Edible Gelatin Diagnosis Using Laser-Induced Breakdown Spectroscopy and Partial Least Square Assisted Support Vector Machine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
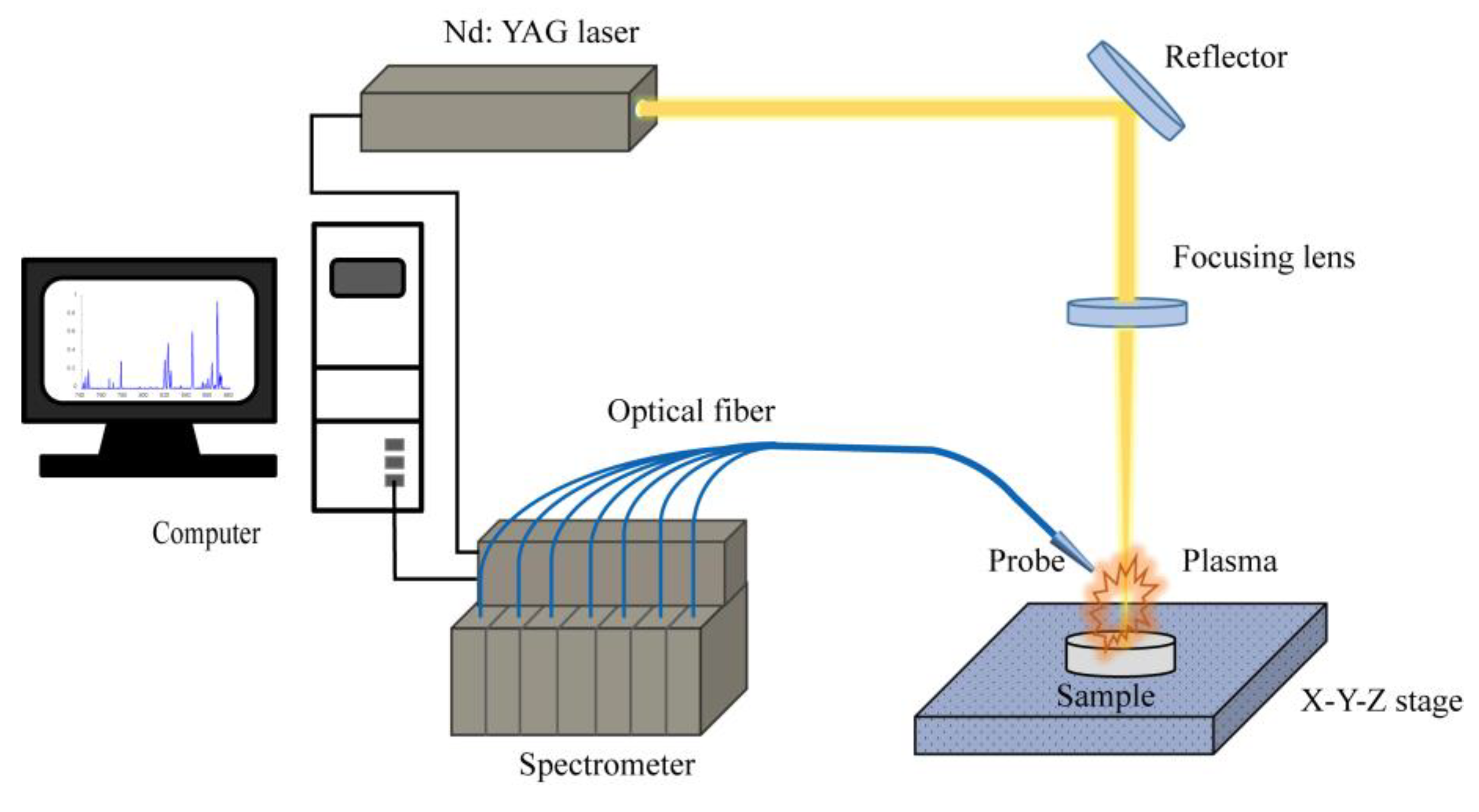
2.2. Experimental Setup
2.3. Data Analysis
2.3.1. PLS Regression
2.3.2. SVM Regression
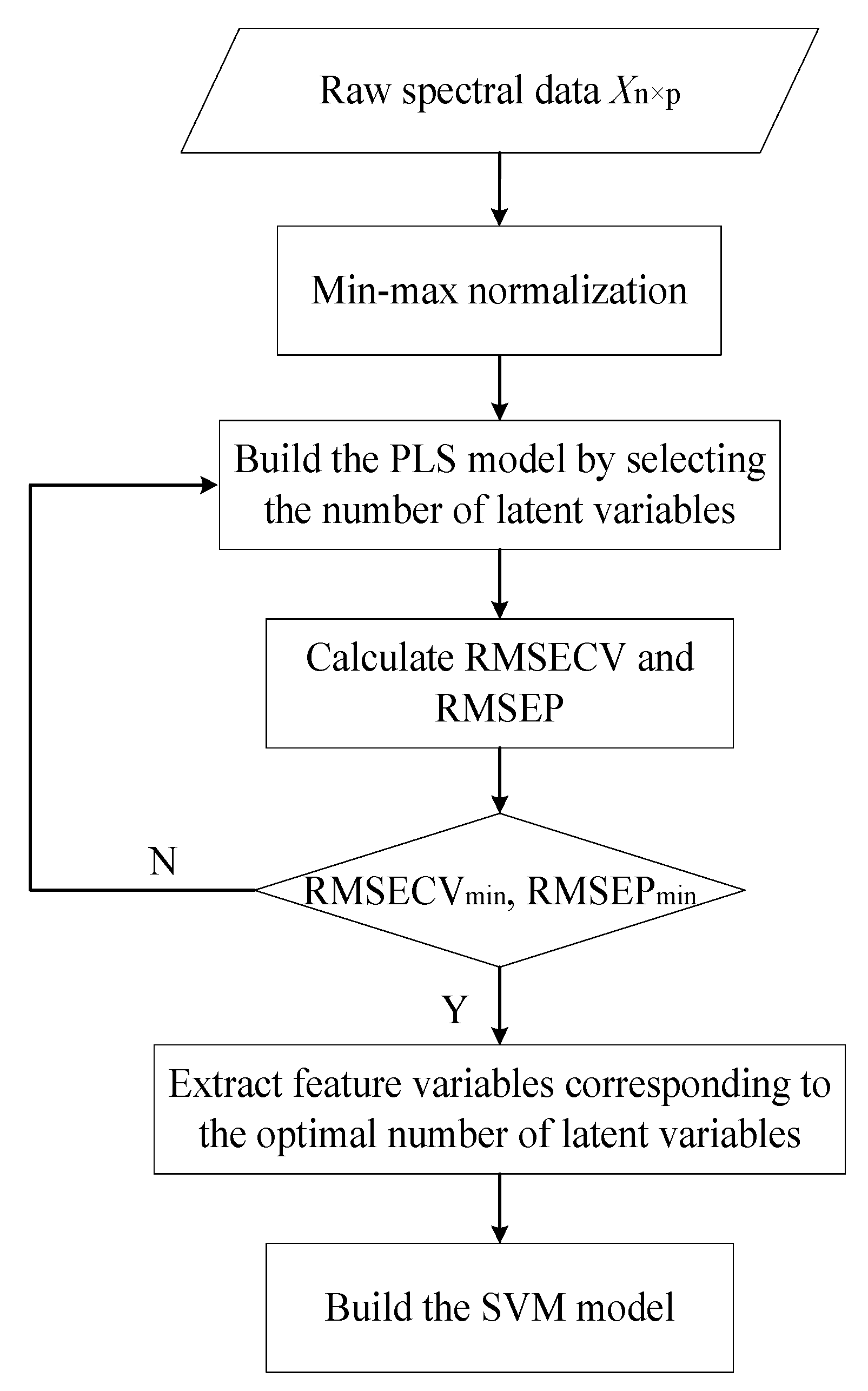
2.3.3. PLS-SVM Regression
2.3.4. Performance Evaluation
3. Results and Discussion
3.1. Spectral Analysis
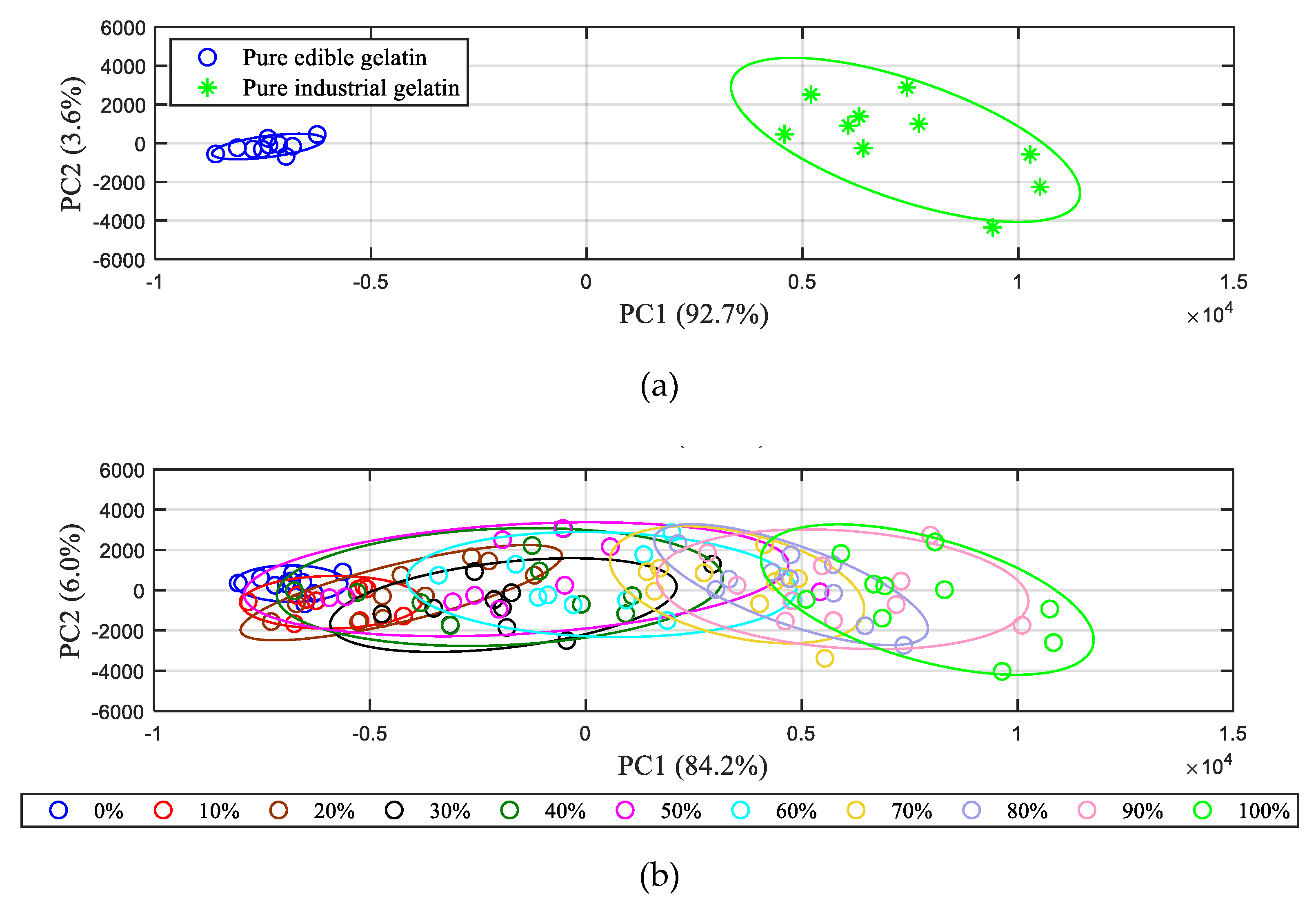
3.2. PCA Results of LIBS Spectra
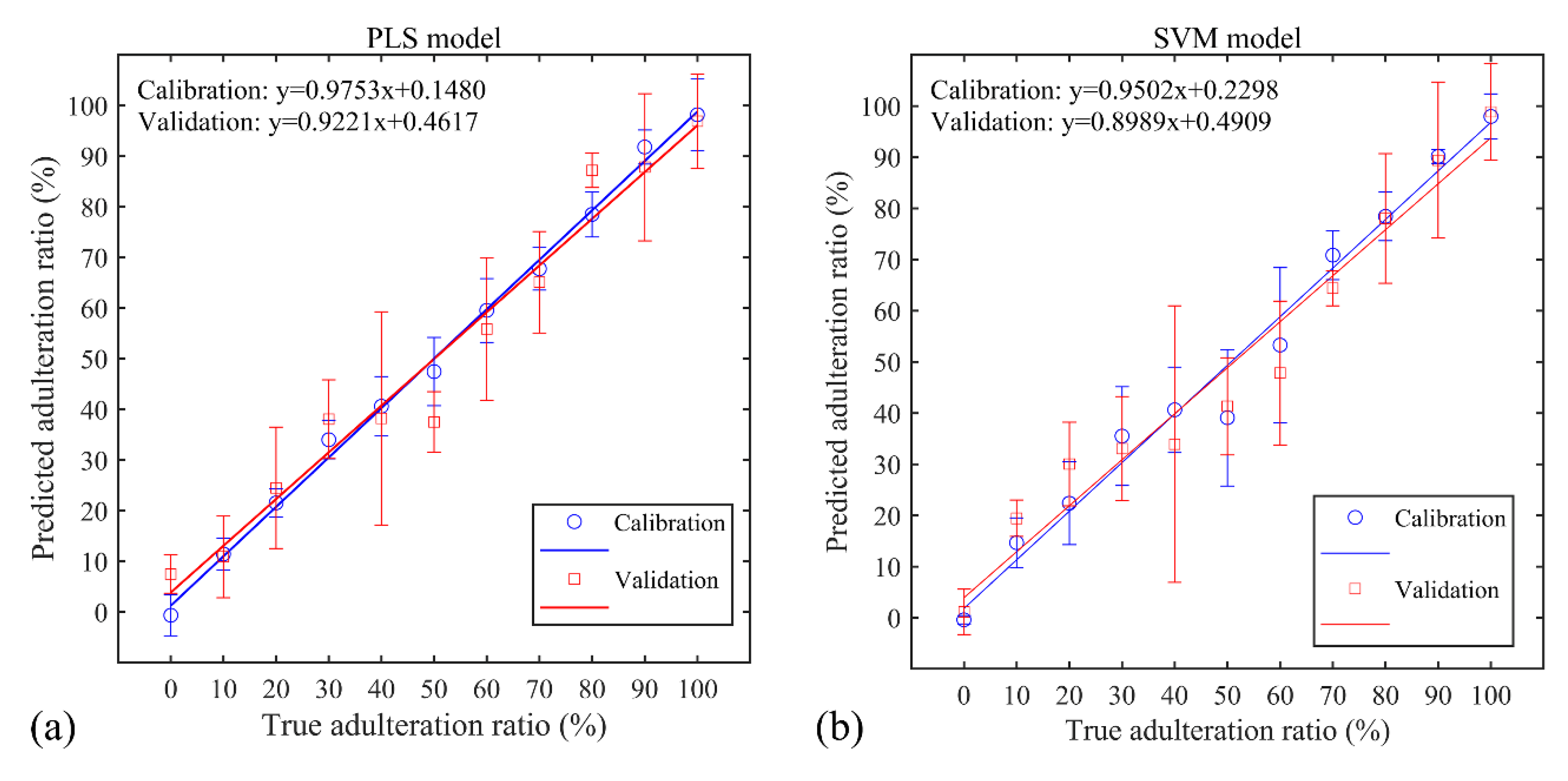
3.3. PLS and SVM Regression Results
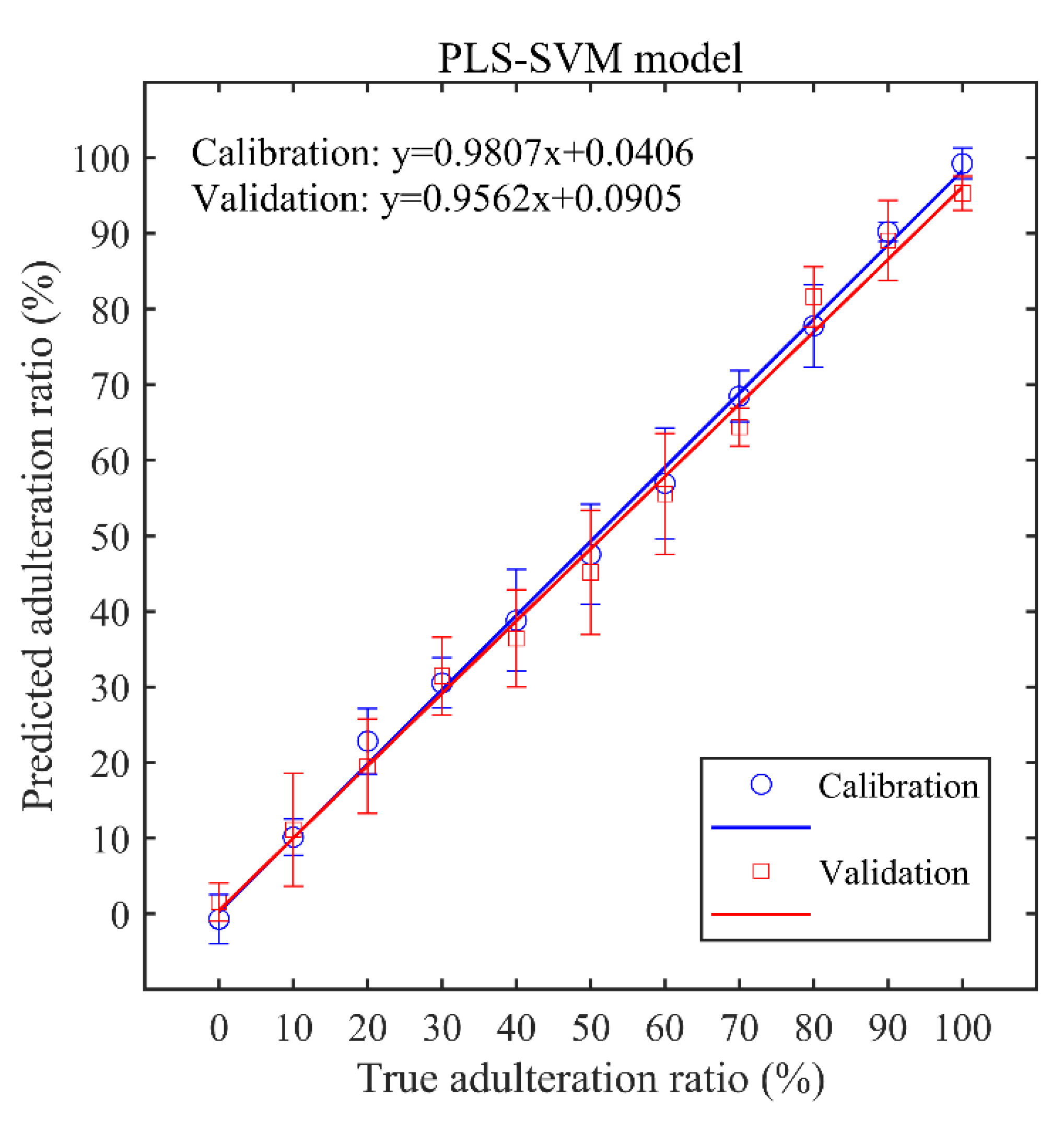
3.4. PLS-SVM Regression Results
3.5. Comparison of SVM Models by Different Variable Selection Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Azira, T.N.; Amin, I.; Man, Y.B.C. Differentiation of bovine and porcine gelatins in processed products via Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and principal component analysis (PCA) techniques. Int. Food Res. J. 2012, 19, 1175–1180. [Google Scholar]
- Azira, T.N.; Man, Y.B.C.; Hafidz, R.N.R.M.; Aina, M.A.; Amin, I. Use of principal component analysis for differentiation of gelatine sources based on polypeptide molecular weights. Food Chem. 2014, 151, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Watanabe, E.; Shibata, H.; Tanabe, S. A reliable enzyme linked immunosorbent assay for the determination of bovine and porcine gelatin in processed foods. J. Agric. Food Chem. 2009, 57, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Tukiran, N.A.; Ismail, A.; Mustafa, S.; Hamid, M. Determination of porcine gelatin in edible bird’s nest by competitive indirect ELISA based on anti-peptide polyclonal antibody. Food Control 2016, 59, 561–566. [Google Scholar] [CrossRef]
