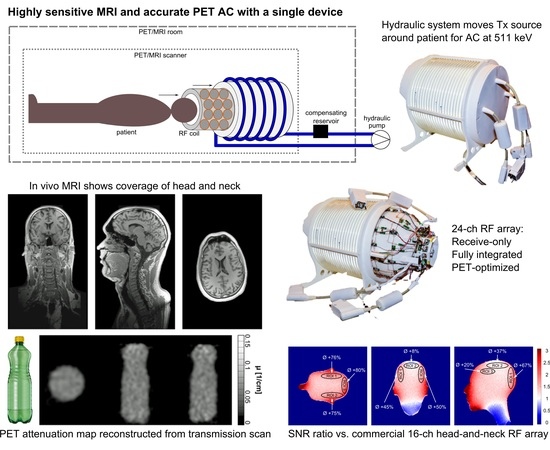
Design, Implementation, and Evaluation of a Head and Neck MRI RF Array Integrated with a 511 keV Transmission Source for Attenuation Correction in PET/MR
Abstract
1. Introduction
2. Materials and Methods
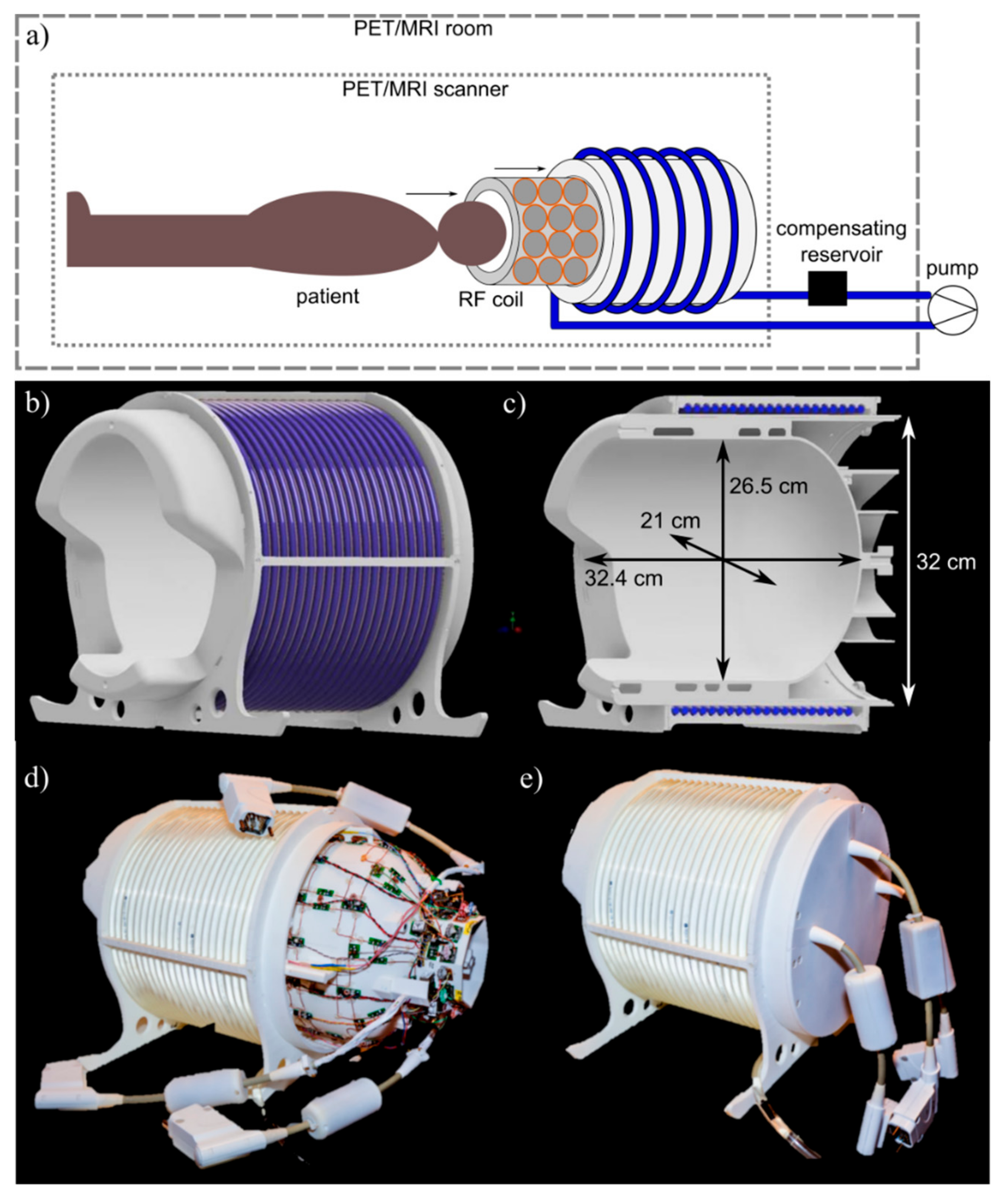
2.1. System Overview
2.2. Mechanical Support and Coil Housing
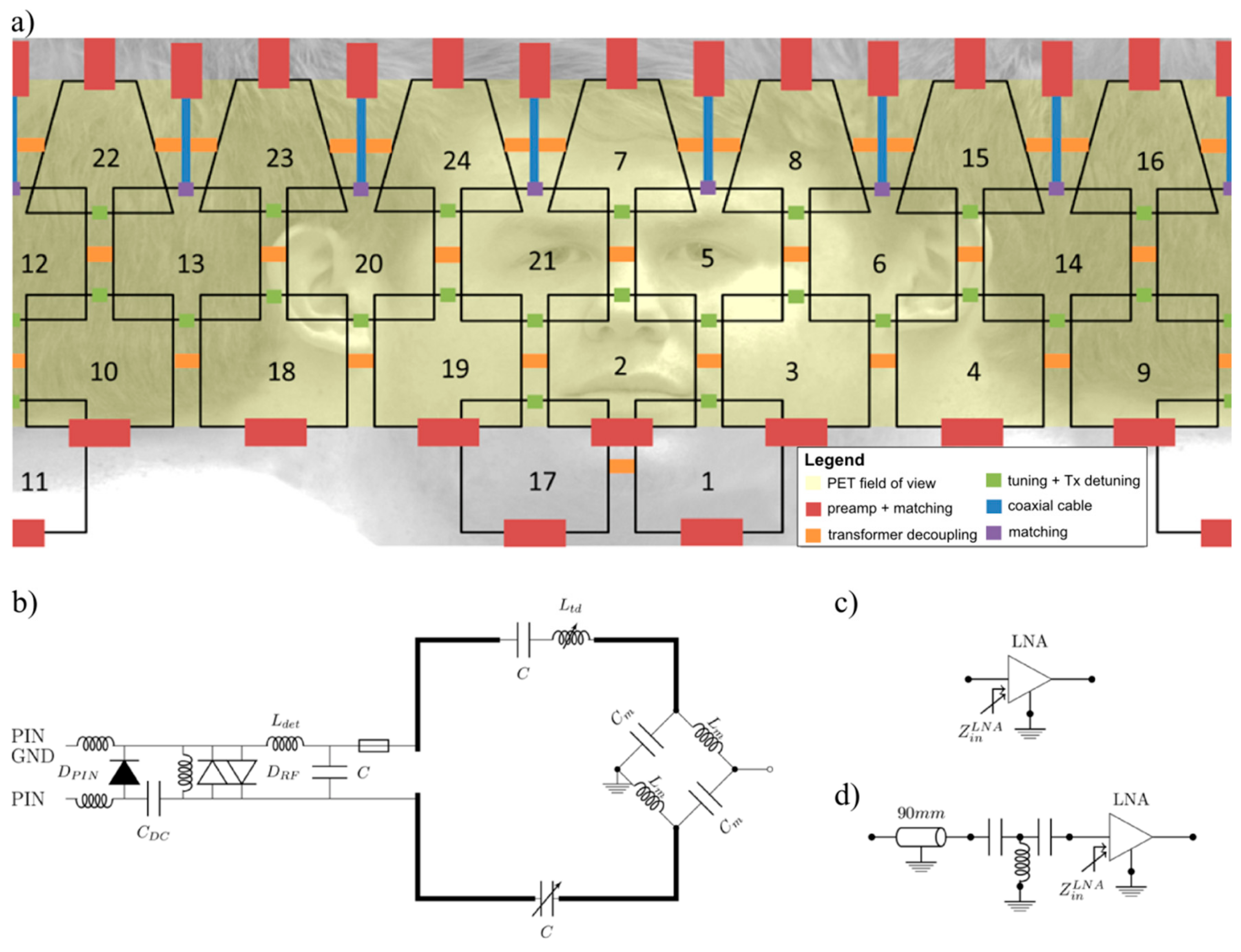
2.3. RF Coil Design and Construction
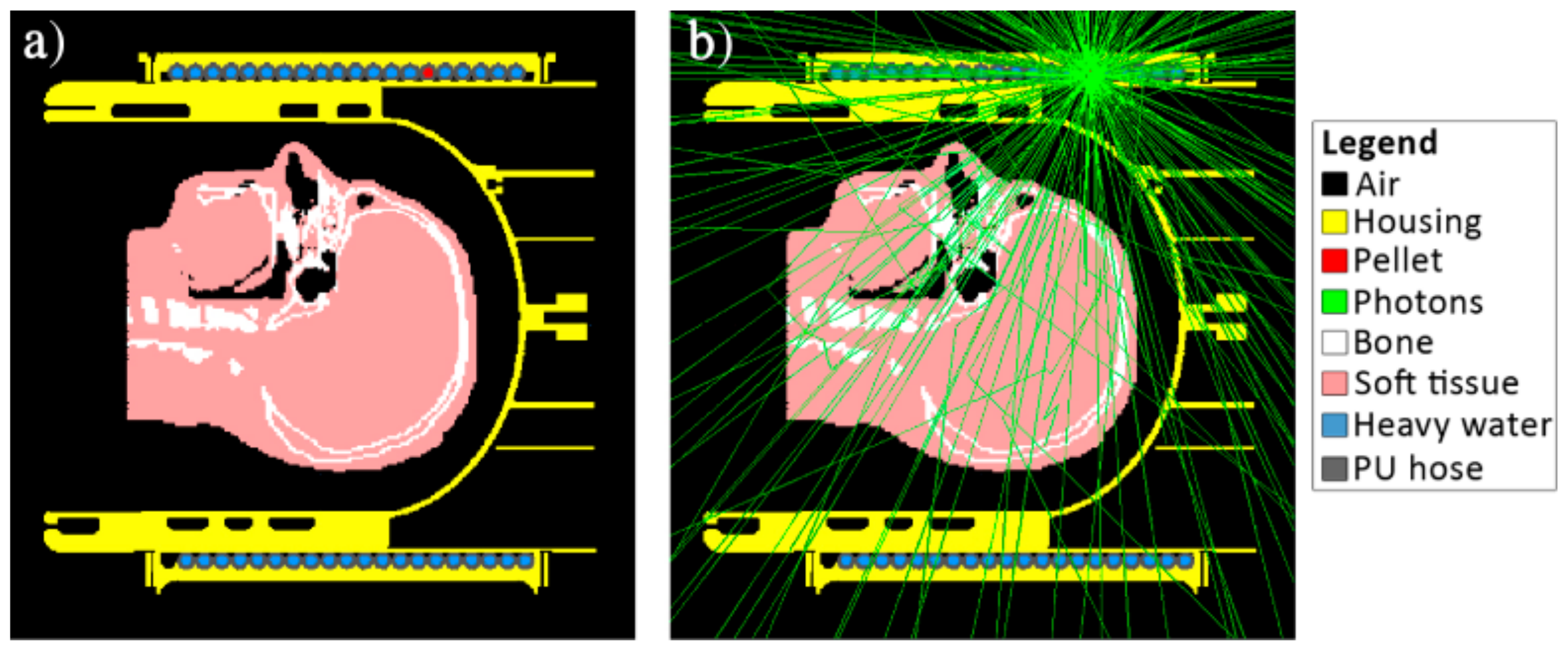
2.4. Monte-Carlo Simulations
2.5. Bench Evaluation of the MR Receiver Array
2.6. MRI Evaluation of the Developed Hardware
2.7. PET Attenuation Coefficient Computation
3. Results
3.1. Monte-Carlo Simulations
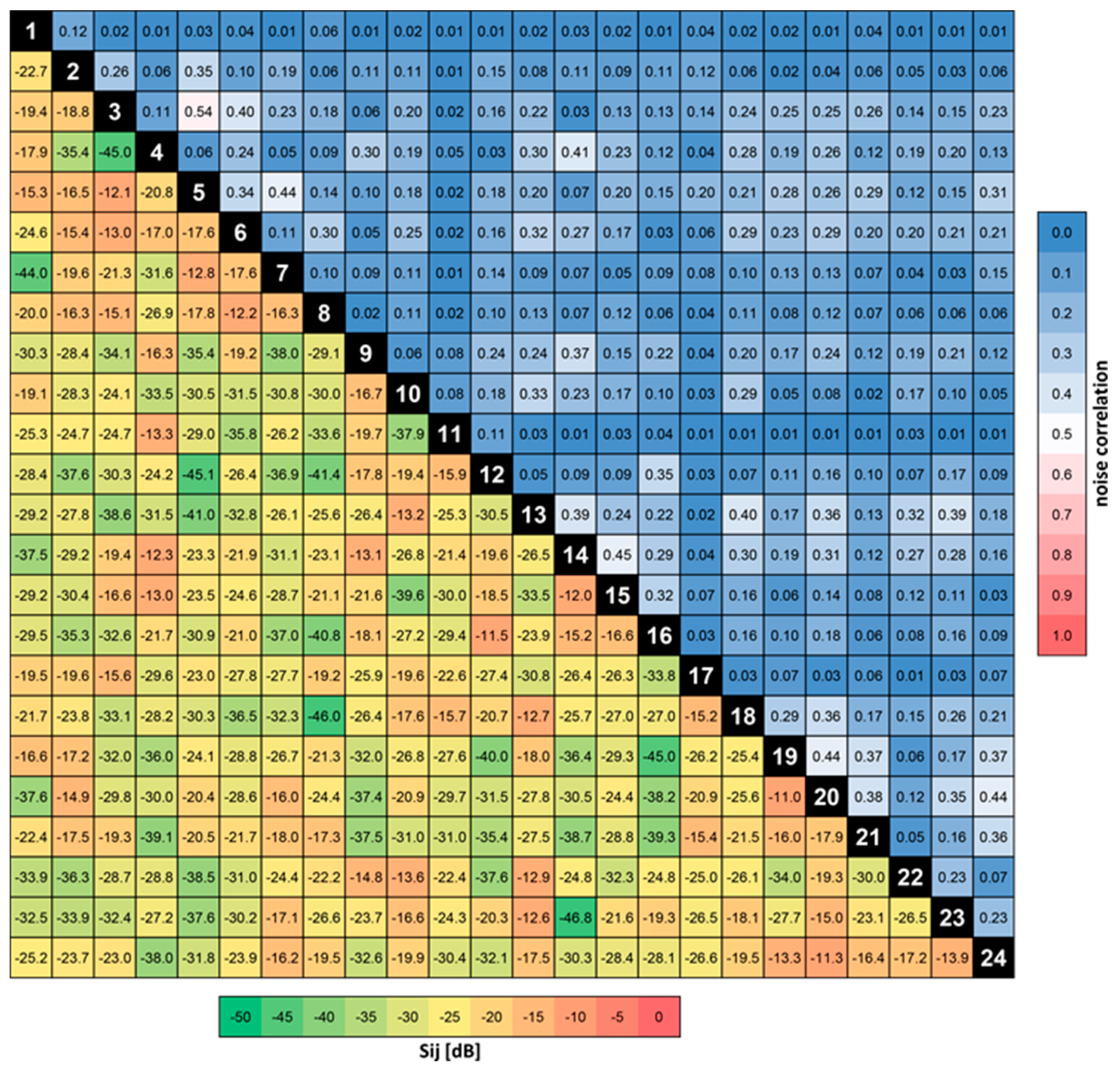
3.2. S-Parameters, Q-Factors, Noise Correlation
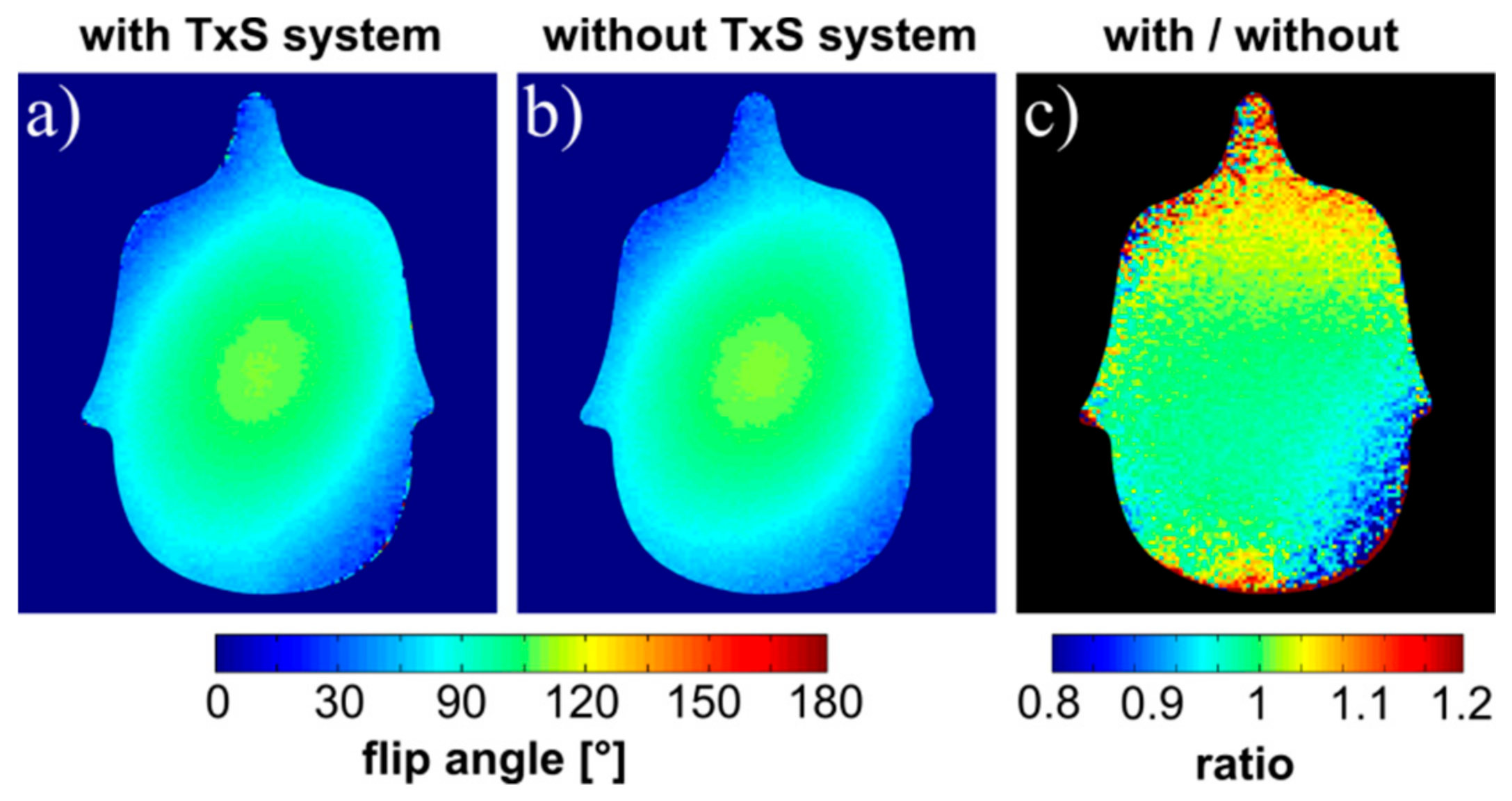
3.3. Influence on Static Magnetic Field (B0) and RF Transmit field (B1+)
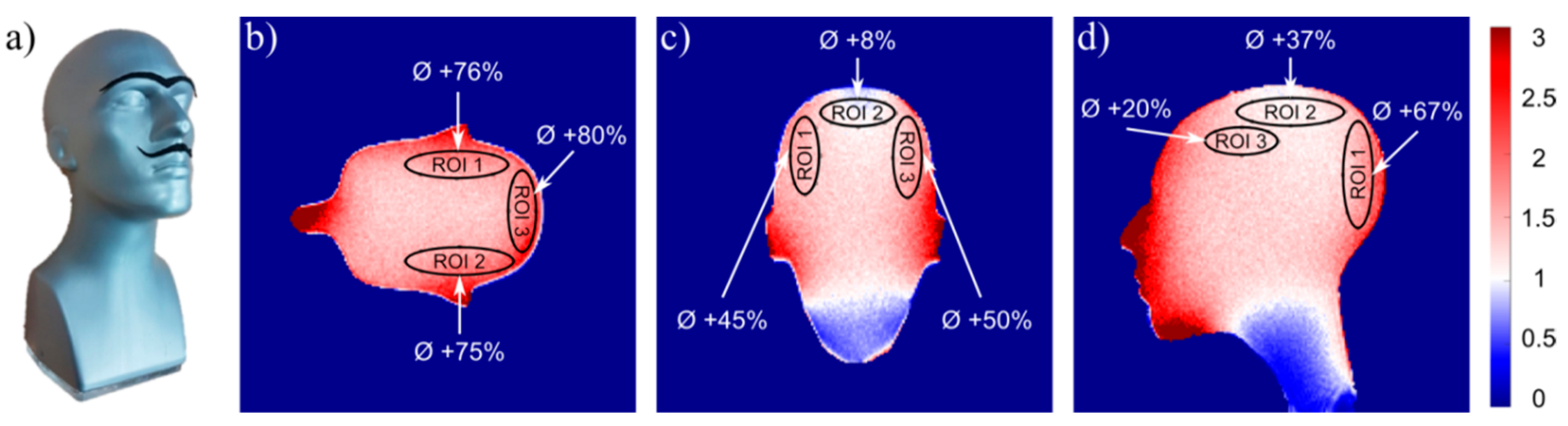
3.4. Performance Comparison with Commercial 16-Channel Coil
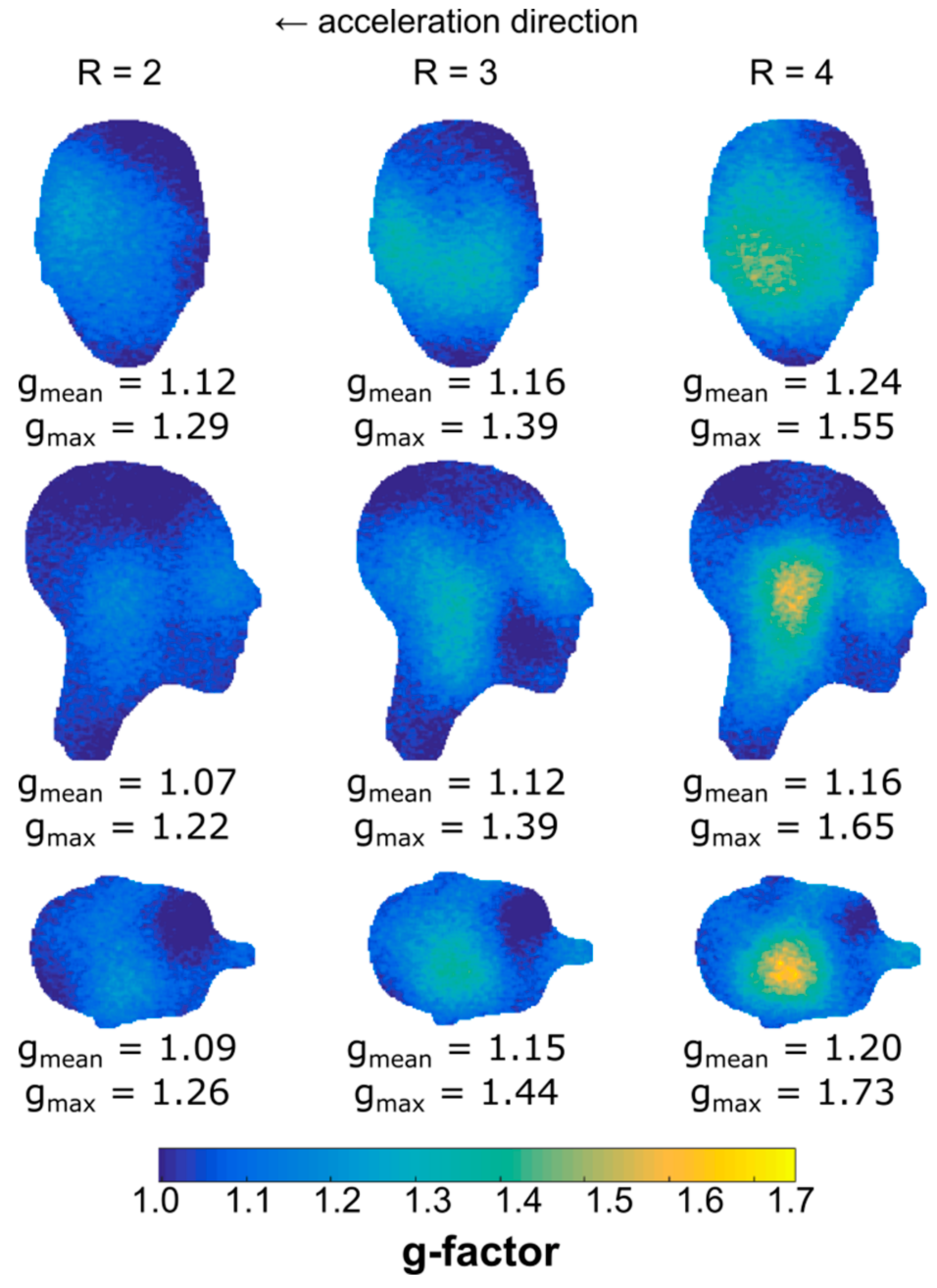
3.5. Parallel Imaging Performance: g-Factor Maps
3.6. In Vivo MRI
3.7. PET Performance: Attenuation Correction Calculation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hammer, B.E. Use of a magnetic field to increase the spatial resolution of positron emission tomography. Med. Phys. 1994, 21, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Torigian, D.A.; Zaidi, H.; Kwee, T.C.; Saboury, B.; Udupa, J.K.; Cho, Z.