Corrosion Detection by Infrared Attenuated Total Reflection Spectroscopy via Diamond-Like Carbon-Coated Silicon Wafers and Iron-Sensitive Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Measurement Parameters
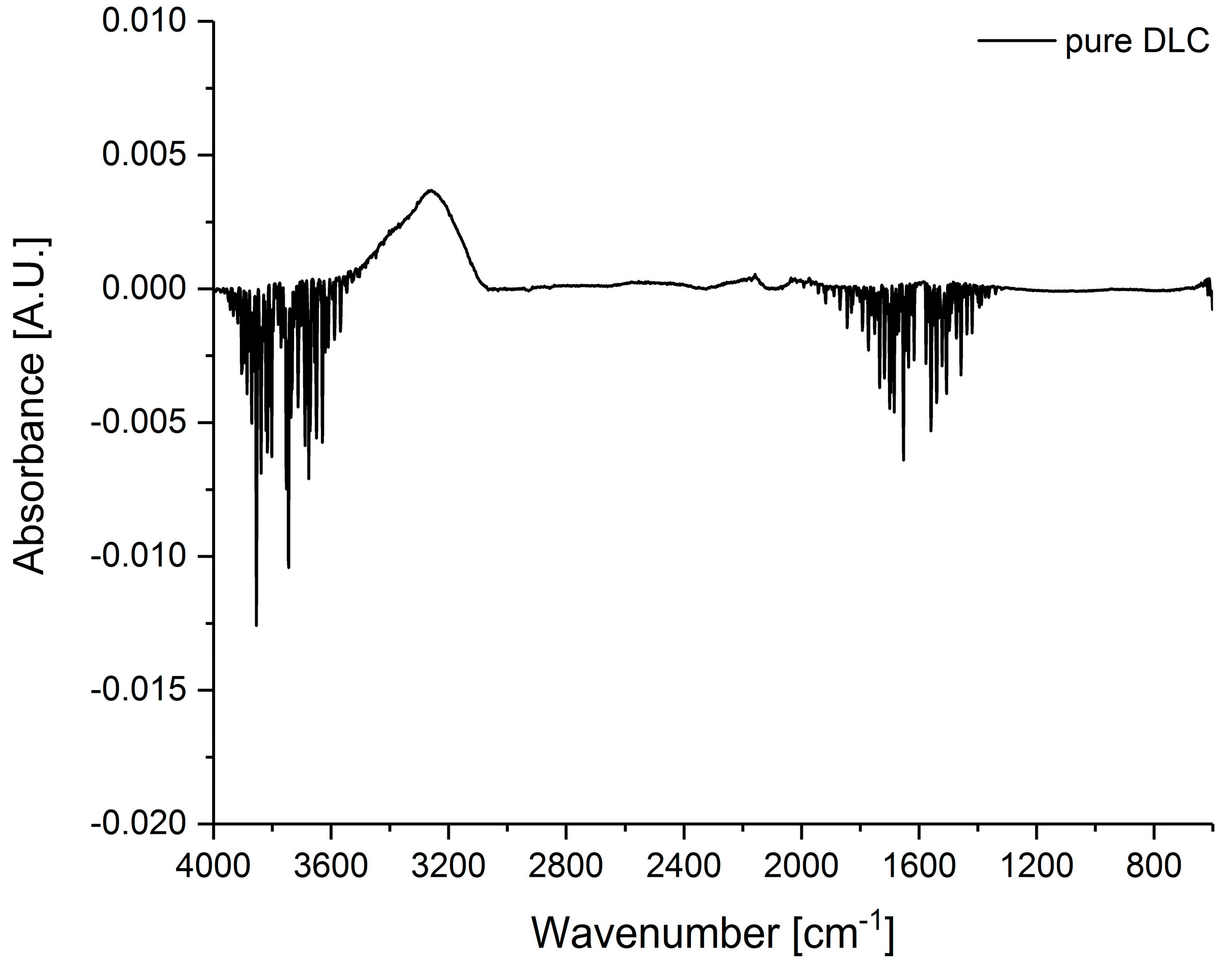
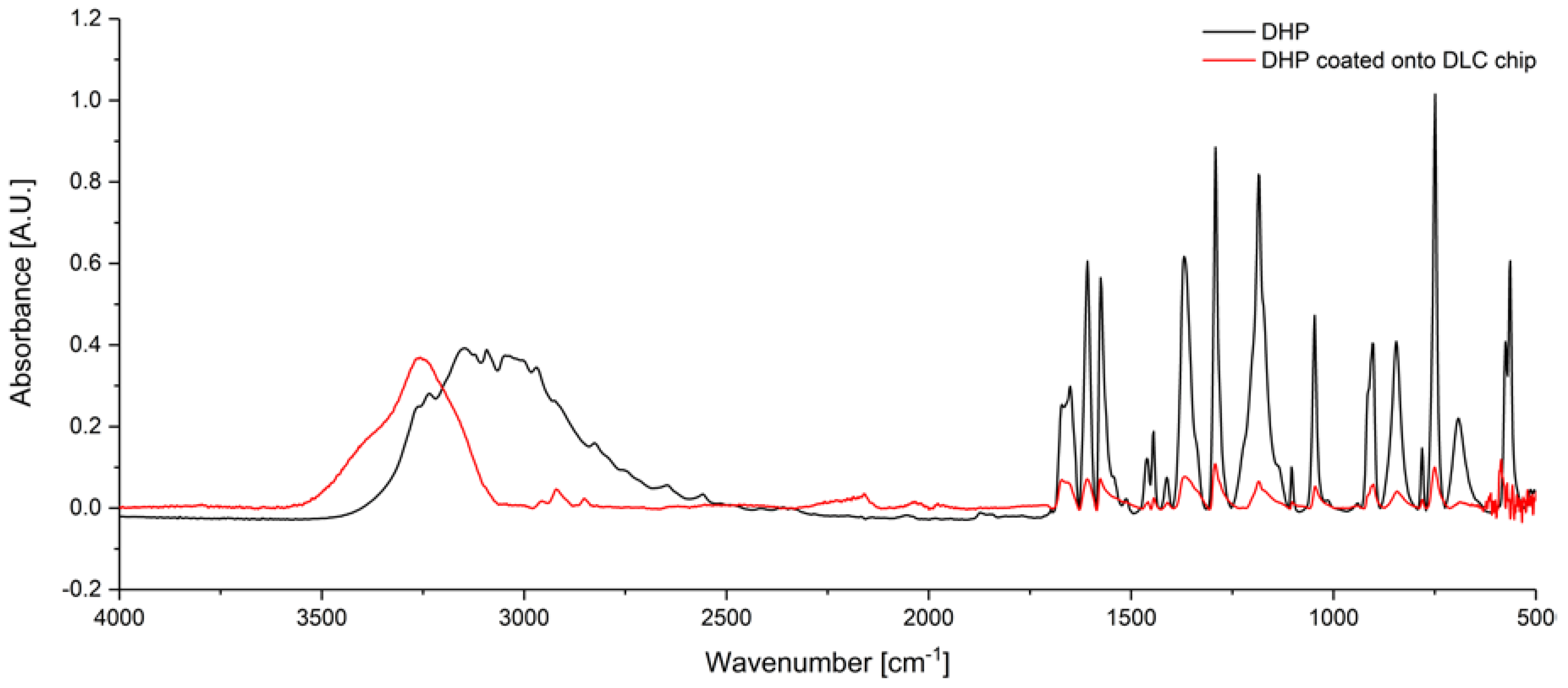
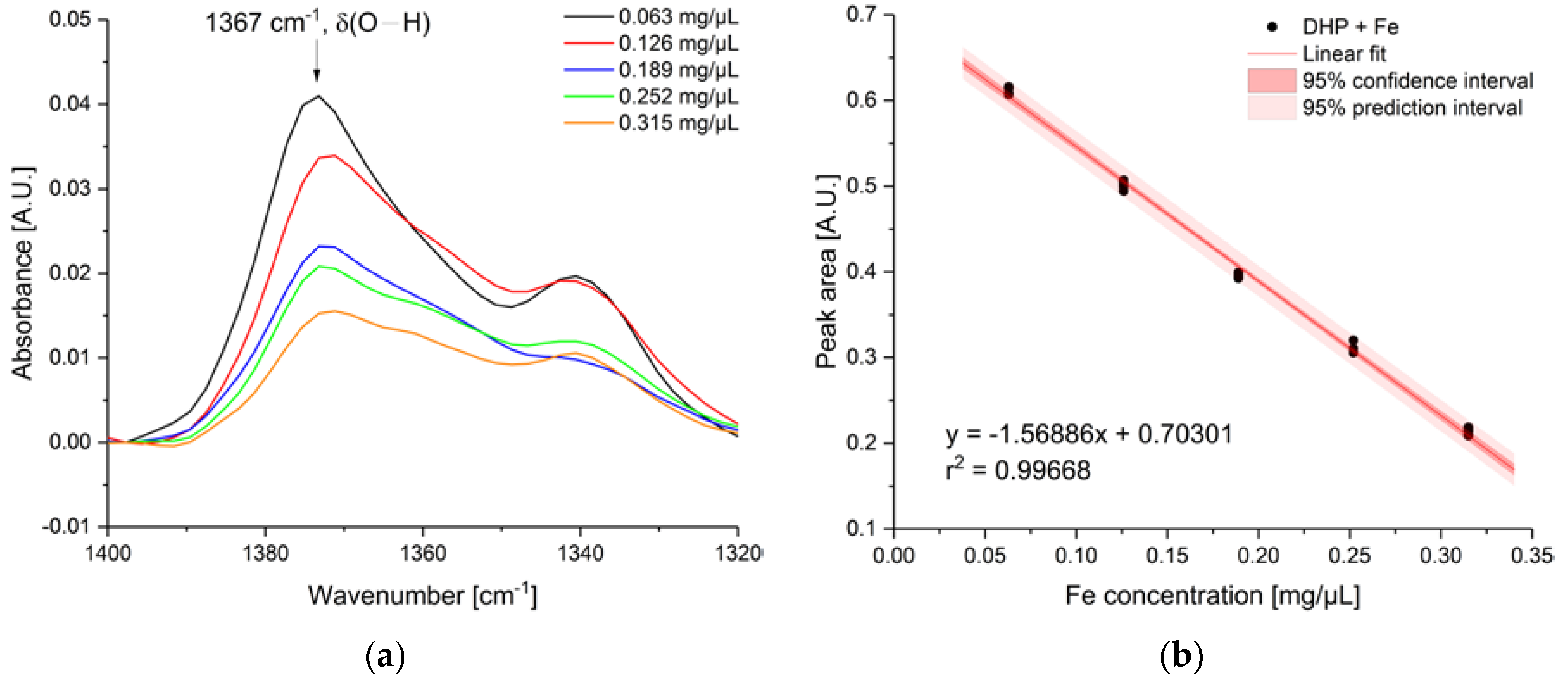
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferreira, H.; Leite, M.G.P. A Life Cycle Assessment study of iron ore mining. J. Clean. Prod. 2015, 108, 1081–1091. [Google Scholar] [CrossRef]
- Kuranovas, A.; Goode, D.; Kvedaras, A.K.; Zhong, S. Load-Bearing Capacity of Concrete-Filled Steel Columns. J. Civ. Eng. Manag. 2009, 15, 21–33. [Google Scholar] [CrossRef]
- Mori, K.; Maki, S.; Tanaka, Y. Warm and Hot Stamping of Ultra High Tensile Strength Steel Sheets Using Resistance Heating. CIRP Ann. 2005, 54, 209–212. [Google Scholar] [CrossRef]
- Neumüller, O.A. Römpps Chemie-Lexikon, 8th ed.; Franckh: Stuttgart, Germany, 1988. [Google Scholar]
- Mcneill, L.S.; Edwards, M. Iron pipe corrosion in distribution systems. J. Am. Water Work. Assoc. 2001, 93, 88–100. [Google Scholar] [CrossRef]
- Li, C.Q.; Mahmoodian, M. Risk based service life prediction of underground cast iron pipes subjected to corrosion. Reliab. Eng. Syst. Saf. 2013, 119, 102–108. [Google Scholar] [CrossRef]
- Sabot, R.; Jeannin, M.; Gadouleau, M.; Guo, Q.; Sicre, E.; Refait, P. Influence of lactate ions on the formation of rust. Corros. Sci. 2007, 49, 1610–1624. [Google Scholar] [CrossRef]