- Tukiran, N.A.; Ismail, A.; Mustafa, S.; Hamid, M. Development of antipeptide enzyme-linked immunosorbent assay for determination of gelatin in confectionery products. Int. J. Food Sci. Technol. 2016, 51, 54–60. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Kesmen, Z.; Baykal, B.; Sagdic, O.; Kulen, O.; Kacar, O.; Yetim, H.; Baykal, A.T. A novel method to differentiate bovine and porcine gelatins in food products: NanoUPLC-ESI-Q-TOF-MS(E) based data independent acquisition technique to detect marker peptides in gelatin. Food Chem. 2013, 141, 2450–2458. [Google Scholar] [CrossRef]
- Azilawati, M.I.; Hashim, D.M.; Jamilah, B.; Amin, I. RP-HPLC method using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate incorporated with normalization technique in principal component analysis to differentiate the bovine, porcine and fish gelatins. Food Chem. 2015, 172, 368–376. [Google Scholar] [CrossRef]
- Cai, H.; Gu, X.L.; Scanlan, M.S.; Ramatlapeng, D.H.; Lively, C.R. Real-time PCR assays for detection and quantitation of porcine and bovine DNA in gelatin mixtures and gelatin capsules. J. Food Compos. Anal. 2012, 25, 83–87. [Google Scholar] [CrossRef]
- Mutalib, S.A.; Muin, N.M.; Dullah, A.A.; Hassan, O.; Mustapha, W.A.W.; Sani, N.A.; Maskat, M.Y. Sensitivity of polymerase chain reaction (PCR)-southern hybridization and conventional PCR analysis for Halal authentication of gelatin capsules. LWT Food Sci. Technol. 2015, 63, 714–719. [Google Scholar] [CrossRef]
- Hashim, D.; Man, Y.; Norakasha, R.; Shuhaimi, M.; Salmah, Y.; Syahariza, Z. Potential use of Fourier transform infrared spectroscopy for differentiation of bovine and porcine gelatins. Food Chem. 2010, 118, 856–860. [Google Scholar] [CrossRef]