-H.; Alavi, A. PET/MR imaging: Technical aspects and potential clinical applications. Radiology 2013, 267, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Wehrl, H.F.; Sauter, A.W.; Divine, M.R.; Pichler, B.J. Combined PET/MR: A Technology Becomes Mature. J. Nucl. Med. 2015, 56, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Roemer, P.B.; Edelstein, W.A.; Hayes, C.E.; Souza, S.P.; Mueller, O.M. The NMR phased array. Magn. Reson. Med. 1990, 16, 192–225. [Google Scholar] [CrossRef] [PubMed]
- Keil, B.; Wald, L.L. Massively Parallel MRI Detector Arrays. J. Magn. Reson. 2013, 229, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.Y.; Keil, B.; Chonde, D.B.; Rosen, B.R.; Catana, C.; Wald, L.L. A 31-channel MR brain array coil compatible with positron emission tomography. Magn. Reson. Med. 2015, 73, 2363–2375. [Google Scholar] [CrossRef]
- Herrick, P.D.E.; Ansorge, R.E.; Hawkes, R.C.; Sawiak, S.J.; Stevick, J.W.; Carpenter, T.A. Radiofrequency coil design for simultaneous PET/MR systems. In Proceedings of the IEEE Nuclear Science Symposium & Medical Imaging Conference, Knoxville, TN, USA, 30 October–6 November 2010; pp. 2560–2567. [Google Scholar]
- Bezrukov, I.; Schmidt, H.; Mantlik, F.; Schwenzer, N.; Brendle, C.; Scholkopf, B.; Pichler, B.J. MR-Based Attenuation Correction Methods for Improved PET Quantification in Lesions Within Bone and Susceptibility Artifact Regions. J. Nucl. Med. 2013, 54, 1768–1774. [Google Scholar] [CrossRef]
- Dixon, W.T. Simple proton spectroscopic imaging. Radiology 1984, 153, 189–194. [Google Scholar] [CrossRef]
