Analysis on the Live Coral Cover around Weizhou Island Using MODIS Data
Abstract
:1. Introduction
2. Materials and Methods
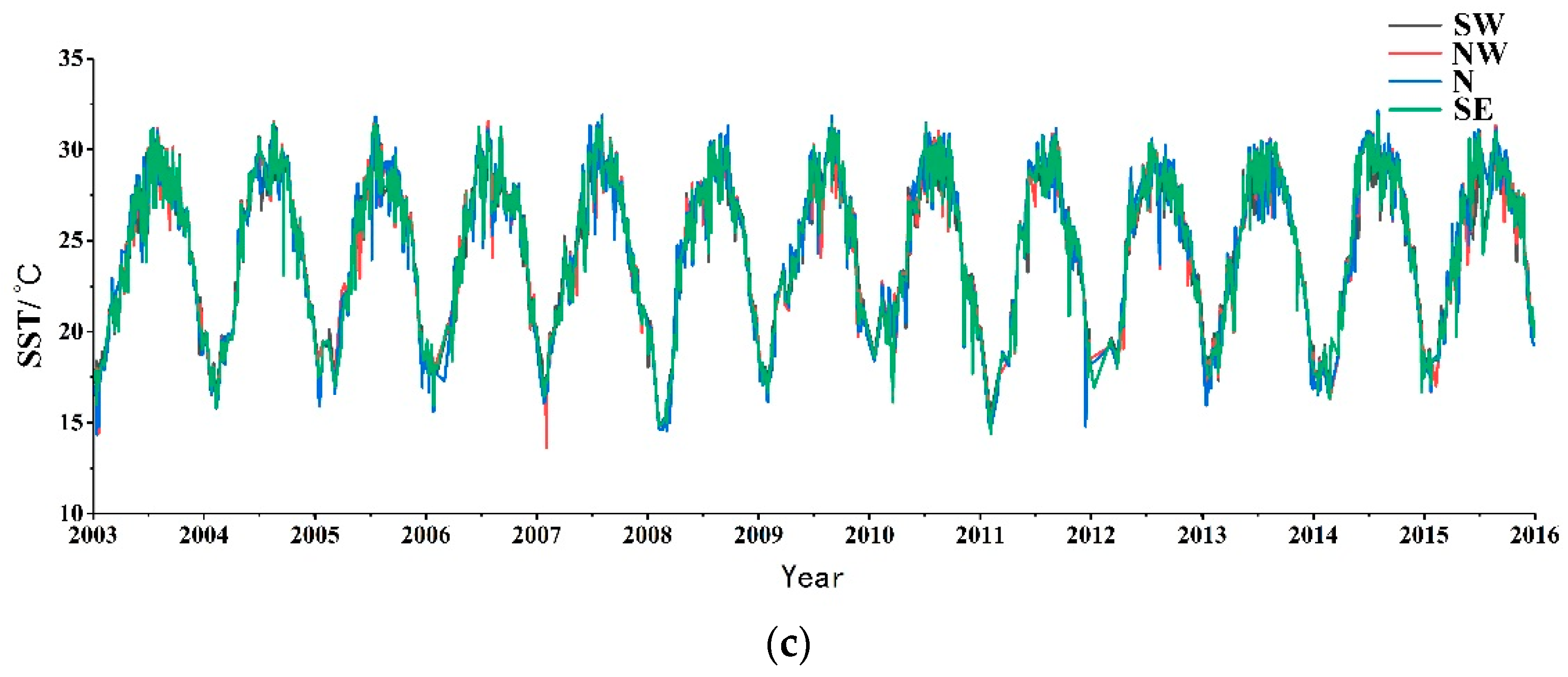
2.1. Study Area and Experimental Data
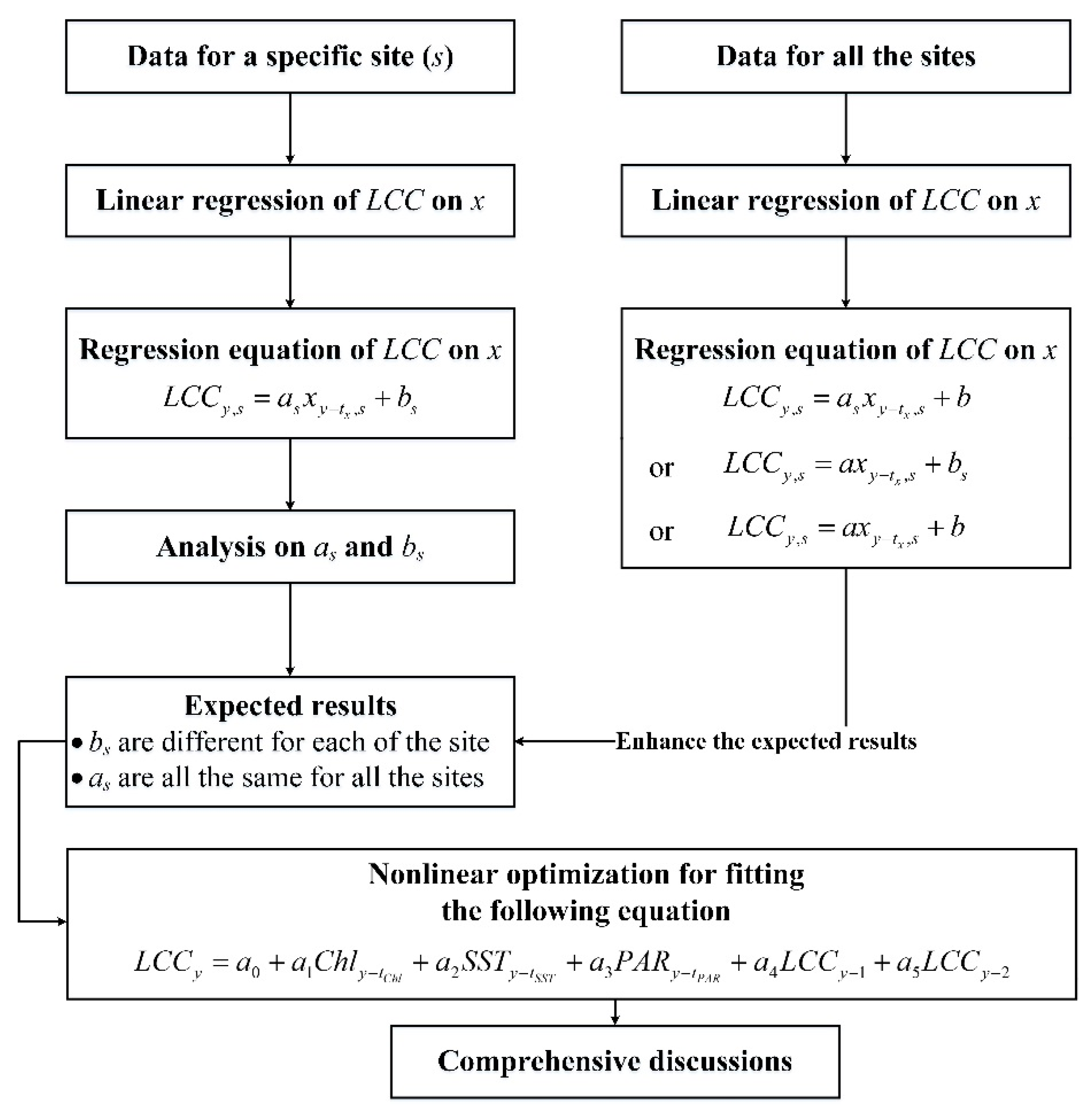
2.2. Processing Methods
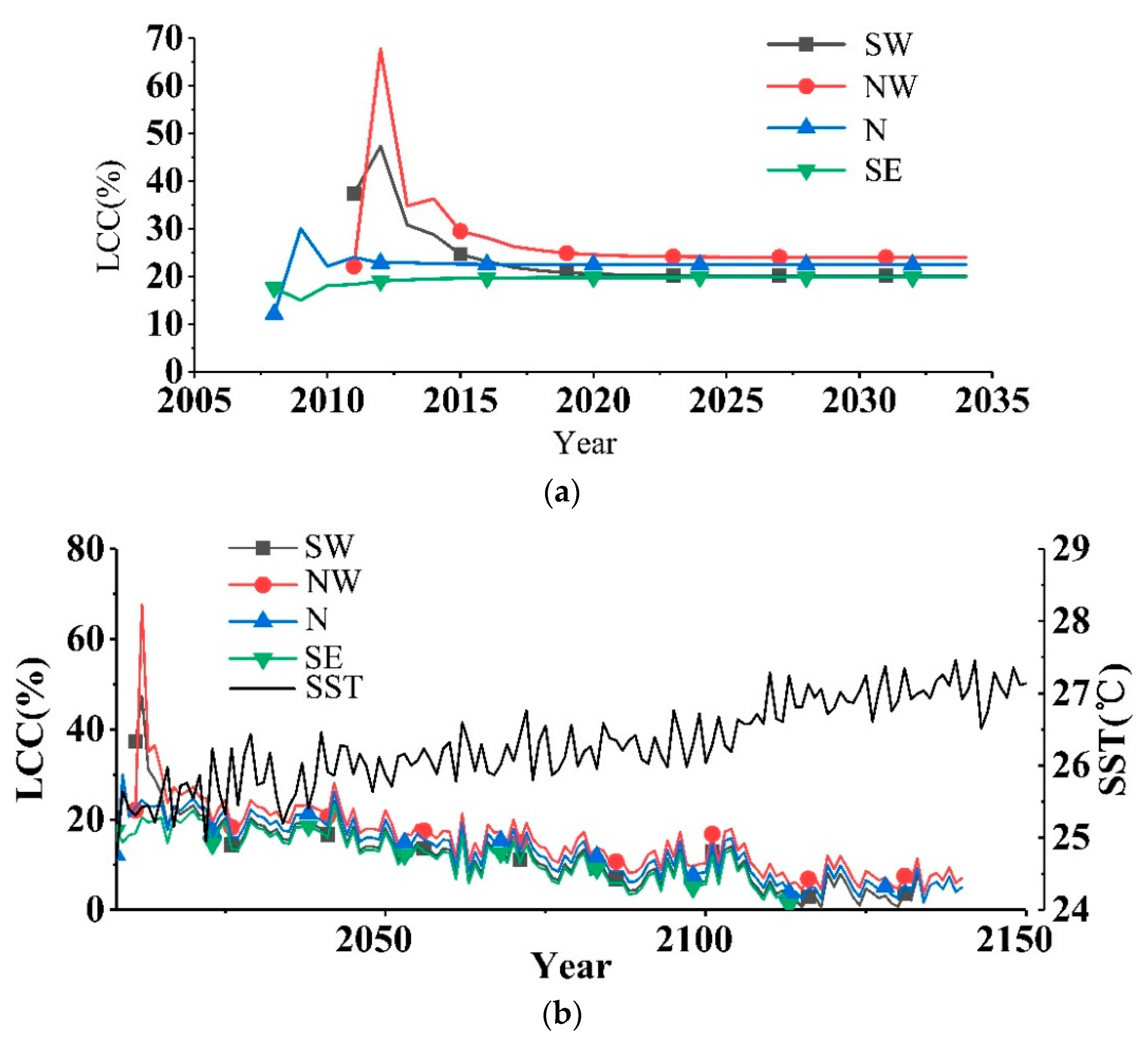
3. Results
4. Discussion
4.1. Supplemental Discussion on the Effectiveness
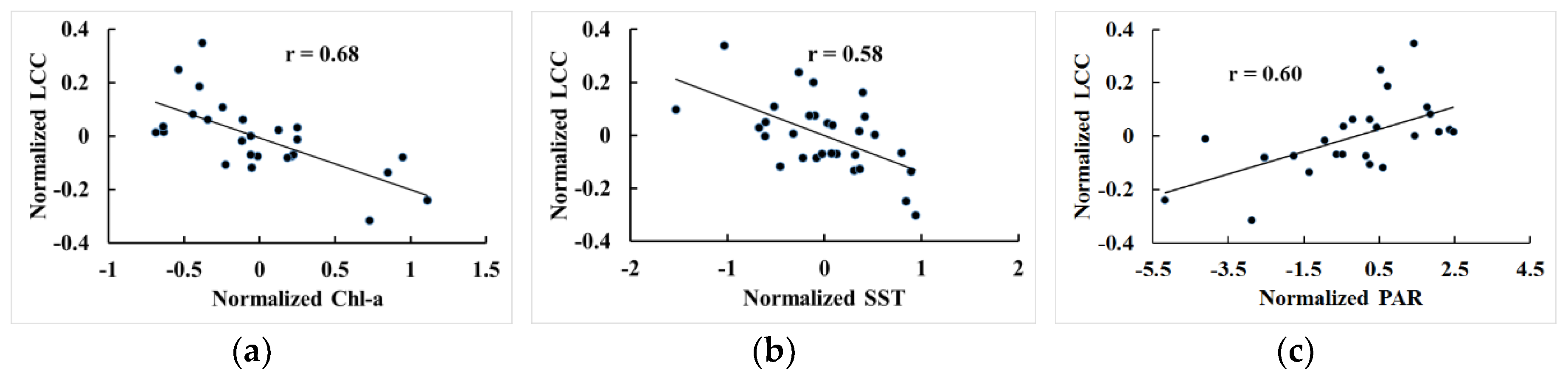
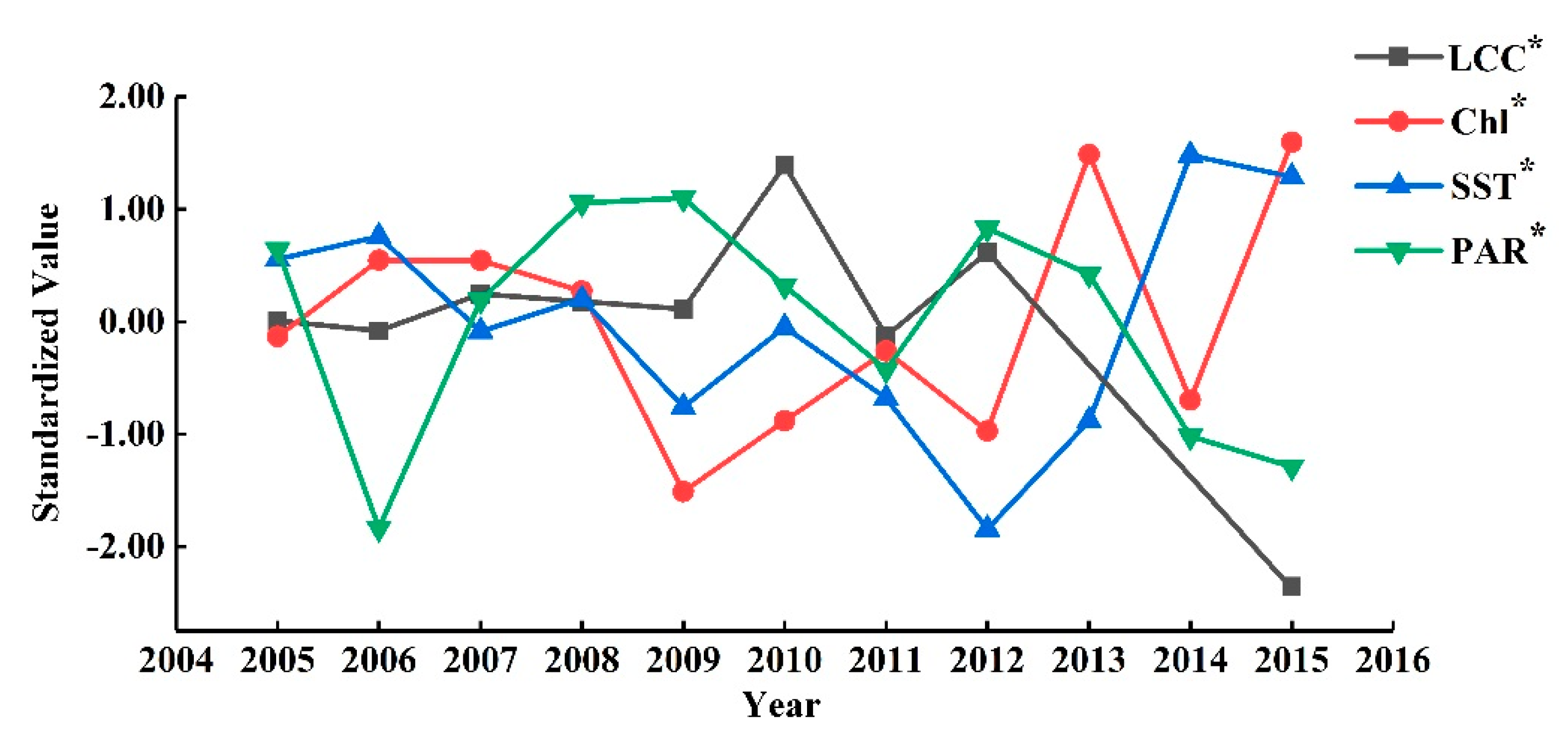
4.2. Discussion on the Responses of the LCCs to the Environments
4.3. Discussion on the Changes of the LCCs to the Environments
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liao, B.; Liu, L.; Liu, C. Research Status and Prospects of Xuwen Coral Reefs. J. Guangdong Ocean Univ. 2011, 31, 91–96. [Google Scholar]
- Carpenter, K.E.; Abrar, M.; Aeby, G.; Aronson, R.B.; Banks, S.; Bruckner, A.; Chiriboga, A.; Cortés, J.; Delbeek, J.C.; DeVantier, L.; et al. One-Third of Reef-Building Corals Face Elevated Extinction Risk from Climate Change and Local Impacts. Sci. 2008, 321, 