- Graham, M.J.; Cohen, M. Analysis of Iron Corrosion Products Using Moessbauer Spectroscopy. Corrosion 1976, 32, 432–437. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part I. Establishment of equivalent circuits for EIS data modelling. Corros. Sci. 2003, 45, 1257–1273. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Matthews, A. EIS comparison on corrosion performance of PVD TiN and CrN coated mild steel in 0.5 N NaCl aqueous solution. Corros. Sci. 2001, 43, 1953–1961. [Google Scholar] [CrossRef]
- Guan, J.-G.; Miao, Y.-Q.; Zhang, Q.-J. Impedimetric biosensors. J. Biosci. Bioeng. 2004, 97, 219–226. [Google Scholar] [CrossRef]
- Mizaikoff, B. Mid-infrared evanescent wave sensors - A novel approach for subsea monitoring. Meas. Sci. Technol. 1999, 10, 1185–1194. [Google Scholar] [CrossRef]
- Wang, X.; Karlsson, M.; Forsberg, P.; Sieger, M.; Nikolajeff, F.; Österlund, L.; Mizaikoff, B. Diamonds are a spectroscopists best friend: Thin-film diamond mid-infrared waveguides for advanced chemical sensors/biosensors. Anal. Chem. 2014, 86, 8136–8141. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P.O.; Viberg, P.; Forsberg, P.; Nikolajeff, F.; Österlund, L.; Karlsson, M. Nanocrystalline diamond sensor targeted for selective CRP detection: An ATR-FTIR spectroscopy study. Anal. Bioanal. Chem. 2016, 408, 3675–3680. [Google Scholar] [CrossRef] [PubMed]
- Fromell, K.; Forsberg, P.; Karlsson, M.; Larsson, K.; Nikolajeff, F.; Baltzer, L. Designed protein binders in combination with nanocrystalline diamond for use in high-sensitivity biosensors. Anal. Bioanal. Chem. 2012, 404, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- López-Lorente, Á.I.; Wang, P.; Sieger, M.; Vargas Catalan, E.; Karlsson, M.; Nikolajeff, F.; Österlund, L.; Mizaikoff, B. Mid-infrared thin-film diamond waveguides combined with tunable quantum cascade lasers for analyzing the secondary structure of proteins. Phys. Status Solidi Appl. Mater. Sci. 2016, 213, 2117–2123. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Karlsson, M.; Österlund, L.; Mizaikoff, B. Diamond Waveguides for Infrared Spectroscopy and Sensing. In Carbon-Based Nanosensor Technology; Kranz, C., Ed.; Springer Series on Chemical Sensors and Biosensors (Methods and Applications); Springer: Cham, Switzerland, 2017; Volume 17. [Google Scholar]
- Andersson, P.O.; Lundquist, M.; Tegler, L.; Börjegren, S.; Baltzer, L.; Österlund, L. A novel ATR-FTIR approach for characterisation and identification of ex situ immobilised species. ChemPhysChem 2007, 8, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, V.; Muthunatesan, S. FT-IR, FT-Raman spectra and scaled quantum mechanical study of 2,3-dihydroxy pyridine and 2,4-dihyroxy-3-nitropyridine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Mikenda, W. Stretching Frequency versus Bond Distance Correlation of O-D(H) Y hydrogen Bonds in Solid Hydrates. J. Mol. Struct. 1986, 147, 1–15. [Google Scholar] [CrossRef]
- Aktaş, N.; Şahiner, N.; Kantoğlu, Ö.; Salih, B.; Tanyolaç, A. Biosynthesis and Characterization of Laccase Catalyzed Poly(Catechol). J. Polym. Environ. 2003, 11, 123–128. [Google Scholar] [CrossRef]