- Cebi, N.; Durak, M.Z.; Toker, O.S.; Sagdic, O.; Arici, M. An evaluation of Fourier transforms infrared spectroscopy method for the classification and discrimination of bovine, porcine and fish gelatins. Food Chem. 2016, 190, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Duconseille, A.; Andueza, D.; Picard, F.; Santé-Lhoutellier, V.; Astruc, T. Molecular changes in gelatin aging observed by NIR and fluorescence spectroscopy. Food Hydrocoll. 2016, 61, 496–503. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.C.; Ma, X.W.; Pan, D.; Zhao, D.M. Quantitative analysis of chromium in edible gelatin by using laser-induced breakdown spectroscopy. Acta Phys. Sin. 2014, 63, 145202. [Google Scholar]
- Zhang, H.; Sun, H.; Wang, L.; Wang, S.; Zhang, W.; Hu, J. Near infrared spectroscopy based on supervised pattern recognition methods for rapid identification of adulterated edible gelatin. J. Spectrosc. 2018, 2018, 7652592. [Google Scholar] [CrossRef]
- Sergio, M.; Umberto, P. Laser-Induced Breakdown Spectroscopy: Theory and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- David, A.C.; Leon, J.R. Handbook of Laser-Induced Breakdown Spectroscopy, 2nd ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Butcher, D.J. Advances in electrothermal atomization atomic absorption spectrometry: Instrumentation, methods, and applications. Appl. Spectrosc. Rev. 2006, 41, 15–34. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Fisher, A.S.; Henon, D.N.; Hill, S.J. Matrix digestion of soil and sediment samples for extraction of lead, cadmium and antimony and their direct determination by inductively coupled plasma-mass spectrometry and atomic emission spectrometry. Microchim. Acta 2004, 144, 277–283. [Google Scholar]
