Measurement System for Lossy Capacitive Sensors: Application to Edible Oils Quality Assessment
Abstract
:1. Introduction
2. Reference Measurements
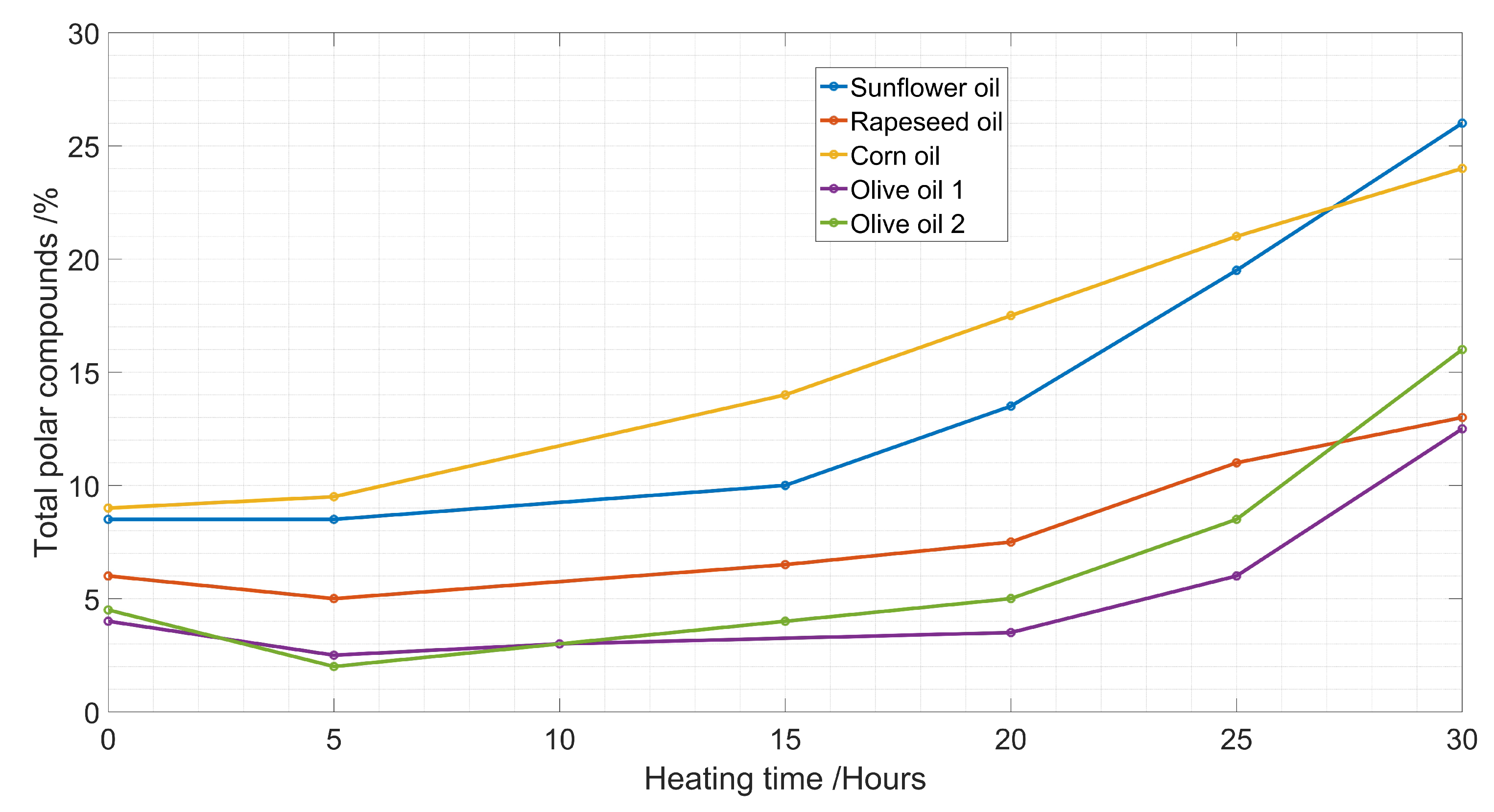
2.1. Chemical Measurement
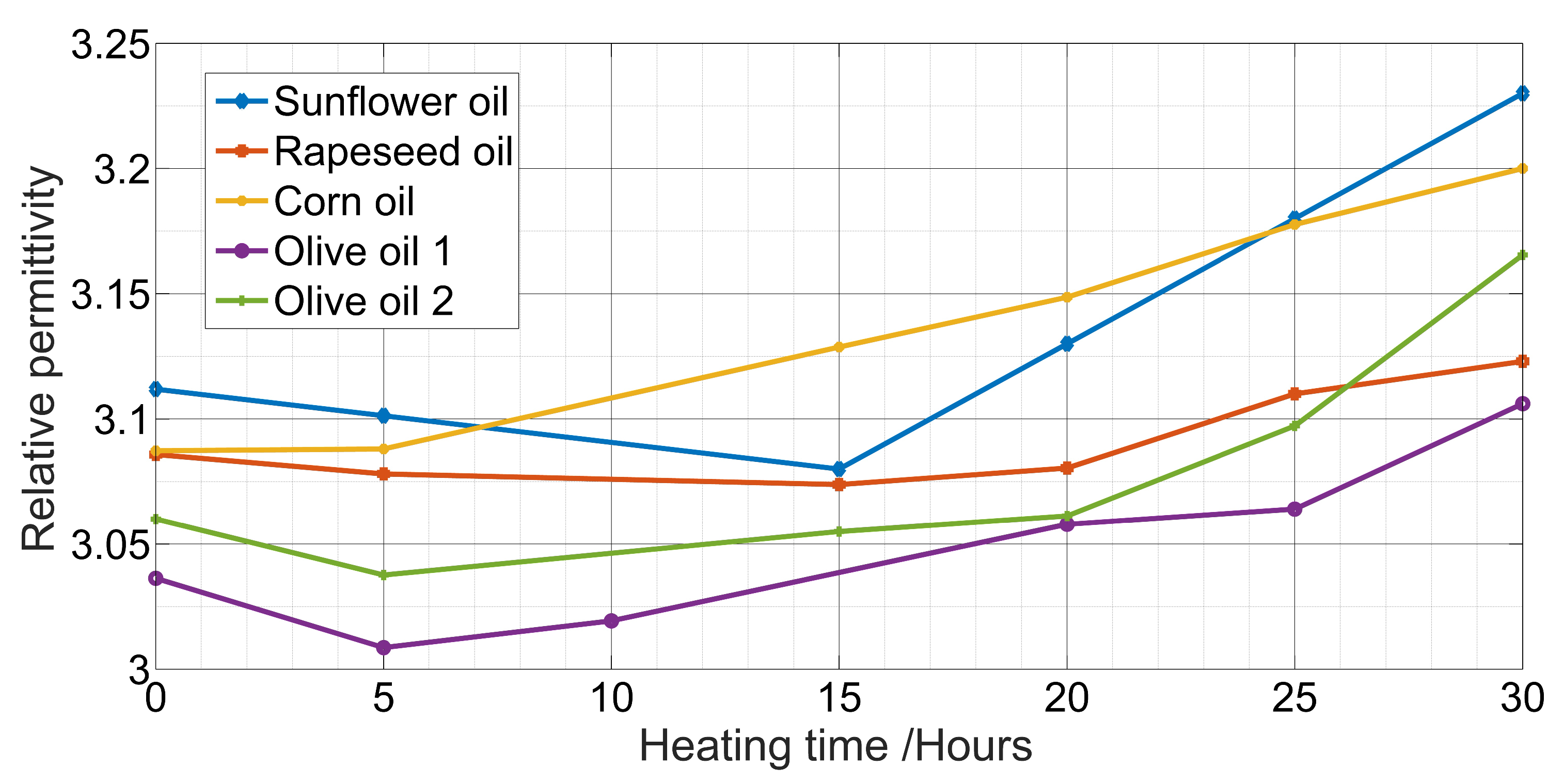
2.2. Dielectric Measurement
2.3. Regression Analysis
3. Measurement Setup
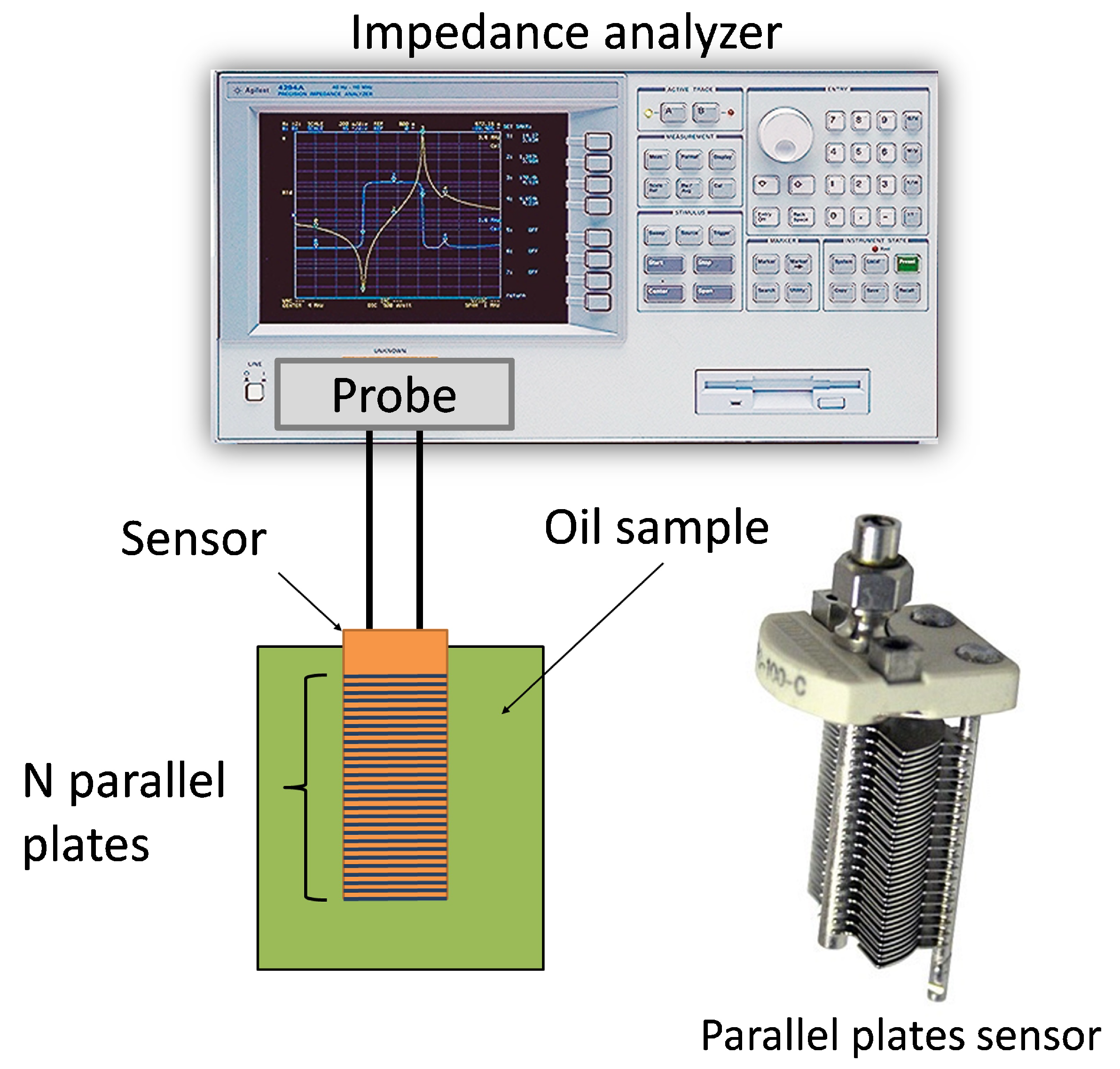
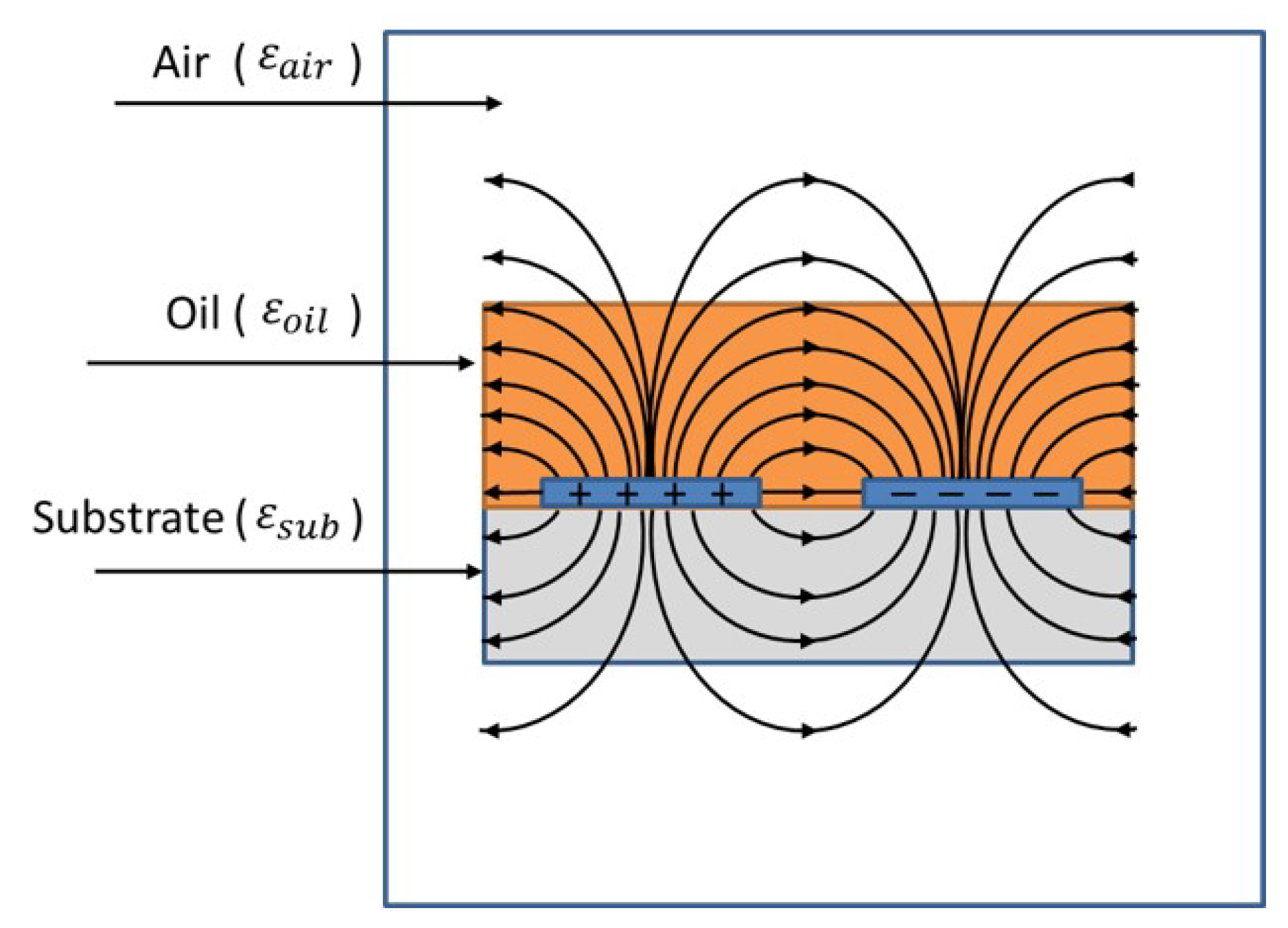
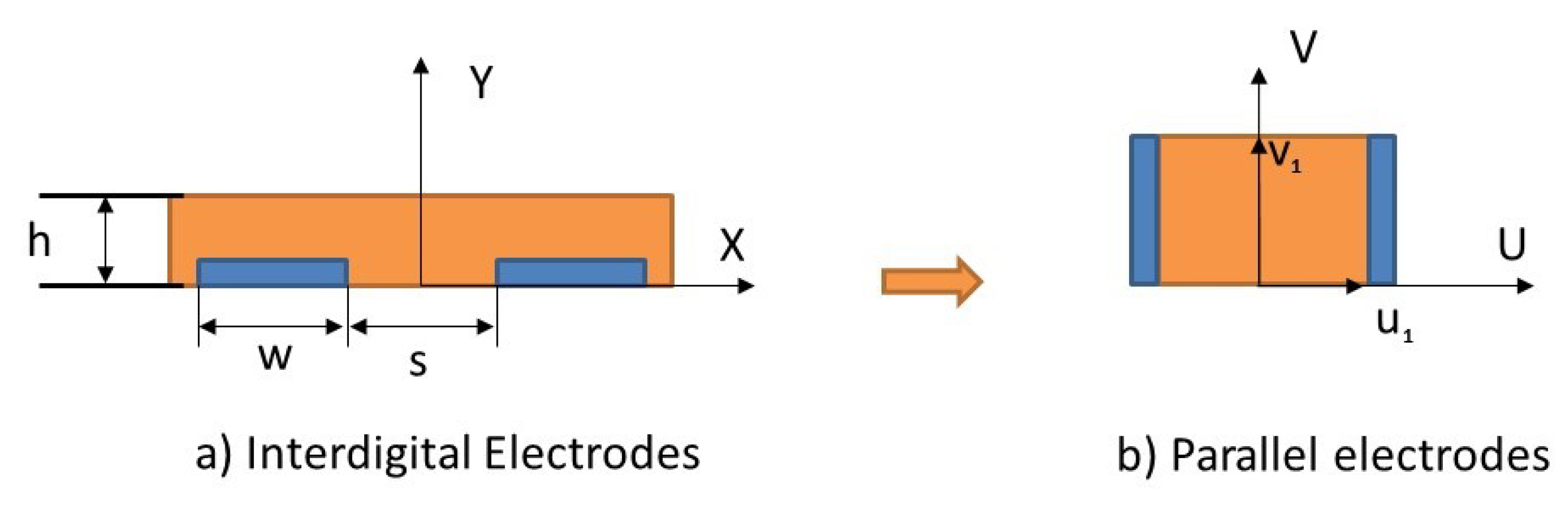
3.1. Capacitive Sensor
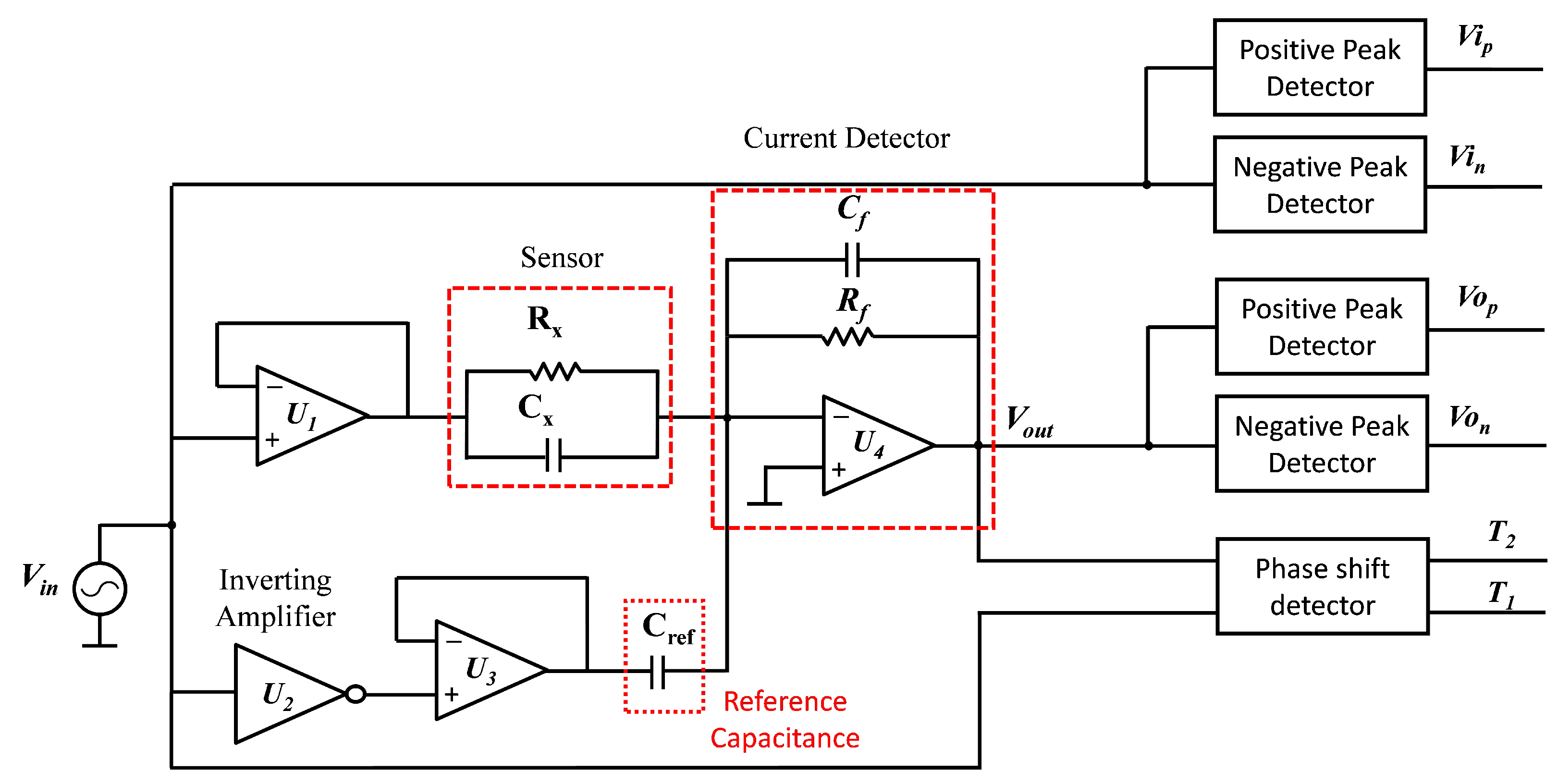
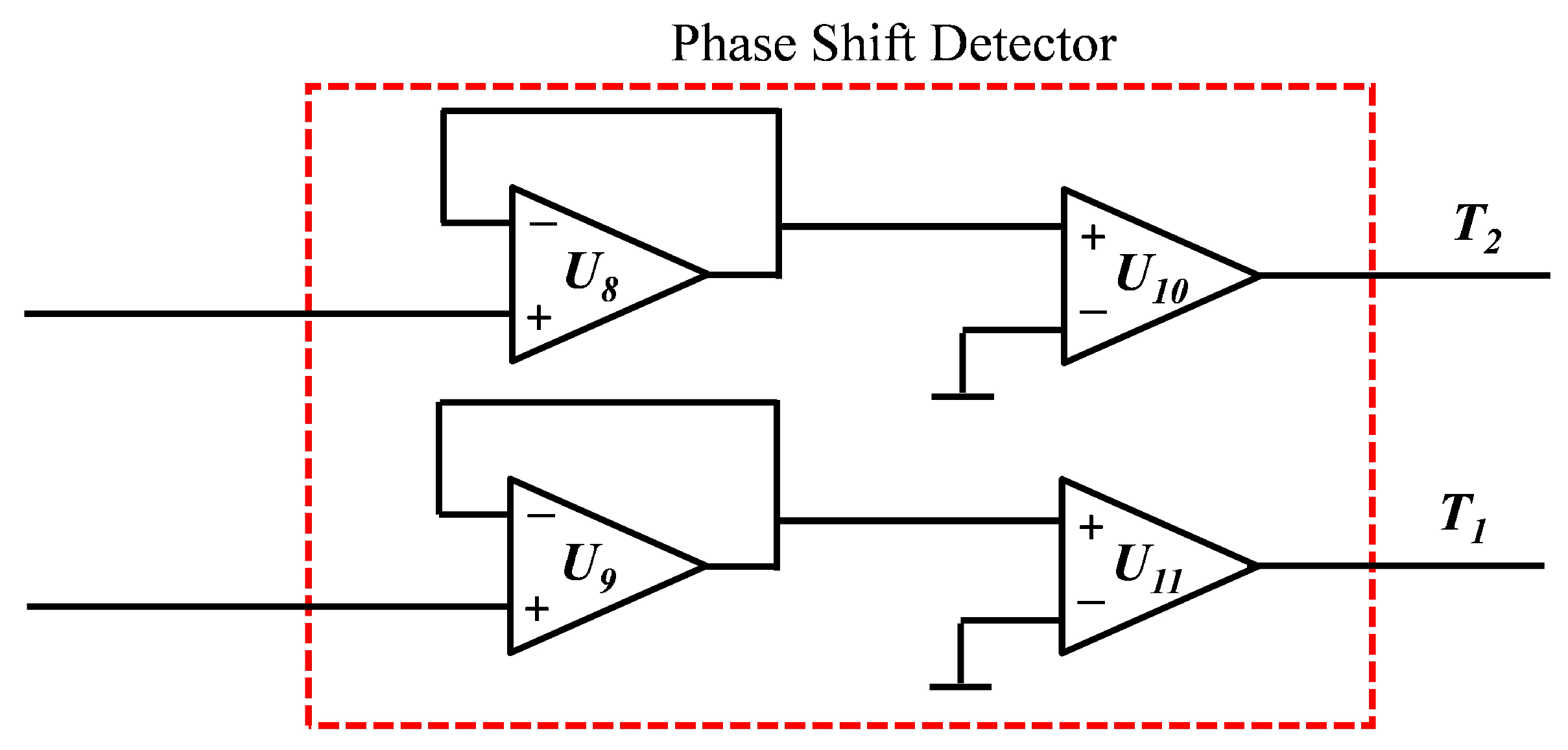
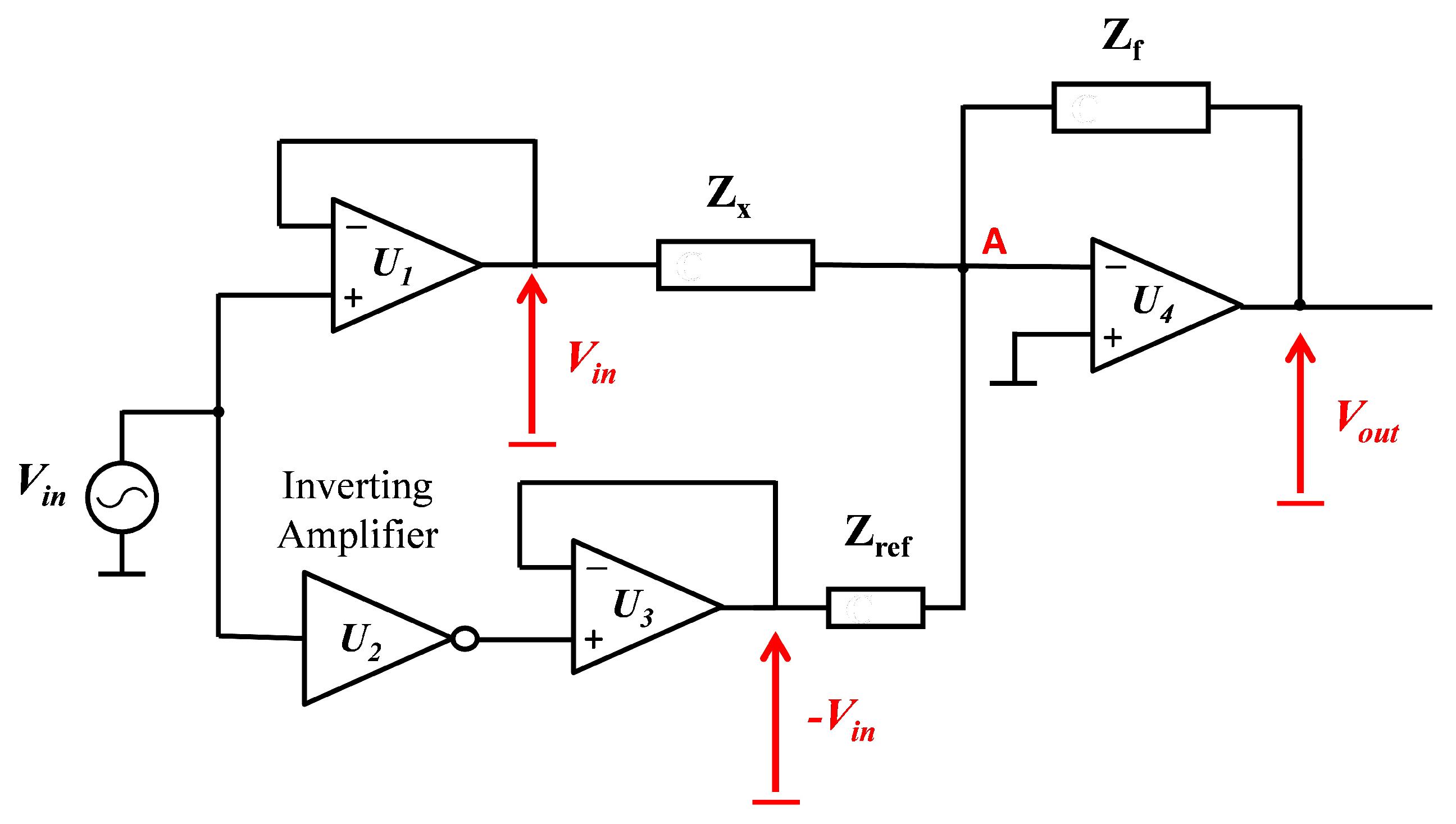
3.2. Measurement Circuit
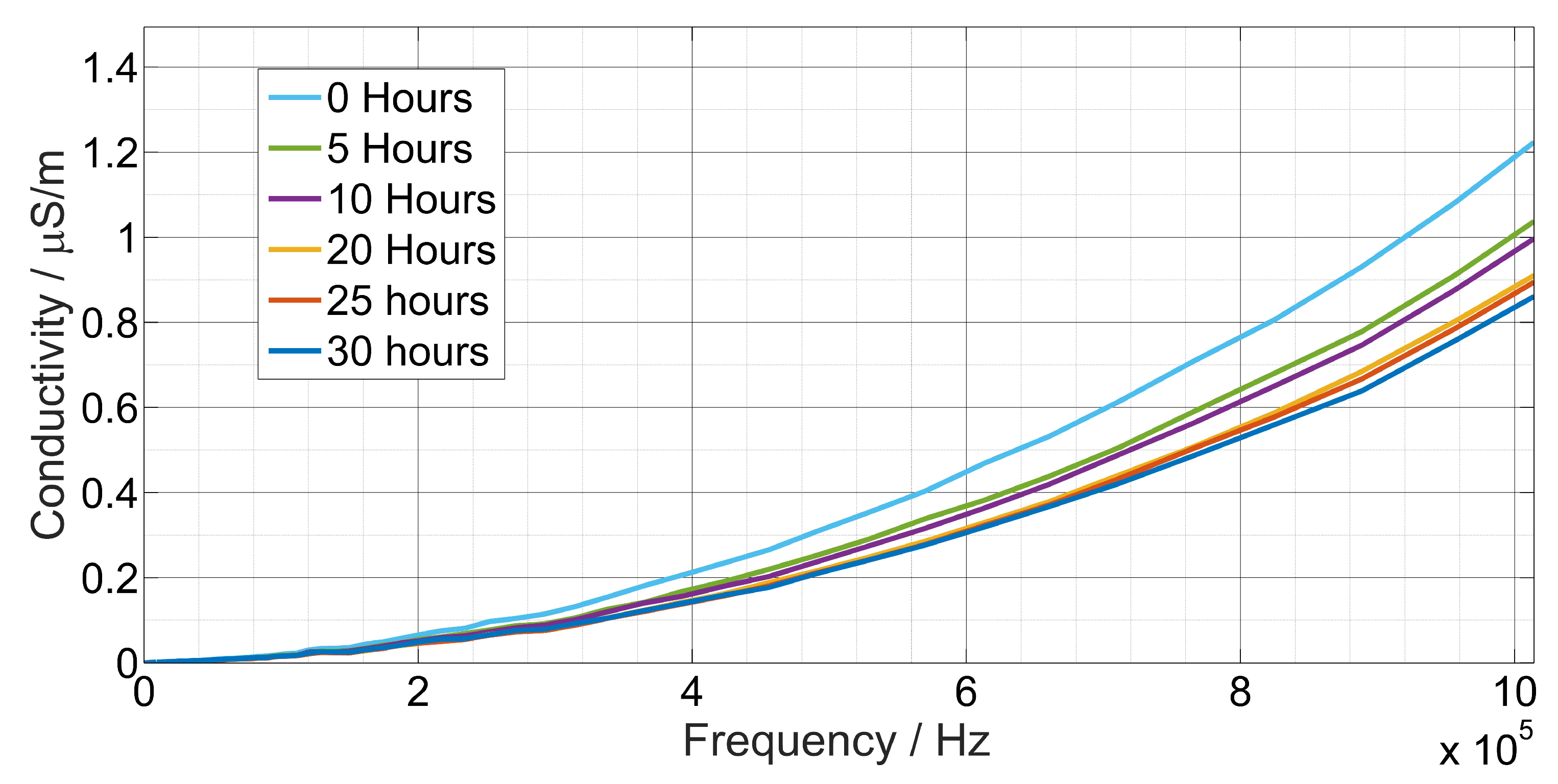
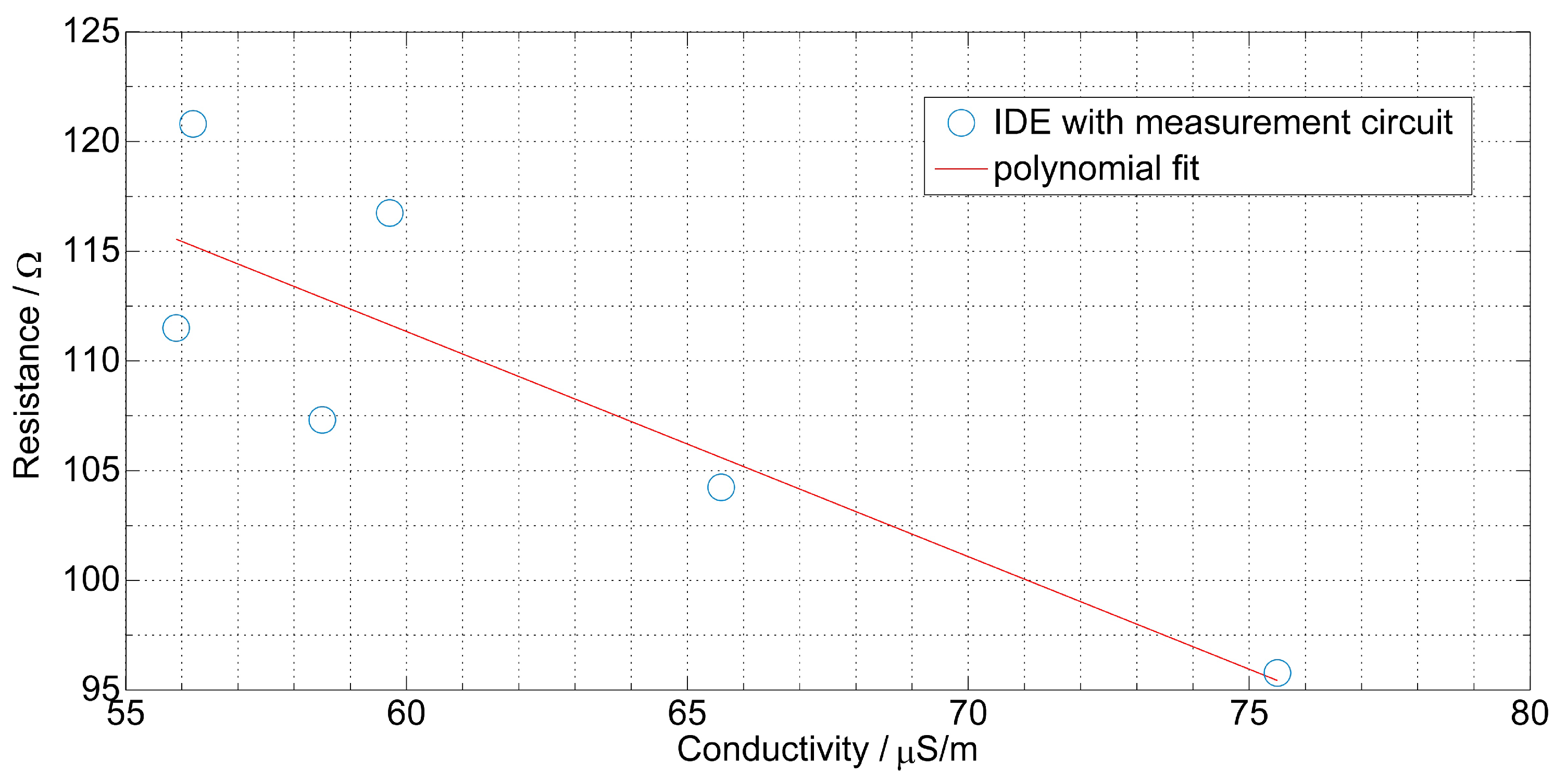
4. Measurement Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kiran, C.R.; Sundresan, A. Thermal Degradation Studies on Edible Oil during Deep Fat Frying Process. Ph.D. Thesis, Cochin University of Science and Technology, Kochi, India, 2015. [Google Scholar]
- Sangdehi, S.K. Quality Evaluation of Frying Oil and Chicken Nuggets Using Visible/Near-Infrared Hyper-Spectral Analysis. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 2005. [Google Scholar]
- Firestone, D. Food technology. Inf. Int. News Fats Oils Relat. Mater. 1993, 4, 1366–1386. [Google Scholar]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, N.; Duponchel, L.; Huvenne, J.P.; Sombret, B.; Legrand, P. Classification of edible fats and oils by principal component analysis of fourier transform infrared spectra. Food Chem. 1996, 57, 245–251. [Google Scholar] [CrossRef]