- Martinez-Moller, A.; Nekolla, S.G. Attenuation correction for PET/MR: Problems, novel approaches and practical solutions. Z. Med. Phys. 2012, 22, 299–310. [Google Scholar] [CrossRef]
- Navalpakkam, B.K.; Braun, H.; Kuwert, T.; Quick, H.H. Magnetic resonance-based attenuation correction for PET/MR hybrid imaging using continuous valued attenuation maps. Investig. Radiol. 2013, 48, 323–332. [Google Scholar] [CrossRef]
- Dickson, J.C.; O’Meara, C.; Barnes, A. A comparison of CT- and MR-based attenuation correction in neurological PET. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Kartmann, R.; Paulus, D.H.; Braun, H.; Aklan, B.; Ziegler, S.; Navalpakkam, B.K.; Lentschig, M.; Quick, H.H. Integrated PET/MR imaging: automatic attenuation correction of flexible RF coils. Med. Phys. 2013, 40, 82301. [Google Scholar] [CrossRef] [PubMed]
- Keereman, V.; Mollet, P.; Berker, Y.; Schulz, V.; Vandenberghe, S. Challenges and current methods for attenuation correction in PET/MR. Magn. Reson. Mater. Phys. 2013, 26, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, L.; Rota Kops, E.; Yun, S.D.; da Silva, N.; Mauler, J.; Weirich, C.; Scheins, J.; Herzog, H.; Tellmann, L.; Lohmann, P.; et al. The Jülich Experience With Simultaneous 3T MR-BrainPET: Methods and Technology. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 3, 352–362. [Google Scholar] [CrossRef]
- Rezaei, A.; Defrise, M.; Bal, G.; Michel, C.; Conti, M.; Watson, C.; Nuyts, J. Simultaneous reconstruction of activity and attenuation in time-of-flight PET. IEEE Trans. Med. Imaging 2012, 31, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.C.; Sersar, R.; Saabye, J.; Ladefoged, C.N.; Andersen, F.L.; Rasmussen, J.H.; Lofgren, J.; Beyer, T. Whole-body PET/MRI: The effect of bone attenuation during MR-based attenuation correction in oncology imaging. Eur. J. Radiol. 