Neural Activities Classification of Human Inhibitory Control Using Hierarchical Model
Abstract
1. Introduction
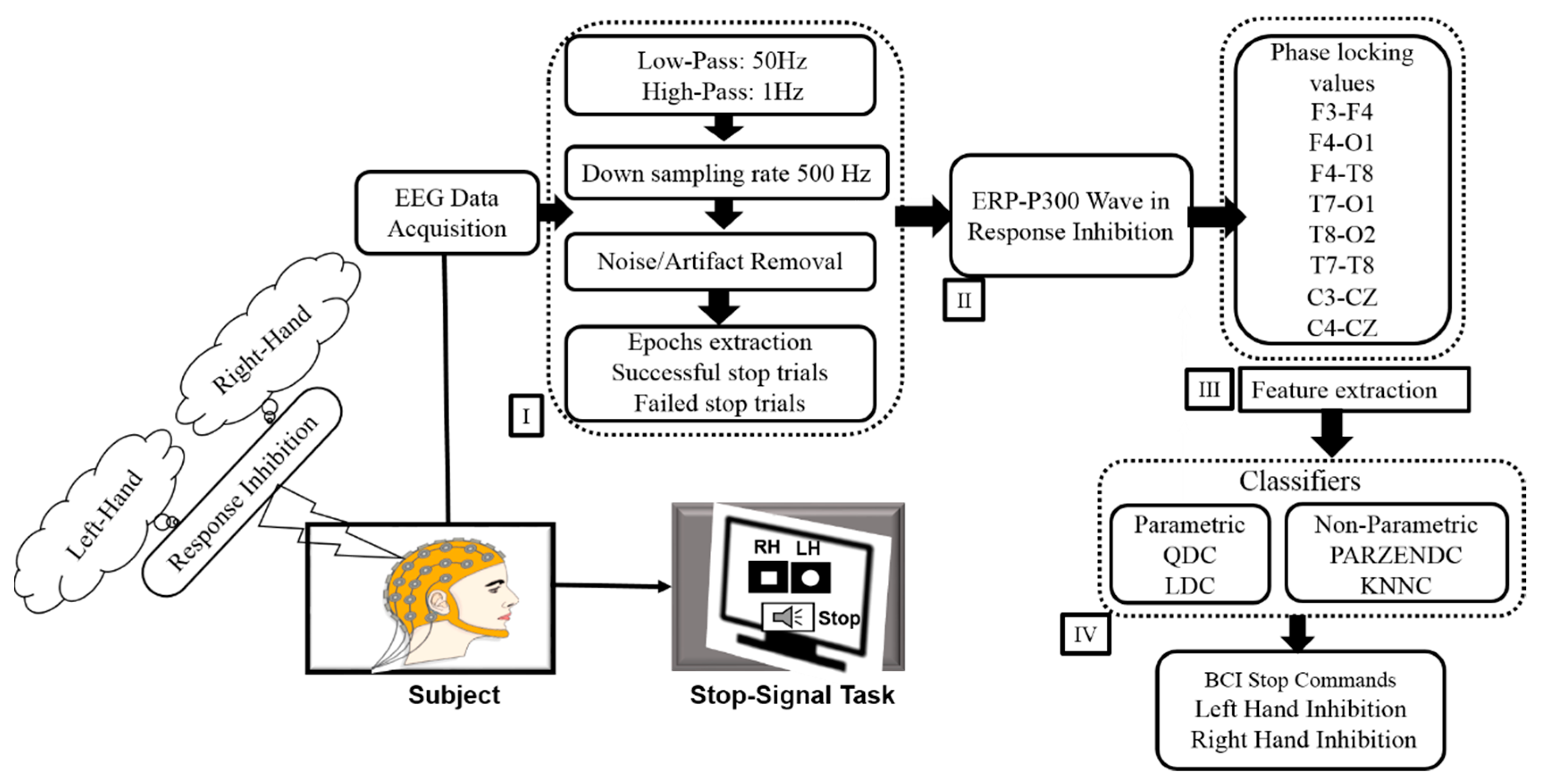
2. Materials and Methods
2.1. Subjects
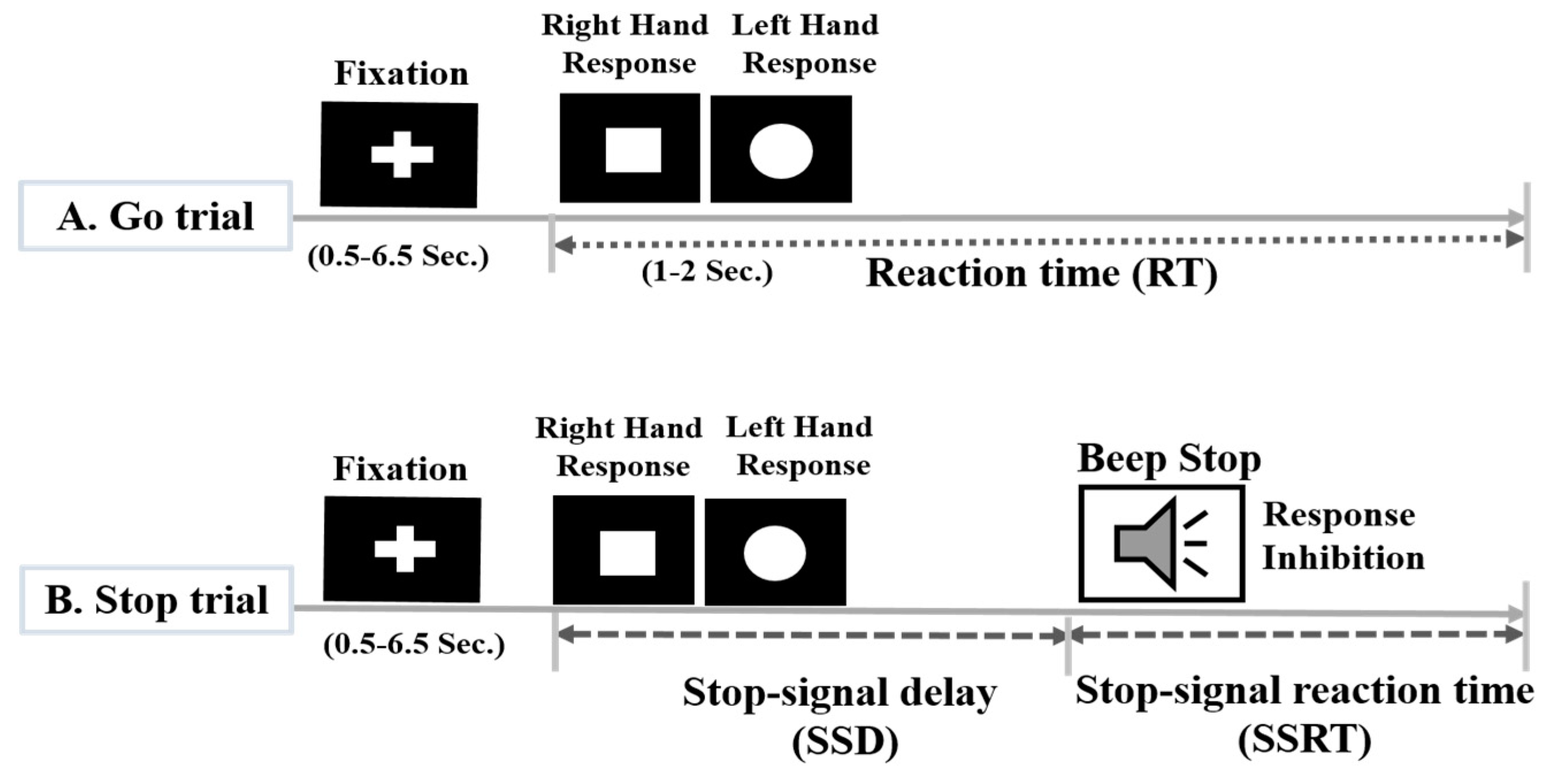
2.2. Experimental Scenario
2.3. Acquisition of EEG Signals
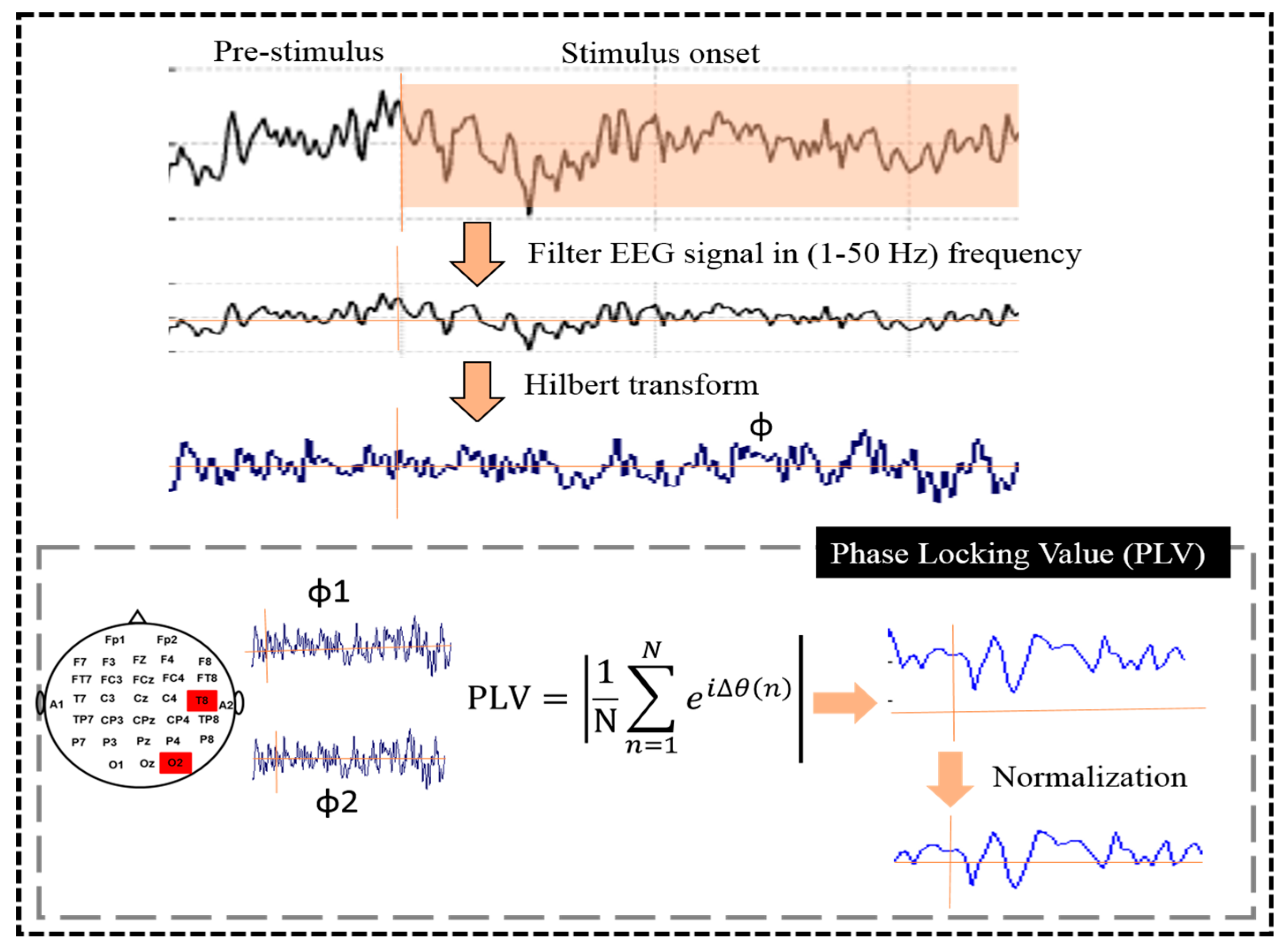
2.4. Phase Locking Value (PLV)
2.5. Feature Extraction for Hierarchical Classification Model
2.6. Forward Feature Selection for Hierarchical Classification Model
2.7. Parametric and Nonparametric Classifier Algorithms in Hierarchical Model
2.8. Accuracy Estimation Method
3. Results
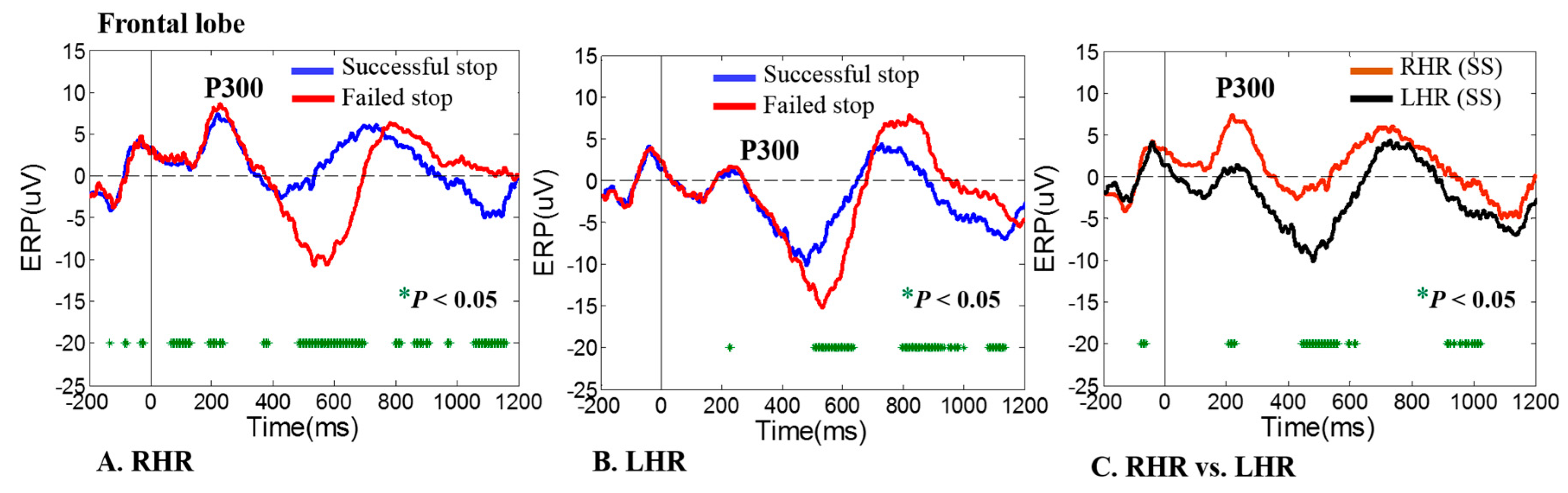
3.1. ERP-P300 Wave during Human Inhibitory Control
3.2. EEG-PLV in Human Inhibitory Control
3.3. Architectural Structures of Hierarchical Classification Model
3.4. Performance of the Hierarchical Classification Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verbruggen, F.; Logan, G.D. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 2008, 12, 11418–11424. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Dowson, J.H.; Sahakian, B.J.; Robbins, T.W. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry 2003, 54, 1465–1468. [Google Scholar] [CrossRef]
- Chambers, C.D.; Garavan, H.; Bellgrove, M.A. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci. Biobehav. Rev. 2009, 33, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Aloise, F.; Schettini, F.; Arico, P.; Leotta, F.; Salinari, S.; Mattia, D.; Babiloni, F.; Cincotti, F. P300-based brain-computer interface for environmental control: An asynchronous approach. J. Neural Eng. 2011, 8, 025025. [Google Scholar] [CrossRef] [PubMed]
- Belitski, A.; Farquhar, J.; Desain, P. P300 audio-visual speller. J. Neural Eng. 2011, 8, 025022. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, N.; Kahya, E. An experiment in use of brain computer interfaces for cognitive researches. Int. J. Intell. Sci. 2015, 5, 80–88. [Google Scholar] [CrossRef]
- Sutton, S.; Braren, M.; John, E.R.; Zubin, J. Evoked potential correlates of stimulus uncertainty. Science 1965, 150, 1187–1188. [Google Scholar] [CrossRef]
- Reinvang, I.; Magnussen, S.; Greenlee, M.W. Hemispheric asymmetry in visual discrimination and memory: ERP evidence for the spatial frequency hypothesis. Exp. Brain Res. 2002, 144, 483–495. [Google Scholar] [CrossRef][Green Version]