- Sinelli, N.; Cerretani, L.; Di Egidio, V.; Bendini, A.; Casiraghi, E. Application of near infrared and mid infrared spectroscopy as a rapid tool to classify extra virgin olive oil on the basis of fruity attribute intensity. Food Res. Int. 2010, 43, 369–375. [Google Scholar] [CrossRef]
- Casale, M.; Zunin, P.; Cosulich, M.E.; Pistarino, E.; Perego, P.; Lanteri, S. Characterisation of table olive cultivar by near infrared spectroscopy. Food Chem. 2010, 122, 1261–1265. [Google Scholar] [CrossRef]
- Yang, H.; Irudayaraj, J. Comparison of near-infrared, Fourier transform-infrared, and Fourier transform-Raman methods for determining olive pomace oil adulteration in extra virgin olive oil. J. Am. Oil Chem. Soc. 2001, 78, 889. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Chen, X.; Yang, Y.; Zhang, J. Application of Fourier transform near-infrared spectroscopy to the quantification and monitoring of carbonyl value in frying oils. Anal. Methods 2014, 6, 7628–7633. [Google Scholar] [CrossRef]
- European Communities. On the Characteristics of Olive Oil and Olive Residue Oil and on the Relevant Methods of Analysis; CONSLEG: 1991R258-01/11/2005; Office for official publications of the European Communities: Brussels, Belgium, 2003. [Google Scholar]
- Lazzerini, C.; Domenici, V. Pigments in extra-virgin olive oils produced in Tuscany (Italy) in different years. Foods 2017, 6, 25. [Google Scholar] [CrossRef]
- Galtier, O.; Dupuy, N.; Le Dréau, Y.; Ollivier, D.; Pinatel, C.; Kister, J.; Artaud, J. Geographic origins and compositions of virgin olive oils determinated by chemometric analysis of NIR spectra. Anal. Chim. Acta 2007, 595, 136–144. [Google Scholar] [CrossRef]