2014, 83, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Andersen, F.L.; Ladefoged, C.N.; Beyer, T.; Keller, S.H.; Hansen, A.E.; Højgaard, L.; Kjær, A.; Law, I.; Holm, S. Combined PET/MR imaging in neurology: MR-based attenuation correction implies a strong spatial bias when ignoring bone. Neuroimage 2014, 84, 206–216. [Google Scholar] [CrossRef]
- Schramm, G.; Maus, J.; Hofheinz, F.; Petr, J.; Lougovski, A.; Beuthien-Baumann, B.; Platzek, I.; van den Hoff, J. Evaluation and automatic correction of metal-implant-induced artifacts in MR-based attenuation correction in whole-body PET/MR imaging. Phys. Med. Biol. 2014, 59, 2713–2726. [Google Scholar] [CrossRef]
- Larsson, A.; Johansson, A.; Axelsson, J.; Nyholm, T.; Asklund, T.; Riklund, K.; Karlsson, M. Evaluation of an attenuation correction method for PET/MR imaging of the head based on substitute CT images. Magn. Reson. Mater. Phys. 2013, 26, 127–136. [Google Scholar] [CrossRef]
- Delso, G.; Carl, M.; Wiesinger, F.; Sacolick, L.; Porto, M.; Hüllner, M.; Boss, A.; Veit-Haibach, P. Anatomic evaluation of 3-dimensional ultrashort-echo-time bone maps for PET/MR attenuation correction. J. Nucl. Med. 2014, 55, 780–785. [Google Scholar] [CrossRef]