- Sha, W.; Li, J.; Xiao, W.; Ling, P.; Lu, C. Quantitative analysis of elements in fertilizer using laser-induced breakdown spectroscopy coupled with support vector regression Model. Sensors 2019, 19, 3277. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, X.; Lv, Y.; Liu, F.; Peng, J.; Shen, T.; Zhao, Y.; Tang, Y.; Luo, S. Quantitative analysis of nutrient elements in soil using single and double-pulse laser-induced breakdown spectroscopy. Sensors 2018, 18, 1526. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.K.; Kumar, R.; Singh, V.K.; Agrawal, R.; Rai, S.; Rai, A.K. Assessment of LIBS for spectrochemical analysis: A review. Appl. Spectrosc. Rev. 2012, 47, 14–40. [Google Scholar] [CrossRef]
- Markiewicz-Keszycka, M.; Cama-Moncunill, X.; Casado-Gavalda, M.P.; Dixit, Y.; Cama-Moncunill, R.; Cullen, P.J.; Sullivan, C. Laser-induced breakdown spectroscopy (LIBS) for food analysis: A review. Trends Food Sci. Technol. 2017, 65, 80–93. [Google Scholar] [CrossRef]
- Gaudiuso, R.; Dell’Aglio, M.; Pascale, O.D.; Senesi, G.S.; Giacomo, A.D. Laser induced breakdown spectroscopy for elemental analysis in environmental, cultural heritage and space applications: A review of methods and results. Sensors 2010, 10, 7434–7468. [Google Scholar] [CrossRef] [PubMed]
- Noll, R.; Fricke-Begemann, C.; Connemann, S.; Meinhardt, C.; Sturm, V. LIBS analyses for industrial applications-an overview of developments from 2014 to 2018. J. Anal. At. Spectrom. 2018, 33, 945–956. [Google Scholar] [CrossRef]
- Rehse, S.J.; Salimnia, H.; Miziolek, A.W. Laser-induced breakdown spectroscopy (LIBS): An overview of recent progress and future potential for biomedical applications. J. Med. Eng. Technol. 2012, 36, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.W.; Omenetto, N. Laser-induced breakdown spectroscopy (LIBS), part II: Review of instrumental and methodological approaches to material analysis and applications to different fields. Appl. Spectrosc. 