560–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K. Coral reefs in the South China Sea: Their response to and records on past environmental changes. Sci. Chin. Earth Sci. 2012, 55, 1217–1229. [Google Scholar] [CrossRef]
- Strong, A.; Liu, G.; Skirving, W.; Markeakin, C. NOAA’s Coral Reef Watch program from satellite observations. Ann. GIS 2011, 17, 83–92. [Google Scholar] [CrossRef]
- Lesser, M.P. Coral Bleaching: Causes and Mechanisms. Coral Reefs: An Ecosystem in Transition; Springer: Berlin/Heidelberg, Germany, 2011; pp. 405–419. [Google Scholar]
- Douglas, A.E. Coral bleaching—how and why? Mar. Pollut. Bull. 2003, 46, 385–392. [Google Scholar] [CrossRef]
- Shaazia, M.; Scott, H.; Rajindra, M.; Ricardo, C. Performance Evaluation of CRW Reef-Scale and Broad-Scale SST-Based Coral Monitoring Products in Fringing Reef Systems of Tobago. Remote Sens.-Basel 2015, 8, 12. [Google Scholar] [Green Version]
- Liu, G.; Heron, S.; Eakin, C.M.; De La Cour, J.; Geiger, E.; Skirving, W.; Burgess, T.; Strong, A. NOAA Coral Reef Watch’s Next-Generation 5 km Satellite Coral Bleaching Thermal Stress Monitoring. News Int. Soc. Reef Stud. 2014, 29, 27–29. [Google Scholar]