- Dimoska, A.; Johnstone, S.J.; Barry, R.J. The auditory-evoked N2 and P3 components in the stop-signal task: Indices of inhibition, response-conflict or error-detection. Brain Cogn. 2006, 62, 98–112. [Google Scholar] [CrossRef]
- Harmony, T.; Alba, A.; Marroqun, J.; GonzálezFrankenberger, B. Time-frequency-topographic analysis of induced power and synchrony of EEG signals during a Go/No-Go task. Int. J. Psychophysiol. 2009, 71, 9–16. [Google Scholar] [CrossRef]
- Maguire, M.; Brier, M.; Moore, P.; Ferree, T.; Ray, D.; Mostofsky, S.; Hart, J., Jr.; Kraut, M. The influence of perceptual and semantic categorization on inhibitory processing as measured by the n2-p3 response. Brain Cogn. 2009, 71, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Chikara, R.K.; Komarov, O.; Ko, L.W. Neural signature of event-related N200 and P300 modulation in parietal lobe during human response inhibition. Int. J. Comput. Biol. Drug Des. 2018, 11, 171–182. [Google Scholar] [CrossRef]
- Cabrera, A.; Farina, D.; Dremstrup, K. Comparison of feature selection and classification methods for a brain—Computer interface driven by non-motor imagery. Med. Biol. Eng. Comput. 2010, 48, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Mekulestr, K.; Krauledat, M.; Dornhege, G.; Curio, G.; Blankertz, B. Machine learning techniques for brain-computer interfaces. Biomed. Technol. 2004, 49, 11–22. [Google Scholar]
- Blankertz, B.; Lemm, S.; Treder, M.; Haufe, S.; Mekulestr, K. Single-trial analysis and classification of ERP components-a tutorial. NeuroImage 2011, 56, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Huster, R.J.; Enriquez-Geppert, S.; Lavallee, C.F.; Falkenstein, M.; Herrmann, C.S. Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. Int. J. Psychophysiol. 2013, 87, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Simson, R.; Vaughan, H.G.; Ritter, W. The scalp topography of potentials in auditory and visual Go/NoGo tasks. Electroencephalogr. Clin. Neurophysiol. 1977, 43, 864–875. [Google Scholar] [CrossRef]
- De Jong, R.; Coles, M.G.; Logan, G.D.; Gratton, G. In search of the point of no return: The control of response processes. J. Exp. Psychol. 1990, 16, 164–182. [Google Scholar] [CrossRef]
- Alegre, M.; Alvarez-Gerriko, I.; Valencia, M.; Iriarte, J.; Artieda, J. Oscillatory changes related to the forced termination of a movement. Clin. Neurophysiol. 2008, 119, 290–300. [Google Scholar] [CrossRef]