2012, 66, 347–419. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, J.L.; De Lucia, F.C.; Munson, C.A.; Miziolek, A.W. Laser-induced breakdown spectroscopy for detection of explosives residues: A review of recent advances, challenges, and future prospects. Anal. Bioanal. Chem. 2009, 395, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Spizzichino, V.; Fantoni, R. Laser induced breakdown spectroscopy in archeometry: A review of its application and future perspectives. Spectrochim. Acta Part B At. Spectrosc. 2014, 99, 201–209. [Google Scholar] [CrossRef]
- Tiwari, P.K.; Rai, N.K.; Kumar, R.; Parigger, C.G.; Rai, A.K. Atomic and molecular laser-induced breakdown spectroscopy of selected pharmaceuticals. Atoms 2019, 7, 71. [Google Scholar] [CrossRef]
- Bilge, G.; Sezer, B.; Eseller, K.E.; Berberoglu, H.; Topcu, A.; Boyaci, I.H. Determination of whey adulteration in milk powder by using laser induced breakdown spectroscopy. Food Chem. 2016, 212, 183–188. [Google Scholar] [CrossRef]
- Velioglu, H.M.; Sezer, B.; Bilge, G.; Baytur, S.E.; Boyaci, I.H. Identification of offal adulteration in beef by laser induced breakdown spectroscopy (LIBS). Meat Sci. 2018, 138, 28–33. [Google Scholar] [CrossRef]
- Temiz, H.T.; Sezer, B.; Berkkan, A.; Tamer, U.; Boyaci, I.H. Assessment of laser induced breakdown spectroscopy as a tool for analysis of butter adulteration. J. Food Compos. Anal. 2018, 67, 48–54. [Google Scholar] [CrossRef]
- Labutin, T.A.; Zaytsev, S.M.; Popov, A.M.; Zorov, N.B. Carbon determination in carbon-manganese steels under atmospheric conditions by laser-induced breakdown spectroscopy. Opt. Express 2014, 22, 22382. [Google Scholar] [CrossRef]