- Maina, J.; Venus, V.; McClanahan, T.R.; Ateweberhan, M. Modelling susceptibility of coral reefs to environmental stress using remote sensing data and GIS models. Ecol. Model. 2008, 212, 180–199. [Google Scholar] [CrossRef]
- Barnes, B.B.; Hallock, P.; Hu, C.; Muller-Karger, F.; Palandro, D.; Walter, C.; Zepp, R. Prediction of coral bleaching in the Florida Keys using remotely sensed data. Coral Reefs 2015, 34, 491–503. [Google Scholar] [CrossRef]
- Qi, S.; Meixia, Z.; Lingying, H.; Hongqiang, Y.; Huiling, Z. Human Activities and Impacts on Coral Reef at the Luhuitou Fringing Reef, Sanya. Trop. Geogr. 2010, 30, 486–490. [Google Scholar]
- Wu, Z.; Wang, D.; Ye, C.; Li, Y.; Chen, M.; Chen, C. Variation tendency and analysis of cause of coral in Sanya. Mar. Environ. Sci. 2012, 31, 682–685. [Google Scholar]
- Zhou, H.; Yao, X.; Li, L.; Geng, T.; Zhang, Y. Scleractinian coral community structure and distribution in the coastal waters surrounding Hainan Island. Biodivers. Sci. 2017, 25, 1123–1130. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Wang, W.; Yu, K.; Wang, Y.; Huang, X.; Huang, R.; Liao, Z.; Xu, S.; Chen, X. Rapid decline of a relatively high latitude coral assemblage at Weizhou Island, northern South China Sea. Biodivers. Conserv. 2019, 1–25. [Google Scholar] [CrossRef]
- Zhao, M.; Yu, K.; Qiaomin, Z.; Shi, Q. Evaluating the sustainability of coral reefs in Sanya of Hainan Island using marine ecological footprint. J. Trop. Oceanogr. 2011, 30, 74–80. [Google Scholar]
- Wang, W.; Yu, K.; Wang, Y. A Review on the Research of Coral Reefs in the Weizhou Island, Beibu Gulf. Trop. Geogr. 2016, 36, 72–79. [Google Scholar]
- Wang, X.; Li, G. The Status and Prospect of Researches on Coral Reef in Weizhou Island. J. Guangxi Academy Sci. 2009, 25, 72–75. [Google Scholar]
- Chen, T.; Li, S.; Yu, K.; Zheng, Z.; Wang, L.; Chen, T. Increasing temperature anomalies reduce coral growth in the Weizhou Island, northern South China Sea. Estuarine, Coast. Shelf Sci. 2013, 130, 121–126. [Google Scholar] [CrossRef]