- Jones, W.; Vaigneur, K.; Young, J.; Reed, J.; Moyers, C.; Nahmias, C. The architectural impact of single photon transmission measurements on full ring 3-D positron tomography. In Proceedings of the 1995 IEEE Nuclear Science Symposium and Medical Imaging Conference Record, San Francisco, CA, USA, 21–28 October 1995; Volume 2, pp. 1026–1030. [Google Scholar]
- Moyers, C.; Nutt, R. Method and Apparatus for Transmission Measurements to Form a 3-D Image in Tomography Applications. U.S. Patent US5296708A, 22 March 1994. [Google Scholar]
- Mollet, P.; Keereman, V.; Clementel, E.; Vandenberghe, S. Simultaneous MR-compatible emission and transmission imaging for PET using time-of-flight information. IEEE Trans. Med. Imaging 2012, 31, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Mollet, P.; Keereman, V.; Bini, J.; Izquierdo-garcia, D.; Fayad, Z.A.; Vandenberghe, S. Improvement of attenuation correction in time-of-flight PET/MR imaging with a positron-emitting source. J. Nucl. Med. 2014, 55, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Renner, A.; Rausch, I.; Cal Gonzalez, J.; Frass-Kriegl, R.; Navarro de Lara, L.I.; Sieg, J.; Laistler, E.; Glanzer, M.; Dungl, D.; Moser, E.; et al. A head coil system with an integrated orbiting transmission point source mechanism for attenuation correction in PET/MRI. Phys. Med. Biol. 2018, 63, 225014. [Google Scholar] [CrossRef] [PubMed]
- Mispelter, J.; Lupu, M.; Briguet, A. NMR Probeheads for Biophysical and Biomedical Experiments: Theoretical Principles and Practical Guidelines; Tjan, K.W., Ed.; Imperial College Press: London, UK, 2006; ISBN 1860946372. [Google Scholar]