- Devangad, P.; Unnikrishnan, V.K.; Tamboli, M.M.; Shameem, K.M.M.; Nayak, R.; Choudhari, K.S.; Chidangil, S. Quantification of Mn in glass matrices using laser induced breakdown spectroscopy (LIBS) combined with chemometric approaches. Anal. Methods 2016, 8, 7177–7184. [Google Scholar] [CrossRef]
- Peng, J.Y.; He, Y.; Jiang, J.D.; Zhao, Z.F.; Zhou, F.; Liu, F. High-accuracy and fast determination of chromium content in rice leaves based on collinear dual-pulse laser-induced breakdown spectroscopy and chemometric methods. Food Chem. 2019, 295, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Karki, V.; Aggarwal, S.K.; Maurya, G.S.; Kumar, R.; Rai, A.K.; Mao, X.L.; Russo, R.E. Evaluation of the prediction precision capability of partial least squares regression approach for analysis of high alloy steel by laser induced breakdown spectroscopy. Spectrochim. Acta B 2015, 108, 8–14. [Google Scholar] [CrossRef]
- Yang, J.H.; Yi, C.C.; Xu, J.W.; Ma, X.H. A laser induced breakdown spectroscopy quantitative analysis method based on the robust least squares support vector machine regression model. J. Anal. At. Spectrom. 2015, 30, 1541–1551. [Google Scholar] [CrossRef]
- Sankaran, S.; Ehsani, R.; Ehsani, R.; Morgan, K.T. Detection of anomalies in citrus leaves using laser-induced breakdown spectroscopy (LIBS). Appl. Spectrosc. 2015, 69, 913–919. [Google Scholar] [CrossRef]
- Yan, C.; Qi, J.; Ma, J.; Tang, H.; Zhang, T.; Li, H. Determination of carbon and sulfur content in coal by laser induced breakdown spectroscopy combined with kernel-based extreme learning machine. Chemometr. Intell. Lab. 2017, 167, 226–231. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, F.; Yang, G.; Chen, H.X.; Song, Z.S. Quantitative analysis of sinters using laser-induced breakdown spectroscopy (LIBS) coupled with kernel-based extreme learning machine (K-ELM). Anal. Methods 2018, 10, 1074–1079. [Google Scholar] [CrossRef]