- Brenes, M.; García, A.; García, P.; Rios, J.J.; Garrido, A. Phenolic compounds in Spanish olive oils. J. Agric. Food Chem. 1999, 47, 3535–3540. [Google Scholar] [CrossRef]
- Lizhi, H.; Toyoda, K.; Ihara, I. Dielectric properties of edible oils and fatty acids as a function of frequency, temperature, moisture and composition. J. Food Eng. 2008, 88, 151–158. [Google Scholar] [CrossRef]
- Lizhi, H.; Toyoda, K.; Ihara, I. Discrimination of olive oil adulterated with vegetable oils using dielectric spectroscopy. J. Food Eng. 2010, 96, 167–171. [Google Scholar] [CrossRef]
- Prevc, T.; Cigic, B.; Vidrih, R.; Poklar Ulrih, N.; Segatin, N. Correlation of basic oil quality indices and electrical properties of model vegetable oil systems. J. Agric. Food Chem. 2013, 61, 11355–11362. [Google Scholar] [CrossRef] [PubMed]
- Grossi, M.; Di Lecce, G.; Toschi, T.G.; Riccò, B. Fast and accurate determination of olive oil acidity by electrochemical impedance spectroscopy. IEEE Sens. J. 2014, 14, 2947–2954. [Google Scholar] [CrossRef]
- Ragni, L.; Iaccheri, E.; Cevoli, C.; Berardinelli, A.; Bendini, A.; Toschi, T.G. A capacitive technique to assess water content in extra virgin olive oils. J. Food Eng. 2013, 116, 246–252. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Nakayama, M.; Watanabe, K. Limitations of a relaxation oscillator in capacitance measurements. IEEE Trans. Instrum. Meas. 2000, 49, 980–983. [Google Scholar] [CrossRef]
- Martin, K. A voltage-controlled switched-capacitor relaxation oscillator. IEEE J. Solid State Circuits 1981, 16, 412–414. [Google Scholar] [CrossRef]
- Toth, F.N.; Meijer, G.C. A low-cost, smart capacitive position sensor. IEEE Trans. Instrum. Meas. 1992, 41, 1041–1044. [Google Scholar] [CrossRef] [Green Version]