- Budd, A.F.; Fukami, H.; Smith, N.D.; Knowlton, N. Taxonomic classification of the reef coral family Mussidae (Cnidaria: Anthozoa: Scleractinia). Zool. J. Linn. Soc. 2012, 166, 465–529. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Zhang, Y.; Lian, J.; Li, X.; You, F.; Yang, J.; Lei, X.; Zhang, C. Structure and diversity of scleractinia coral communities along the west seashore of Xuwen County. Biodivers. Sci. 2011, 19, 505–510. [Google Scholar]
- Zhao, M.; Yu, K.; Zhang, Q.; Shi, Q.; Price, G.J. Long-term Decline of a Fringing Coral Reef in the Northern South China Sea. J. Coast. Res. 2012, 28, 1088–1099. [Google Scholar]
- Zhao, M.; Yu, K.; Shi, Q.; Yang, H.; Riegl, B.; Zhang, Q.; Yan, H.; Chen, T.; Liu, G.; Lin, Z. Comparison of coral diversity between big and small atolls: A case study of Yongle atoll and Lingyang reef, Xisha Islands, central of South China Sea. Biodivers. Conserv. 2017, 26, 1143–1159. [Google Scholar] [CrossRef]
- Huang, R.; Yu, K.; Wang, Y.; Wang, J.; Mu, L.; Wang, W. Bathymetry of the Coral Reefs of Weizhou Island Based on Multispectral Satellite Images. Remote Sens. 2017, 9, 750. [Google Scholar] [CrossRef]
- Huang, R.; Yu, K.; Wang, Y.; Wang, W.; Mu, L.; Wang, J. Method to design a live coral cover sensitive index for multispectral satellite images. Opt. Express 2018, 26, A374–A397. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhao, M.; Liu, B.; Zhang, C.; Liang, Q. Ecological Situation of Coral Reefs in the Weizhou Island Based on Reef Check. Trop. Geogr. 2016, 36, 66–71. [Google Scholar]
- Hodgson, G.; Kiene, W.; Mihaly, J.; Liebeler, J.; Shuman, C.; Maun, L. Reef Check Instruction Manual: A Guide to Reef Check Coral Reef Monitoring; Reef Check Foundation: Los Angeles, CA, USA, 2004. [Google Scholar]
- Huang, H.; Ma, B.; Lian, J.; Yang, J.; Liang, W. Status and conservation strategies of the coral reef in Weizhou Island, Guangxi. Trop. Geogr. 2009, 29, 307–312. [Google Scholar]
- English, S.; Wilkinson, C.; Baker, V. Survey Manual for Tropical Marine Resources; English, S., Wilkinson, C., Baker, V., Eds.; Australian Institute of Marine Science: Townsville, Australia, 1997.