- Krämer, U.M.; Knight, R.T.; Münte, T.F. Electrophysiological evidence for different inhibitory mechanisms when stopping or changing a planned response. J. Cogn. Neurosci. 2011, 23, 2481–2493. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, Y.; Gao, X.; Gao, S. Amplitude and phase coupling measures for feature extraction in an EEG-based brain-computer interface. J. Neural Eng. 2007, 4, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.P.; Makeig, S.; McKeown, M.J.; Bell, A.J.; Lee, T.W.; Sejnowski, T.J. Imaging brain dynamics using independent component analysis. Proc. IEEE 2001, 89, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.J.; Sejnowski, T.J. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995, 7, 1129–1159. [Google Scholar] [CrossRef] [PubMed]
- Le Van, M.Q.; Foucher, J.; Lauchaux, J.P.; Rodriguez, E.; Lutz, A.; Martinerie, J.; Varela, F. Comparison of Hilbert transform and wavelet methods for the analysis of neuronal synchrony. J. Neurosci. Methods 2001, 111, 83–98. [Google Scholar] [CrossRef]
- Lachaux, J.P.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999, 8, 194–208. [Google Scholar] [CrossRef]
- Palva, S.; Palva, J. New vistas for α-frequency band oscillations. Trends Neurosci. 2007, 4, 4–158. [Google Scholar] [CrossRef] [PubMed]
- Swann, N.; Poizner, H.; Houser, M.; Gould, S.; Greenhouse, I.; Cai, W.; Strunk, J.; George, J.; Aron, A.R. Deep Brain Stimulation of the Subthalamic Nucleus Alters the Cortical Profile of Response Inhibition in the Beta Frequency Band: A Scalp EEG Study in Parkinson’s Disease. J. Neurosci. 2011, 186, 5721–5729. [Google Scholar] [CrossRef]
- Bermingham, M.L.; Pong-Wong, R.; Spiliopoulou, A.; Hayward, C.; Rudan, I.; Campbell, H.; Wright, A.F.; Wilson, J.F.; Agakov, F.; Navarro, P.; et al. Application of high-dimensional feature selection: Evaluation for genomic prediction in man. Sci. Rep. 2015, 5, 10312. [Google Scholar] [CrossRef]
- Guyon, I.; Elisseeff, A. An Introduction to Variable and Feature Selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Fisher, R.A. The use of multiple measurements in taxonomic problems. Ann. Eugen. 1936, 7, 179–188. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Tsuruoka, S.; Kimura, F.; Miyake, Y. Increasing the Feature size in handwritten Numeral Recognition to improve accuracy. Syst. Comput. Jpn. 1995, 26, 35–44. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhou, X.; Huang, T.S.; Hasegawa-Johnson, M. Feature analysis and selection for acoustic event detection. In Proceedings of the 2008 IEEE International Conference on Acoustics, Speech and Signal Processing, Las Vegas, NV, USA, 31 March–4 April 2008. [Google Scholar]