- de Jong, G.W.; Meijer, G.C.; van der Lingen, K.; Spronck, J.W.; Aalsma, A.M.; Bertels, D.J.A.M. A smart capacitive absolute angular-position sensor. Sens. Actuators A Phys. 1994, 41, 212–216. [Google Scholar] [CrossRef]
- Brookhuis, R.A.; Lammerink, T.S.; Wiegerink, R.J. Differential capacitive sensing circuit for a multi-electrode capacitive force sensor. Sens. Actuators A Phys 2015, 234, 168–179. [Google Scholar] [CrossRef]
- Iliev, B.P.; Nihtianov, S.N.; Shterev, G.P.; Meijer, G.C. A multi-period interface system for impedance measurements. In Proceedings of the First ISA/IEEE Conference on Sensors for Industry, Rosemont, IL, USA, 7 November 2001; pp. 276–280. [Google Scholar]
- Van der Goes, F.M.; Meijer, G.C. A novel low-cost capacitive-sensor interface. IEEE Trans. Instrum. Meas. 1996, 45, 536–540. [Google Scholar] [CrossRef] [Green Version]
- Cichocki, A.; Unbehauen, R. A switched-capacitor interface for capacitive sensors based on relaxation oscillators. IEEE Trans. Instrum. Meas. 1990, 39, 797–799. [Google Scholar] [CrossRef]
- Cao, Y.; Temes, G.C. High-accuracy circuits for on-chip capacitance ratio testing or sensor readout. IEEE Trans. Circuits Syst. II Analog. Digit. Signal Process. 1994, 41, 637–639. [Google Scholar]
- Sreenath, V.; George, B. An easy-to-interface CDC with an efficient automatic calibration. IEEE Trans. Instrum. Meas. 2016, 65, 960–967. [Google Scholar] [CrossRef]
- Sreenath, V.; George, B. A Switched-Capacitor Circuit-Based Digitizer for Efficient Interfacing of Parallel RC Sensors. IEEE Sens. J. 2017, 17, 2109–2119. [Google Scholar] [CrossRef]