- Zhao, M.; Yu, K.; Shi, Q.; Yang, H.; Riegl, B.; Zhang, Q.; Yan, H.; Chen, T.; Liu, G.; Lin, Z. The coral communities of Yongle atoll: Status, threats and conservation significance for coral reefs in South China Sea. Mar. Freshw. Res. 2016, 67, 1888–1896. [Google Scholar] [CrossRef]
- Liang, W.; Li, G.Z.; Fan, H.Q.; Wang, X.; Nong, H.Q.; Huang, H.; Li, X.B.; Lan, G.B. Species Composition and Distribution of Coral on Weizhou Island Guangxi. Guangxi Sci. 2010, 17, 93–96. [Google Scholar]
- Zhang, Q.; Shi, Q.; Chen, G.; Fong, T.C.W.; Wong, D.C.C.; Huang, H.; Wang, H.; Zhao, M. Status monitoring and health assessment of Luhuitou fringing reef of Sanya, Hainan, China. Chin. Sci. Bull. 2006, 51, 81–88. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, J.; Fu, L.; Zhou, C.; Haffner, D.G. Temporal and Spatial Variation of Nutrients and Chlorophyll-a, and Their Relationship in Pengxi River Backwater Area, Three Gorges Reservoir. Environ. Sci. 2015, 36, 2061–2069. [Google Scholar]
- D Angelo, C.; Wiedenmann, J. Impacts of nutrient enrichment on coral reefs: New perspectives and implications for coastal management and reef survival. Curr. Opin. Env. Sust. 2014, 7, 82–93. [Google Scholar] [CrossRef]
- Szmant, A.M. Nutrient enrichment on coral reefs: Is it a major cause of coral reef decline? Estuaries 2002, 25, 743–766. [Google Scholar] [CrossRef]
- Li, Y.; Yu, K.; Wang, Y.; Guo, J.; Huang, X.; Pei, J.; Luo, Y. Distribution Characteristics of Surface Seawater Nutrients in Summer around Luhuitou Reef in Sanya. Trop. Geogr. 2017, 37, 708–717. [Google Scholar]
- Li, S.; Yu, K. Recent development in coral reef bleaching research. Acta Ecol. Sin. 2007, 27, 2059–2069. [Google Scholar]
- Fabricius, K.E. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Pollut. Bull. 2005, 50, 125–146. [Google Scholar] [CrossRef]
- Voss, J.D.; Richardson, L.L. Nutrient enrichment enhances black band disease progression in corals. Coral Reefs 2006, 25, 569–576. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, Z.; Mo, S.; Tang, C.; Zhou, X. Bioerosion In Porites Corals at Weizhou Island and Its Environmental Significance. Chin. Sci. Bull. 2013, 58, 1574–1582. [Google Scholar]
- Aronson, R.B.; Bruno, J.F.; Precht, W.F.; Glynn, P.W.; Harvell, C.D.; Kaufman, L.; Rogers, C.S.; Shinn, E.A.; Valentine, J.F. Causes of coral reef degradation. Science 2003, 302, 1502–1504. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zheng, X.; Lan, W.; Shi, X.; Li, T. Variations of Nutrients Concentration in Surface Seawater in Adjacent Area of Weizhou Island in Recent 10 Years. J. Appl. Oceanogr. 2015, 34, 65–72. [Google Scholar]
- Chen, T.; Zheng, Z.; Mo, S.; Zhou, X.; Chen, T. Variation of Skeletal Extension Rate for Porites Corals around Weizhou Island in Response to Global Warming and Increase of Extreme Events. J. Trop. Oceanogr. 2013, 32, 79–84. [Google Scholar]
- Zhou, J.; Shi, Q.; Yu, K. Exploration of factors that influence photosynthetic efficiency of symbiotic zooxanthellae of scleractinian corals in a Sanya fringing reef. J. Trop. Oceanogr. 2014, 33, 81–89. [Google Scholar]