- Guezenoc, J.; Bassel, L.; Gallet-Budynek, A.; Bousquet, B. Variables selection: A critical issue for quantitative laser-induced breakdown spectroscopy. Spectrochim. Acta B 2017, 134, 6–10. [Google Scholar] [CrossRef]
- Yan, C.; Qi, J.; Ma, J.; Tang, H.; Zhang, T.; Li, H. A hybrid variable selection method based on wavelet transform and mean impact value for calorific value determination of coal using laser-induced breakdown spectroscopy and kernel extreme learning machine. Spectrochim. Acta B 2019, 154, 75–81. [Google Scholar] [CrossRef]
- Luna, A.S.; Gonzaga, F.B.; da Rocha, W.F.; Lima, I.C. A comparison of different strategies in multivariate regression models for the direct determination of Mn, Cr, and Ni in steel samples using laser-induced breakdown spectroscopy. Spectrochim. Acta B 2018, 139, 20–26. [Google Scholar] [CrossRef]
- Vapnik, V. Statistical Learning Theory; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Hsu, C.W.; Lin, C.J. A simple decomposition method for support vector machines. Mach. Learn. 2002, 46, 291–314. [Google Scholar] [CrossRef]
- Atomic Spectra Database, National Institute of Standards and Technology (NIST). Available online: http://www.nist.gov/pml/atomic-spectra-database (accessed on 28 April 2019).
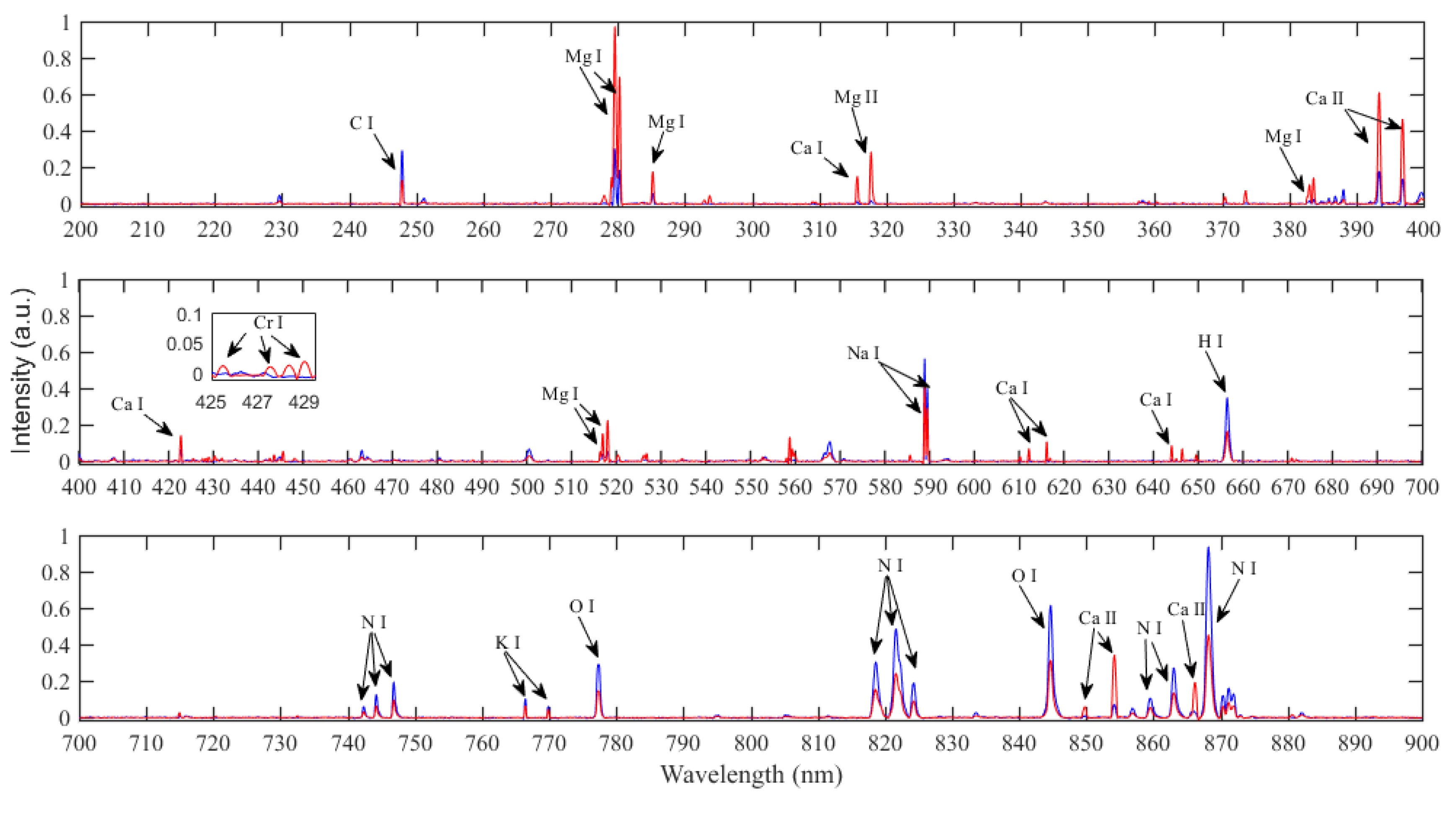


| Elements | Emission Lines (nm) |
|---|---|
| C | I 247.86 |
| N | I 742.36, I 744.23, I 746.83, I 818.80, I 821.63, I 824.24, I 849.80, I 859.40, I 862.92, I 868.34 |
| O | I 777.19, I 844.64 |
| H | I 656.28 |
| K | I 766.49, I 769.90 |
| Na | I 588.99, I 589.59 |
| Ca | I 315.77, II 393.37, II 396.85, I 422.67, I 612.22, I 616.22, I 643.91, II 854.21, II 866.21 |
| Mg | II 279.55, II 280.27, I 285.21, II 317.58, I 382.94, I 516.73, I 517.27, I 518.36 |