- Atmanand, M.; Kumar, V.J.; Murti, V. A microcontroller-based scheme for measurement of L and C. Meas. Sci. Technol. 1995, 6, 576. [Google Scholar] [CrossRef]
- Wolffenbuttel, R.F.; Regtien, P.P. Capacitance-to-phase angle conversion for the detection of extremely small capacities. IEEE Trans. Instrum. Meas. 1987, 1001, 868–872. [Google Scholar] [CrossRef]
- Aslam, M.Z.; Tang, T.B. A high resolution capacitive sensing system for the measurement of water content in crude oil. Sensors 2014, 14, 11351–11361. [Google Scholar] [CrossRef]
- Marioli, D.; Sardini, E.; Taroni, A. High-accuracy measurement techniques for capacitance transducers. Meas. Sci. Technol. 1993, 4, 337. [Google Scholar] [CrossRef]
- Yang, W. A self-balancing circuit to measure capacitance and loss conductance for industrial transducer applications. IEEE Trans. Instrum. Meas. 1996, 45, 955–958. [Google Scholar] [CrossRef]
- Huang, S.; Stott, A.; Green, R.; Beck, M. Electronic transducers for industrial measurement of low value capacitances. J. Phys. E Sci. Instrum. 1988, 21, 242. [Google Scholar] [CrossRef]
- Huang, S.; Xie, C.; Stott, A.; Green, R.; Beck, M. A capacitance-based solids concentration transducer with high immunity to interference from the electrostatic charge generated in solids/air two-component flows. Trans. Inst. Meas. Control 1988, 10, 98–102. [Google Scholar] [CrossRef]
- Mochizuki, K.; Masuda, T.; Watanabe, K. An interface circuit for high-accuracy signal processing of differential-capacitance transducers. IEEE Trans. Instrum. Meas. 1998, 47, 823–827. [Google Scholar] [CrossRef] [Green Version]