- Wu, H. Study on the Responses of the Growth and Photosynthetic Functions of Marine Macroalgae to Diverse Light Environments. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2016. [Google Scholar]
- Liao, Z.; Yu, K.; Wang, Y. Review on the Effect of Macroalgae on the Degeneration of Coral Reefs. Acta Ecol. Sin. 2016, 36, 6687–6695. [Google Scholar]
- Zheng, Z. Studies on Water Temperature Variations in Weizhou Island & Coral Bleaching. Ph.D. Thesis, Ocean University of China, Qingdao, Chnia, 2013. [Google Scholar]
- Zhang, W.; Zhou, G.C.; Gao, X.Y. Analyses of Global Sea Surface Temperature Variance and Correlation. Period. Ocean Univ. Chin. 2012, 42, 17–22. [Google Scholar]
- Riegl, B.; Piller, W.E. Possible refugia for reefs in times of environmental stress. Acta Diabetol. 2003, 92, 520–531. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral Reefs Under Rapid Climate Change and Ocean Acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [Green Version]
- Veron, J.; Hoegh-Guldberg, O.; Lenton, T.; Lough, J.; Obura, D.; Pearce-Kelly, P.; Sheppard, C.; Spalding, M.; Stafford-Smith, M.; Rogers, A.; et al. The coral reef crisis: The critical importance of <350 ppm CO2. Mar. Pollut. Bull. 2009, 58, 1428–1436. [Google Scholar] [CrossRef]


| Site | Pair ID | Year | LCC (%) | ||||
|---|---|---|---|---|---|---|---|
| y | y-1 | y-2 | LCCy | LCCy-1 | LCCy-2 | ||
| South-west | 1 | 2006 | 2005 | 2004 | 37.9 | 39.1 | 24.3 |
| 2 | 2007 | 2006 | 2005 | 42.3 | 37.9 | 39.1 | |
| 3 | 2008 | 2007 | 2006 | 41.4 | 42.3 | 37.9 | |
| 4 | 2009 | 2008 | 2007 | 40.5 | 41.4 | 42.3 | |
| 5 | 2010 | 2009 | 2008 | 57.7 | 40.5 | 41.4 | |
| 6 | 2011 | 2010 | 2009 | 37.32 | 57.7 | 40.5 | |
| 7 | 2012 | 2011 | 2010 | 47.3 | 37.32 | 57.7 | |
| North-west | 8 | 2009 | 2008 | 2007 | 34.4 | 25.3 | 25.3 |
| 9 | 2010 | 2009 | 2008 | 57.7 | 34.4 | 25.3 | |
| 10 | 2011 | 2010 | 2009 | 22.1 | 57.7 | 34.4 | |
| 11 | 2012 | 2011 | 2010 | 67.7 | 22.1 | 57.7 | |
| North | 12 | 2009 | 2008 | 2007 | 30.0 | 12.21 | 12.21 |
| South-east | 13 | 2008 | 2007 | 2006 | 17.58 | 17.58 | 3.3 |
| 14 | 2009 | 2008 | 2007 | 15.0 | 17.58 | 17.58 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Zhang, H.; Yu, K. Analysis on the Live Coral Cover around Weizhou Island Using MODIS Data. Sensors 2019, 19, 4309. https://doi.org/10.3390/s19194309
Huang R, Zhang H, Yu K. Analysis on the Live Coral Cover around Weizhou Island Using MODIS Data. Sensors. 2019; 19(19):4309. https://doi.org/10.3390/s19194309
Chicago/Turabian StyleHuang, Rongyong, Huiya Zhang, and Kefu Yu. 2019. "Analysis on the Live Coral Cover around Weizhou Island Using MODIS Data" Sensors 19, no. 19: 4309. https://doi.org/10.3390/s19194309
APA StyleHuang, R., Zhang, H., & Yu, K. (2019). Analysis on the Live Coral Cover around Weizhou Island Using MODIS Data. Sensors, 19(19), 4309. https://doi.org/10.3390/s19194309