- Avdievich, N.I.; Hetherington, H.P. 4 T Actively-Detuneable Double-Tuned 1H/31P Head Volume Coil and Four-Channel 31P Phased Array for Human Brain Spectroscopy. J. Magn. Reson. 2007, 186, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Reykowski, A.; Wright, S.M.; Porter, J.R. Design of matching networks for low noise preamplifiers. Magn. Reson. Med. 1995, 33, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Santin, G.; Strul, D.; Staelens, S.; Assié, K.; Autret, D.; Avner, S.; Barbier, R.; Bardiès, M.; Bloomfield, P.M.; et al. GATE: A simulation toolkit for PET and SPECT. Phys. Med. Biol. 2004, 49, 4543–4561. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Kim, D.; Breton, E.; Axel, L. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn. Reson. Med. 2010, 64, 439–446. [Google Scholar] [CrossRef]
- Eckstein, K.; Dymerska, B.; Bachrata, B.; Bogner, W.; Poljanc, K.; Trattnig, S.; Robinson, S.D. Computationally Efficient Combination of Multi-channel Phase Data From Multi-echo Acquisitions (ASPIRE). Magn. Reson. Med. 2018, 79, 2996–3006. [Google Scholar] [CrossRef]
- Robinson, S.; Schödl, H.; Trattnig, S. A Method for Unwrapping Highly Wrapped Multi-echo Phase Images at Very High Field: UMPIRE. Magn. Reson. Med. 2014, 72, 80–92. [Google Scholar] [CrossRef]
- Robson, P.M.; Grant, A.K.; Madhuranthakam, A.J.; Lattanzi, R.; Sodickson, D.K.; McKenzie, C.A. Comprehensive quantification of signal-to-noise ratio and g-factor for image-based and k-space-based parallel imaging reconstructions. Magn. Reson. Med. 2008, 60, 895–907. [Google Scholar] [CrossRef]