| Cr | I 425.43, I 427.48, I 428.97 |
| Model | Optimized Parameters | RMSECV | Rc2 | RMSEP | Rp2 | LOD | ||
|---|---|---|---|---|---|---|---|---|
| PLS | LVs = 6 | 4.97% | 0.9876 | 10.96% | 0.9390 | 12.4% | ||
| SVM | C = 11.5362 | γ= 2.7634 | MSE = 1.6461 | 8.81% | 0.9237 | 12.22% | 0.8544 | 14.8% |
| Model | Variables | Time | RMSECV | Rc2 | RMSEP | Rp2 | LOD |
|---|---|---|---|---|---|---|---|
| CARS-SVM | 88 | 59 s | 5.26% | 0.9736 | 7.89% | 0.9453 | 15.8% |
| MC-UVE-SVM | 84 | 165 s | 5.54% | 0.9695 | 12.23% | 0.8521 | 36.5% |
| RF-SVM | 42 | 123 s | 5.06% | 0.9745 | 6.85% | 0.9544 | 29.8% |
| PCA-SVM | 15 | 25 s | 5.48% | 0.9701 | 11.11% | 0.8853 | 19.7% |
| PLS-SVM | 6 | 3 s | 4.64% | 0.9790 | 5.69% | 0.9708 | 7.9% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, S.; Li, D.; Zhang, Y.; Hu, J.; Wang, L. Edible Gelatin Diagnosis Using Laser-Induced Breakdown Spectroscopy and Partial Least Square Assisted Support Vector Machine. Sensors 2019, 19, 4225. https://doi.org/10.3390/s19194225
Zhang H, Wang S, Li D, Zhang Y, Hu J, Wang L. Edible Gelatin Diagnosis Using Laser-Induced Breakdown Spectroscopy and Partial Least Square Assisted Support Vector Machine. Sensors. 2019; 19(19):4225. https://doi.org/10.3390/s19194225
Chicago/Turabian StyleZhang, Hao, Shun Wang, Dongxian Li, Yanyan Zhang, Jiandong Hu, and Ling Wang. 2019. "Edible Gelatin Diagnosis Using Laser-Induced Breakdown Spectroscopy and Partial Least Square Assisted Support Vector Machine" Sensors 19, no. 19: 4225. https://doi.org/10.3390/s19194225
APA StyleZhang, H., Wang, S., Li, D., Zhang, Y., Hu, J., & Wang, L. (2019). Edible Gelatin Diagnosis Using Laser-Induced Breakdown Spectroscopy and Partial Least Square Assisted Support Vector Machine. Sensors, 19(19), 4225. https://doi.org/10.3390/s19194225





