cui, T.; Goyal, S.M. Carbon nanotube based sensors for the detection of viruses. Sensors Actuators B Chem. 2011, 155, 67–74. [Google Scholar] [CrossRef]
- Tam, P.D.; Van Hieu, N.; Chien, N.D.; Le, A.-T.; Anh Tuan, M. DNA sensor development based on multi-wall carbon nanotubes for label-free influenza virus (type A) detection. J. Immunol. Methods 2009, 350, 118–124. [Google Scholar] [CrossRef]
- Hideshima, S.; Hinou, H.; Ebihara, D.; Sato, R.; Kuroiwa, S.; Nakanishi, T.; Nishimura, S.-I.; Osaka, T. Attomolar Detection of Influenza A Virus Hemagglutinin Human H1 and Avian H5 Using Glycan-Blotted Field Effect Transistor Biosensor. Anal. Chem. 2013, 85, 5641–5644. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.; Yoon, J.-S.; Rim, T.; Meyyappan, M.; Baek, C.-K. Optimization of Signal to Noise Ratio in Silicon Nanowire ISFET Sensors. IEEE Sens. J. 2017, 17, 2792–2796. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhm, M.; Lee, J.-M.; Lee, J.; Lee, J.H.; Choi, S.; Park, B.-G.; Kim, D.M.; Choi, S.-J.; Mo, H.-S.; Jeong, Y.-J.; et al. Ultrasensitive Electrical Detection of Hemagglutinin for Point-of-Care Detection of Influenza Virus Based on a CMP-NANA Probe and Top-Down Processed Silicon Nanowire Field-Effect Transistors. Sensors 2019, 19, 4502. https://doi.org/10.3390/s19204502
Uhm M, Lee J-M, Lee J, Lee JH, Choi S, Park B-G, Kim DM, Choi S-J, Mo H-S, Jeong Y-J, et al. Ultrasensitive Electrical Detection of Hemagglutinin for Point-of-Care Detection of Influenza Virus Based on a CMP-NANA Probe and Top-Down Processed Silicon Nanowire Field-Effect Transistors. Sensors. 2019; 19(20):4502. https://doi.org/10.3390/s19204502
Chicago/Turabian StyleUhm, Mihee, Jin-Moo Lee, Jieun Lee, Jung Han Lee, Sungju Choi, Byung-Gook Park, Dong Myong Kim, Sung-Jin Choi, Hyun-Sun Mo, Yong-Joo Jeong, and et al. 2019. "Ultrasensitive Electrical Detection of Hemagglutinin for Point-of-Care Detection of Influenza Virus Based on a CMP-NANA Probe and Top-Down Processed Silicon Nanowire Field-Effect Transistors" Sensors 19, no. 20: 4502. https://doi.org/10.3390/s19204502
APA StyleUhm, M., Lee, J.-M., Lee, J., Lee, J. H., Choi, S., Park, B.-G., Kim, D. M., Choi, S.-J., Mo, H.-S., Jeong, Y.-J., & Kim, D. H. (2019). Ultrasensitive Electrical Detection of Hemagglutinin for Point-of-Care Detection of Influenza Virus Based on a CMP-NANA Probe and Top-Down Processed Silicon Nanowire Field-Effect Transistors. Sensors, 19(20), 4502. https://doi.org/10.3390/s19204502