- Parzen, E. On estimation of a probability density function and mode. Ann. Math. Stat. 1962, 1065–1076. [Google Scholar] [CrossRef]
- Webb, A.R. Statistical Pattern Recognition; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Efron, B.; Gong, G. A leisurely look at the bootstrap the jackknife and cross-validation. Am. Stat. 1983, 37, 36–48. [Google Scholar]
- Salazar-Ramirez, A.; Martin, J.I.; Martinez, R.; Arruti, A.; Muguerza, J.; Sierra, B. A hierarchical architecture for recognising intentionality in mental tasks on a brain-computer interface. PLoS ONE 2019, 14, e0218181. [Google Scholar] [CrossRef] [PubMed]
- Keith, S.; Javier, E. The complex hierarchical topology of EEG functional connectivity. J. Neurosci. Methods 2017, 276, 1–12. [Google Scholar]
- Huang, C.S.; Lin, C.L.; Ko, L.W.; Liu, S.Y.; Sua, T.P.; Lin, C.T. A hierarchical classification system for sleep stage scoring via forehead EEG signals. In Proceedings of the 2013 IEEE Symposium on Computational Intelligence, Cognitive Algorithms, Mind, and Brain (CCMB), Singapore, 16–19 April 2013; pp. 1–5. [Google Scholar] [CrossRef]
- Ko, L.W.; Shih, Y.C.; Chikara, R.K.; Chuang, Y.T.; Chang, E.C. Neural Mechanisms of Inhibitory Response in a Battlefield Scenario: A Simultaneous fMRI-EEG Study. Front. Hum. Neurosci. 2016, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Chikara, R.K.; Chang, E.C.; Lu, Y.-C.; Lin, D.-S.; Lin, C.-T.; Ko, L.-W. Monetary Reward and Punishment to Response Inhibition Modulate Activation and Synchronization Within the Inhibitory Brain Network. Front. Hum. Neurosci. 2018, 12, 27. [Google Scholar] [CrossRef]
- Washizawa, Y.; Higashi, H.; Rutkowski, T.; Tanaka, T.; Cichocki, A. Tensor based simultaneous feature extraction and sample weighting for EEG classification. Neural Inf. Process. Models Appl. 2010, 61, 26–33. [Google Scholar]
- Woehrle, H.; Krell, M.M.; Straube, S.; Kim, S.K.; Kirchner, E.A.; Kirchner, F. An adaptive spatial filter for user-independent single trial detection of event-related potentials. IEEE Trans. Biomed. Eng. 2015, 62, 1696–1705. [Google Scholar] [CrossRef]