- Lotters, J.C.; Olthuis, W.; Veltink, P.H.; Bergveld, P. A sensitive differential capacitance to voltage converter for sensor applications. IEEE Trans. Instrum. Meas. 1999, 48, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Melton, S.; Jafar, S.; Sykes, D.; Trigiano, M. Review of stability measurements for frying oils and fried food flavor. J. Am. Oil Chem. Soc. 1994, 71, 1301–1308. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Vasishtha, A.K. Changes in the characteristics and composition of oils during deep-fat frying. Am. Oil Chem. Soc. 1996, 73, 499–506. [Google Scholar] [CrossRef]
- Choe, E.; Min, D. Chemistry of deep-fat frying oils. Food Sci. 2007, 72, 77–86. [Google Scholar] [CrossRef]
- Choe, E.; Min, D. Production and Composition of Frying Fats. Deep. Fry. 2007, 2, 3–24. [Google Scholar]
- Houhoula, D.P.; Oreopoulou, V.; Tzia, C. The effect of process time and temperature on the accumulation of polar compounds in cottonseed oil during deep-fat frying. J. Sci. Food Agric. 2003, 83, 314–319. [Google Scholar] [CrossRef]
- Karakaya, S.; Simsek, S. Changes in Total Polar Compounds, Peroxide Value, Total Phenols and Antioxidant Activity of Various Oils Used in Deep Fat Frying. Am. Oil Chem. Soc. 2011, 88, 1361–1366. [Google Scholar] [CrossRef]
- Chang, S.S.; Peterson, R.J.; Ho, C.T. Chemical reactions involved in the deep-fat frying of foods1. J. Am. Oil Chem. Soc. 1978, 55, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Zahir, E.; Saeed, R.; Hameed, M.A.; Yousuf, A. Study of physicochemical properties of edible oil and evaluation of frying oil quality by Fourier Transform-Infrared (FT-IR) Spectroscopy. Arab. J. Chem. 2017, 10, S3870–S3876. [Google Scholar] [CrossRef] [Green Version]