- Kellman, P.; McVeigh, E.R. Image reconstruction in SNR units: A general method for SNR measurement. Magn. Reson. Med. 2005, 54, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.A.; Jakob, P.M.; Heidemann, R.M.; Nittka, M.; Jellus, V.; Wang, J.; Kiefer, B.; Haase, A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn. Reson. Med. 2002, 47, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Moser, E.; Stadlbauer, A.; Windischberger, C.; Quick, H.H.; Ladd, M.E. Magnetic resonance imaging methodology. Eur. J. Nucl. Med. Mol. Imaging 2009, 36 (Suppl. 1), S30–S41. [Google Scholar] [CrossRef]
- Daube-Witherspoon, M.E.; Muehllehner, G. Treatment of Axial Data in Three-Dimensional PET. J. Nucl. Med. 1987, 28, 1717–1724. [Google Scholar] [PubMed]
- Hubbell, J.H. Photon Cross-Sections, Attenuation Coefficients, and Energy Absorption Coefficients from 10-KeV to 100-GeV; Technical Report; U.S. Department of Commerce, National Bureau of Standards: Washington, DC, USA, 1969.
- Queiroz, M.A.; Huellner, M.W. PET/MR in cancers of the head and neck. Semin. Nucl. Med. 2015, 45, 248–265. [Google Scholar] [CrossRef] [PubMed]
- Szyszko, T.A.; Cook, G.J.R. PET/CT and PET/MRI in head and neck malignancy. Clin. Radiol. 2018, 73, 60–69. [Google Scholar] [CrossRef] [PubMed]



| Standard Deviation of ∆f (Hz) | |||
|---|---|---|---|
| with TxS System | without TxS System | ||
| tune-up shim | phantom | 97.0 | 97.5 |
| slice | 40.4 | 36.8 | |
| automatic shim | phantom | 72.5 | 75.9 |