- Panicker, R.C.; Puthusserypady, S.; Sun, Y. Adaptation in P300 brain–computer interfaces: A two-classifier cotraining approach. IEEE Trans. Biomed. Eng. 2010, 57, 2927–2935. [Google Scholar] [CrossRef]
- Furman, D.; Reichart, R.; Pratt, H. Finger flexion imagery: EEG classification through physiologically-inspired feature extraction and hierarchical voting. In Proceedings of the 4th International Winter Conference on Brain-Computer Interface (BCI), Yongpyong, Korea, 22–24 February 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Ko, L.W.; Lu, Y.C.; Bustince, H.; Chang, Y.C.; Chang, Y.; Ferandez, J.; Wang, Y.K.; Sanz, J.A.; Dimuro, G.P.; Lin, C.T. Multimodal Fuzzy Fusion for Enhancing the Motor-Imagery-Based Brain Computer Interface. IEEE Comput. Intell. Mag. 2019, 14, 96–106. [Google Scholar] [CrossRef]
- Speier, W.; Deshpande, A.; Cui, L.; Chandravadia, N.; Roberts, D.; Pouratian, N. A comparison of stimulus types in online classification of the P300 speller using language models. PLoS ONE 2017, 12, e0175382. [Google Scholar] [CrossRef] [PubMed]
- Murugavel, A.S.M.; Ramakrishnan, S. Hierarchical multi-class SVM with ELM kernel for epileptic EEG signal classification. Med. Biol. Eng. Comput. 2016, 54, 149. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Lin, K.L.; Ko, L.W.; Sheng-Fu Liang, S.F.; Kuo, B.C.; Chung, I.F. Nonparametric Single-Trial EEG Feature Extraction and Classification of Driver’s Cognitive Responses. EURASIP J. Adv. Signal. Process. 2008, 849040. [Google Scholar] [CrossRef]
- Kok, A.; Ramautar, J.; de Ruiter, M.; Band, G.P.H.; Ridderinkhof, K.R. ERP components associated with successful and unsuccessful inhibition in a stop-signal task. Psychophysiology 2004, 41, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Ko, L.W.; Chikara, R.K.; Shih, Y.; Chang, E.C. Neural Oscillations in Temporoparietal Lobes under Inhibitory Control in a Naturalistic Situation. In Proceedings of the 2016 IEEE 16th International Conference on Bioinformatics and Bioengineering (BIBE), Taichung, Taiwan, 31 October–2 November 2016; 2016; pp. 373–376. [Google Scholar] [CrossRef]
- Ko, L.W.; Chikara, R.K.; Liu, Y. Brain activities changes in typical and realistic scenario-compatible stop-signal inhibitory tasks and classification performance. In Proceedings of the 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER), San Diego, CA, USA, 6–8 November 2013; pp. 501–504. [Google Scholar] [CrossRef]