- Wei, J. Distributed capacitance of planar electrodes in optic and acoustic surface wave devices. IEEE J. Quantum Electron. 1977, 13, 152–158. [Google Scholar] [CrossRef]
- Olthuis, W.; Streekstra, W.; Bergveld, P. Theoretical and experimental determination of cell constants of planar-interdigitated electrolyte conductivity sensors. Sens. Actuators B Chem. 1995, 24, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Fendri, A.; Ghariani, H.; Kanoun, O. Dielectric spectroscopy for assessment of water content in edible oils. In Proceedings of the 2017 14th International Multi-Conference on Systems, Signals & Devices (SSD), Marrakech, Morocco, 28–31 March 2017; pp. 728–732. [Google Scholar]
- Grossi, M.; Di Lecce, G.; Toschi, T.G.; Riccò, B. A novel electrochemical method for olive oil acidity determination. Microelectron. J. 2014, 45, 1701–1707. [Google Scholar] [CrossRef]
- Li, J.; Cai, W.; Sun, D.; Liu, Y. A quick method for determining total polar compounds of frying oils using electric conductivity. Food Anal. Methods 2016, 9, 1444–1450. [Google Scholar] [CrossRef]
- Khaled, A.Y.; Aziz, S.A.; Rokhani, F.Z. Capacitive sensor probe to assess frying oil degradation. Inf. Process. Agric. 2015, 2, 142–148. [Google Scholar] [CrossRef] [Green Version]

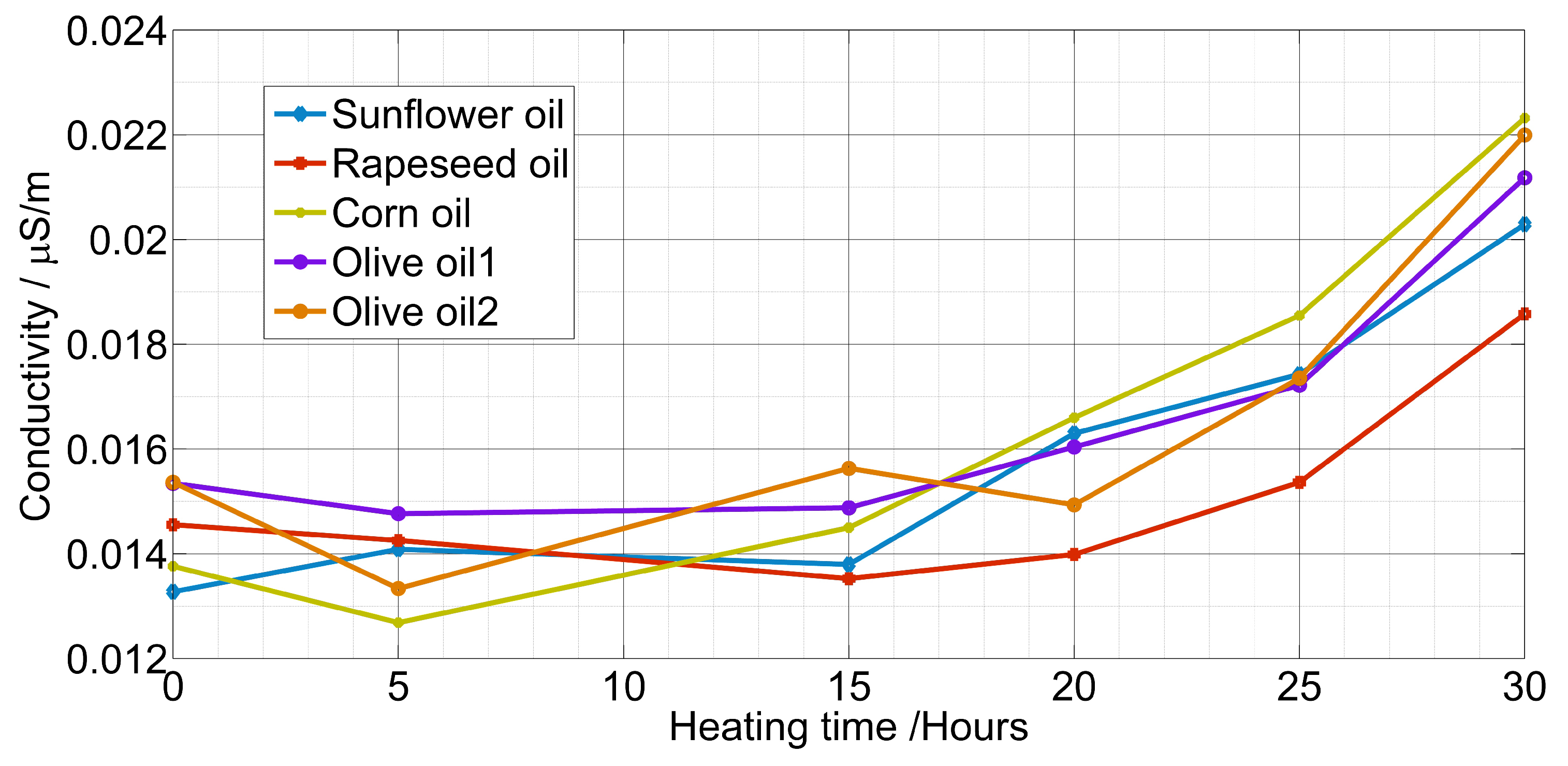




| Sample | Relationship | R2 |
|---|---|---|
| Sunflower oil | AC = 0.9948 + 0.0005751 | 0.99 |
| Rapeseed oil | AC = 0.9458 + 0.007019 | 0.94 |
| Corn oil | AC = 0.9669 + 0.007539 | 0.96 |
| Olive oil 1 | AC = 0.9982 + 0.0005035 | 0.99 |
| Olive oil 2 | AC = 0.9938 + 0.00193 | 0.99 |
| Sample | Relationship | R2 |
|---|---|---|
| Sunflower oil | TPC = 122.1 − 369 | 0.93 |