| slice | 45.4 | 43.0 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro de Lara, L.I.; Frass-Kriegl, R.; Renner, A.; Sieg, J.; Pichler, M.; Bogner, T.; Moser, E.; Beyer, T.; Birkfellner, W.; Figl, M.; et al. Design, Implementation, and Evaluation of a Head and Neck MRI RF Array Integrated with a 511 keV Transmission Source for Attenuation Correction in PET/MR. Sensors 2019, 19, 3297. https://doi.org/10.3390/s19153297
Navarro de Lara LI, Frass-Kriegl R, Renner A, Sieg J, Pichler M, Bogner T, Moser E, Beyer T, Birkfellner W, Figl M, et al. Design, Implementation, and Evaluation of a Head and Neck MRI RF Array Integrated with a 511 keV Transmission Source for Attenuation Correction in PET/MR. Sensors. 2019; 19(15):3297. https://doi.org/10.3390/s19153297
Chicago/Turabian StyleNavarro de Lara, Lucia Isabel, Roberta Frass-Kriegl, Andreas Renner, Jürgen Sieg, Michael Pichler, Thomas Bogner, Ewald Moser, Thomas Beyer, Wolfgang Birkfellner, Michael Figl, and et al. 2019. "Design, Implementation, and Evaluation of a Head and Neck MRI RF Array Integrated with a 511 keV Transmission Source for Attenuation Correction in PET/MR" Sensors 19, no. 15: 3297. https://doi.org/10.3390/s19153297
APA StyleNavarro de Lara, L. I., Frass-Kriegl, R., Renner, A., Sieg, J., Pichler, M., Bogner, T., Moser, E., Beyer, T., Birkfellner, W., Figl, M., & Laistler, E. (2019). Design, Implementation, and Evaluation of a Head and Neck MRI RF Array Integrated with a 511 keV Transmission Source for Attenuation Correction in PET/MR. Sensors, 19(15), 3297. https://doi.org/10.3390/s19153297