- Greenhouse, I.; Wessel, J.R. EEG signatures associated with stopping are sensitive to preparation. Psychophysiology 2013, 50, 900–908. [Google Scholar] [CrossRef]
- Bostanov, V. BCI competition 2003-data sets Ib and IIb: Feature extraction from event-related brain potentials with the continuous wavelet transform and the t-value scalogram. IEEE Trans. Biomed. Eng. 2004, 51, 1057–1061. [Google Scholar] [CrossRef]
- Atum, Y.; Gareis, I.; Gentiletti, G.; Acevedo, R.; Rufiner, L. Genetic feature selection to optimally detect P300 in brain computer interfaces. In Proceedings of the IEEE Engineering in Medicine and Biology 27th Annual Conference (EMBC 10), Buenos Aires, Argentina, 31 August–4 September 2010; pp. 3289–3292. [Google Scholar]
- Krusienski, D.J.; Sellers, E.W.; Vaughan, T.M. Common spatio-temporal patterns for the P300 speller. In Proceedings of the 3rd International IEEE EMBS Conference on Neural Engineering (CNE ’07), Kohala Coast, HI, USA, 2–5 May 2007; pp. 421–424. [Google Scholar]
- Krusienski, D.J.; Sellers, E.W.; Cabestaing, F.; Bayoudh, S.; McFarland, D.J.; Vaughan, T.M.; Wolpaw, J.R. A comparison of classification techniques for the P300 Speller. J. Neural Eng. 2006, 3, 299–305. [Google Scholar] [CrossRef]
- Wang, Y.T.; Huang, K.-C.; Wei, C.-S.; Huang, T.-Y.; Ko, L.W.; Lin, C.T.; Cheng, C.-K.; Jung, T.P. Developing an EEG-based on-line closed-loop lapse detection and mitigation system. Front. Neurosci. 2014, 8, 321. [Google Scholar] [CrossRef]
- Lee, Y.; Lin, W.; Cherng, F.; Ko, L.W. A Visual Attention Monitor Based on Steady-State Visual Evoked Potential. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wu, X.; Cai, L.; Deng, B.; Wang, J. Modulation of Spectral Power and Functional Connectivity in Human Brain by Acupuncture Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Edelman, B.J.; Meng, J.; Gulachek, N.; Cline, C.C.; He, B. Exploring Cognitive Flexibility with a Noninvasive BCI Using Simultaneous Steady-State Visual Evoked Potentials and Sensorimotor Rhythms. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Nicolelis, M.A. Brain-machine interfaces to restore motor function and probe neural circuits. Nat. Rev. Neurosci. 2003, 4, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.B. Cortical neural prosthetics. Annu. Rev. Neurosci. 2004, 27, 487–507. [Google Scholar] [CrossRef]