| Rapeseed oil | TPC = 149.5 − 454 | 0.89 |
| Corn oil | TPC = 131.9 − 398.1 | 0.99 |
| Olive oil1 | TPC = 96.71 − 289.6 | 0.82 |
| Olive oil2 | TPC = 108.4 − 327.2 | 0.99 |
| Sample | Relationship | R2 |
|---|---|---|
| Sunflower oil | C = 20.24 + 164.4 | 0.91 |
| Rapeseed oil | C = 28.66 + 140.6 | 0.79 |
| Corn oil | C = 25.04 + 151.2 | 0.97 |
| Olive oil | C = 21.63 + 162.1 | 0.90 |
| Olive oil | C = 14.45 + 183.8 | 0.51 |
| Sample | Relationship | R2 |
|---|---|---|
| Sunflower oil | R = −1.026 + 172.9 | 0.74 |
| Rapeseed oil | R = −5.607 + 148.2 | 0.45 |
| Corn oil | R = −0.932 + 163.9 | 0.69 |
| Olive oil | R = −1.212 + 230.1 | 0.91 |
| Olive oil | R = −1.171 + 232.4 | 0.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fendri, A.; Kallel, A.Y.; Nouri, H.; Ghariani, H.; Kanoun, O. Measurement System for Lossy Capacitive Sensors: Application to Edible Oils Quality Assessment. Sensors 2019, 19, 4299. https://doi.org/10.3390/s19194299
Fendri A, Kallel AY, Nouri H, Ghariani H, Kanoun O. Measurement System for Lossy Capacitive Sensors: Application to Edible Oils Quality Assessment. Sensors. 2019; 19(19):4299. https://doi.org/10.3390/s19194299
Chicago/Turabian StyleFendri, Ahmed, Ahmed Yahia Kallel, Hanen Nouri, Hamadi Ghariani, and Olfa Kanoun. 2019. "Measurement System for Lossy Capacitive Sensors: Application to Edible Oils Quality Assessment" Sensors 19, no. 19: 4299. https://doi.org/10.3390/s19194299
APA StyleFendri, A., Kallel, A. Y., Nouri, H., Ghariani, H., & Kanoun, O. (2019). Measurement System for Lossy Capacitive Sensors: Application to Edible Oils Quality Assessment. Sensors, 19(19), 4299. https://doi.org/10.3390/s19194299