- Makuraza, J.; Pogrebnaya, T.; Pogrebnoi, A. Vibrational and Electronic Spectra of Natural Dyes Constituents for Solar Cell Application: DFT and TDDFT Study. Int. J. Mater. Sci. Appl. 2015, 4, 314–324. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türkmen, D.; Dettenrieder, C.; Forsberg, P.; Mattsson, A.; Nikolajeff, F.; Österlund, L.; Karlsson, M.; Mizaikoff, B. Corrosion Detection by Infrared Attenuated Total Reflection Spectroscopy via Diamond-Like Carbon-Coated Silicon Wafers and Iron-Sensitive Dyes. Sensors 2019, 19, 3373. https://doi.org/10.3390/s19153373
Türkmen D, Dettenrieder C, Forsberg P, Mattsson A, Nikolajeff F, Österlund L, Karlsson M, Mizaikoff B. Corrosion Detection by Infrared Attenuated Total Reflection Spectroscopy via Diamond-Like Carbon-Coated Silicon Wafers and Iron-Sensitive Dyes. Sensors. 2019; 19(15):3373. https://doi.org/10.3390/s19153373
Chicago/Turabian StyleTürkmen, Dervis, Carina Dettenrieder, Pontus Forsberg, Andreas Mattsson, Fredrik Nikolajeff, Lars Österlund, Mikael Karlsson, and Boris Mizaikoff. 2019. "Corrosion Detection by Infrared Attenuated Total Reflection Spectroscopy via Diamond-Like Carbon-Coated Silicon Wafers and Iron-Sensitive Dyes" Sensors 19, no. 15: 3373. https://doi.org/10.3390/s19153373
APA StyleTürkmen, D., Dettenrieder, C., Forsberg, P., Mattsson, A., Nikolajeff, F., Österlund, L., Karlsson, M., & Mizaikoff, B. (2019). Corrosion Detection by Infrared Attenuated Total Reflection Spectroscopy via Diamond-Like Carbon-Coated Silicon Wafers and Iron-Sensitive Dyes. Sensors, 19(15), 3373. https://doi.org/10.3390/s19153373