- Dobkin, B.H. Brain-computer interface technology as a tool to augment plasticity and outcomes for neurological rehabilitation. J. Physiol. 2007, 579, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.J.; Wolpaw, J.R. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008, 7, 1032–1043. [Google Scholar] [CrossRef]
- Millán, J.D.R.; Rupp, R.; Müller-Putz, G.; Murray-Smith, R.; Giugliemma, C.; Tangermann, M.; Vidaurre, C.; Cincotti, F.; Kubler, A.; Leeb, R.; et al. Combining brain–computer interfaces and assistive technologies: State-of-the-art and challenges. Front. Neurosci. 2010, 4, 161. [Google Scholar]
- Wang, W.; Collinger, J.L.; Perez, M.A.; Tyler-Kabara, E.C.; Cohen, L.G.; Birbaumer, N.; Brose, S.W.; Schwartz, A.B.; Boninger, M.L.; Weber, D.J. Neural interface technology for rehabilitation: Exploiting and promoting neuroplasticity. Phys. Med. Rehabil. Clin. N. Am. 2010, 21, 157–178. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Sahakian, B.J. The neuropsychiatry of impulsivity. Curr. Opin. Psychiatry 2007, 20, 255–261. [Google Scholar] [CrossRef]
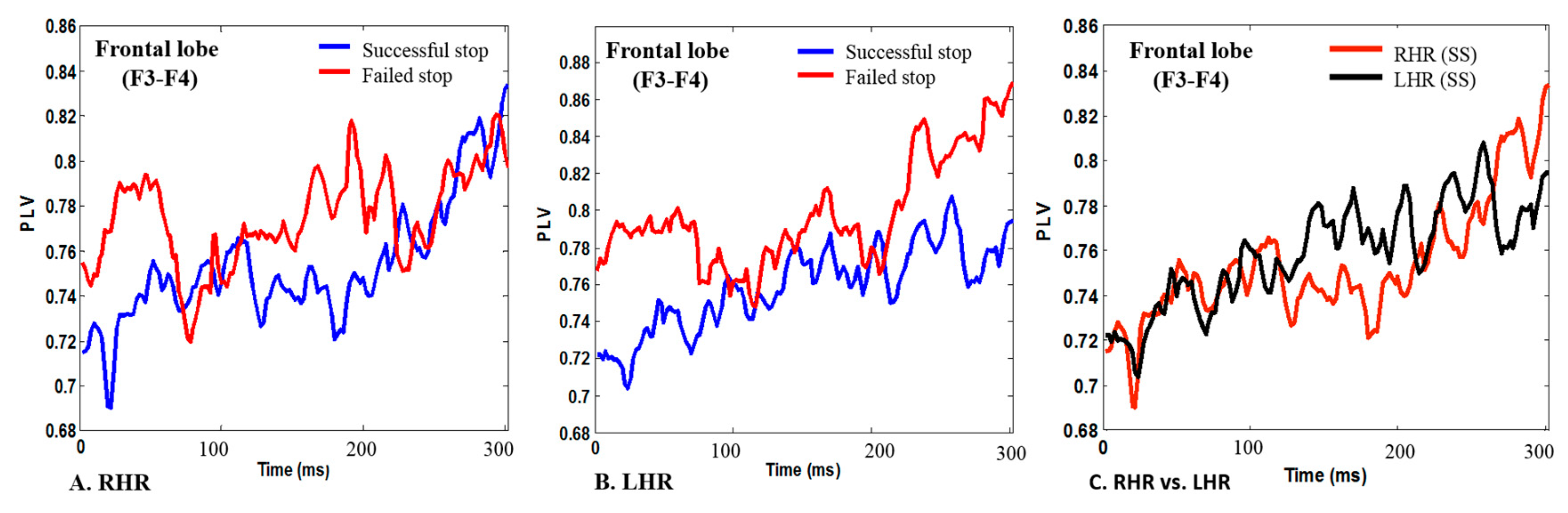
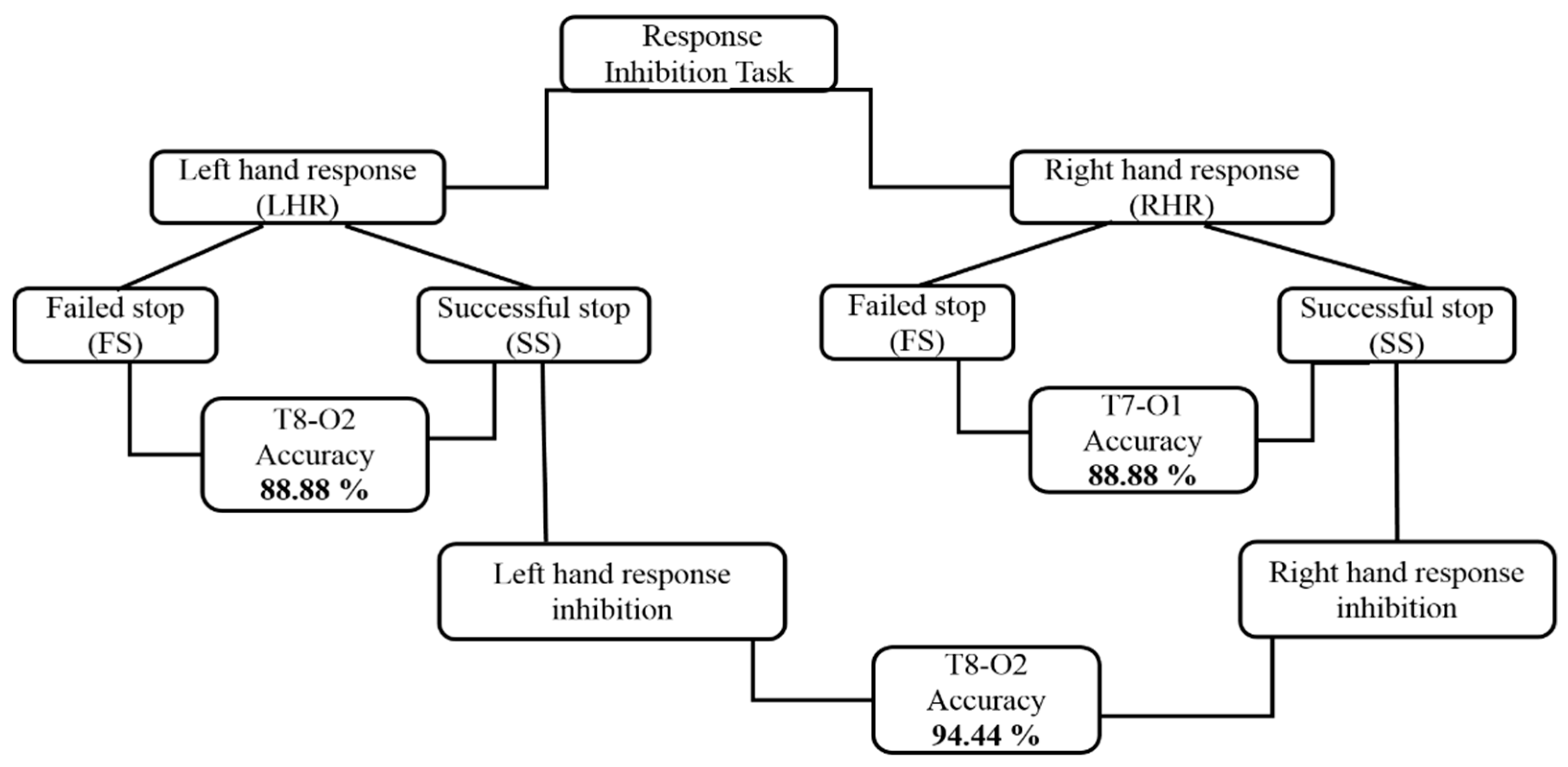

| Outcomes | Hierarchical Model Outcomes Using Brain Connectivity during Right Hand Inhibition | |||||||
|---|---|---|---|---|---|---|---|---|
| F3-F4 | F4-O1 | F4-T8 | T7-O1 | T8-O2 | T7-T8 | C3-CZ | C4-CZ | |
| Classifier | QDA | QDA | LDA | QDA | QDA | QDA | LDA | QDA |
| Sensitivity | 0.58 | 0.58 | 0.77 | 0.88 | 1.00 | 0.88 | 0.83 | 0.91 |
| Specificity | 0.91 | 0.75 | 0.44 | 0.88 | 0.66 | 0.77 | 0.33 | 0.41 |
| FPR | 0.09 | 0.25 | 0.56 | 0.12 | 0.34 | 0.23 | 0.67 | 0.59 |
| PPV | 0.87 | 0.70 | 0.58 | 0.88 | 0.62 | 0.80 | 0.55 | 0.61 |
| F-measure | 0.68 | 0.63 | 0.65 | 0.87 | 0.76 | 0.83 | 0.65 | 0.74 |
| Accuracy (Mean ± SD) | 75 ± 7.46 | 66.66 ± 11.22 | 61.11 ± 8.27 | 88.88 ± 3.32 | 61.11 ± 7.78 | 83.33 ± 3.63 | 58.33 ± 5.69 | 66.66 ± 5.83 |
| Outcomes | Hierarchical Model Outcomes Using Brain Connectivity during Left Hand Inhibition | |||||||
|---|---|---|---|---|---|---|---|---|
| F3-F4 | F4-O1 | F4-T8 | T7-O1 | T8-O2 | T7-T8 | C3-CZ | C4-CZ | |
| Classifier | LDA | LDA | QDA | QDA | QDA | QDA | QDA | QDA |
| Sensitivity | 0.91 | 0.66 | 0.66 | 0.77 | 1.00 | 0.88 | 0.50 | 0.83 |
| Specificity | 0.50 | 0.58 | 0.55 | 0.66 | 0.77 | 0.55 | 0.75 | 0.41 |
| FPR | 0.50 | 0.42 | 0.45 | 0.34 | 0.23 | 0.45 | 0.25 | 0.59 |
| PPV | 0.64 | 0.61 | 0.60 | 0.70 | 0.81 | 0.66 | 0.66 | 0.58 |
| F-measure | 0.74 | 0.62 | 0.62 | 0.72 | 0.89 | 0.75 | 0.56 | 0.68 |
| Accuracy (Mean ± SD) | 70.8 ± 5.50 | 62.50 ± 6.02 | 61.11 ± 6.29 | 72.22 ± 3.21 | 88.88 ± 2.48 | 72.22 ± 2.25 | 62.50 ± 3.49 | 62.50 ± 2.65 |
| Outcomes | Hierarchical Model Outcomes Using Brain Connectivity in RHR and LHR Inhibitions | |||||||
|---|---|---|---|---|---|---|---|---|
| F3-F4 | F4-O1 | F4-T8 | T7-O1 | T8-O2 | T7-T8 | C3-CZ | C4-CZ | |
| Classifier | KNNC | QDA | QDA | QDA | QDA | QDA | QDA | QDA |
| Sensitivity | 0.58 | 0.50 | 0.66 | 0.91 | 0.88 | 0.50 | 0.66 | 0.91 |
| Specificity | 0.66 | 0.83 | 0.75 | 0.75 | 1.00 | 0.75 | 0.50 | 0.58 |
| FPR | 0.34 | 0.17 | 0.25 | 0.25 | 0.00 | 0.25 | 0.50 | 0.42 |
| PPV | 0.46 | 0.75 | 0.72 | 0.78 | 1.00 | 0.66 | 0.57 | 0.68 |
| F-measure | 0.50 | 0.29 | 0.68 | 0.83 | 0.93 | 0.56 | 0.60 | 0.77 |
| Accuracy (Mean ± SD) | 62.50 ± 2.24 | 66.66 ± 5.44 | 70.83 ± 3.52 | 83.34 ± 1.71 | 94.44 ± 1.00 | 62.50 ± 2.70 | 58.33 ± 1.95 | 75 ± 2.09 |
| EEG Signal | Features | Classifier | References |
|---|---|---|---|
| Sleep stage, Fp1, Fp2 | Band power | Hierarchical modal, SVM | [38] |
| Motor imagery | Band power | adaptive LDA/QDA | [41] |
| P300 | Time points | adaptive LDA/SVM | [42] |
| P300 | Time Points | Co-training LDA | [43] |
| Motor imagery | Time point, Power spectral | Hierarchical modal, SVM | [44] |
| Motor imagery | Band power | Hierarchical, KNN, SVM | [45] |
| P300, Motor imagery | Band power | QDA, LDA, KNNC | [46] |
| P300 | Time points | SWLDA | [47] |
| Epileptic seizures | Wavelet transformation | Hierarchical modal, SVM | [47] |
| Virtual-reality (VR) | NWFE, PCA | NBC, KNNC | [48] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikara, R.K.; Ko, L.-W. Neural Activities Classification of Human Inhibitory Control Using Hierarchical Model. Sensors 2019, 19, 3791. https://doi.org/10.3390/s19173791
Chikara RK, Ko L-W. Neural Activities Classification of Human Inhibitory Control Using Hierarchical Model. Sensors. 2019; 19(17):3791. https://doi.org/10.3390/s19173791
Chicago/Turabian StyleChikara, Rupesh Kumar, and Li-Wei Ko. 2019. "Neural Activities Classification of Human Inhibitory Control Using Hierarchical Model" Sensors 19, no. 17: 3791. https://doi.org/10.3390/s19173791
APA StyleChikara, R. K., & Ko, L.-W. (2019). Neural Activities Classification of Human Inhibitory Control Using Hierarchical Model. Sensors, 19(17), 3791. https://doi.org/10.3390/s19173791




