Laplacian Eigenmaps Network-Based Nonlocal Means Method for MR Image Denoising
Abstract
1. Introduction
2. Methods
2.1. Rician Noise Model
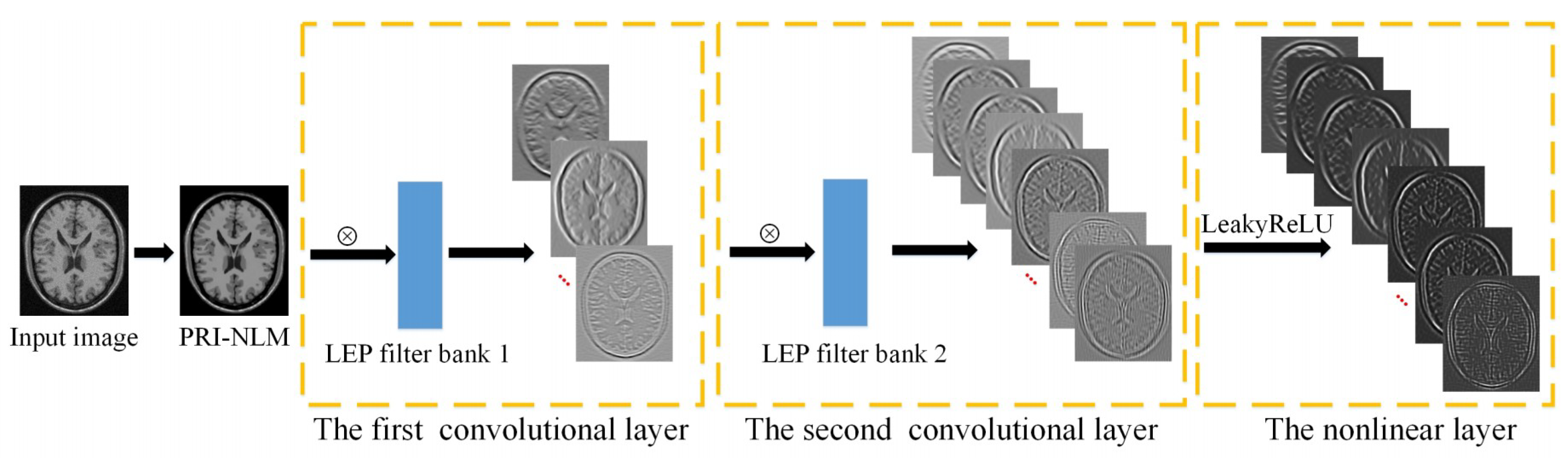
2.2. The Proposed LEPNet Model
2.2.1. The First Convolutional Layer
2.2.2. The Second Convolutional Layer
2.2.3. The Nonlinear Processing Layer
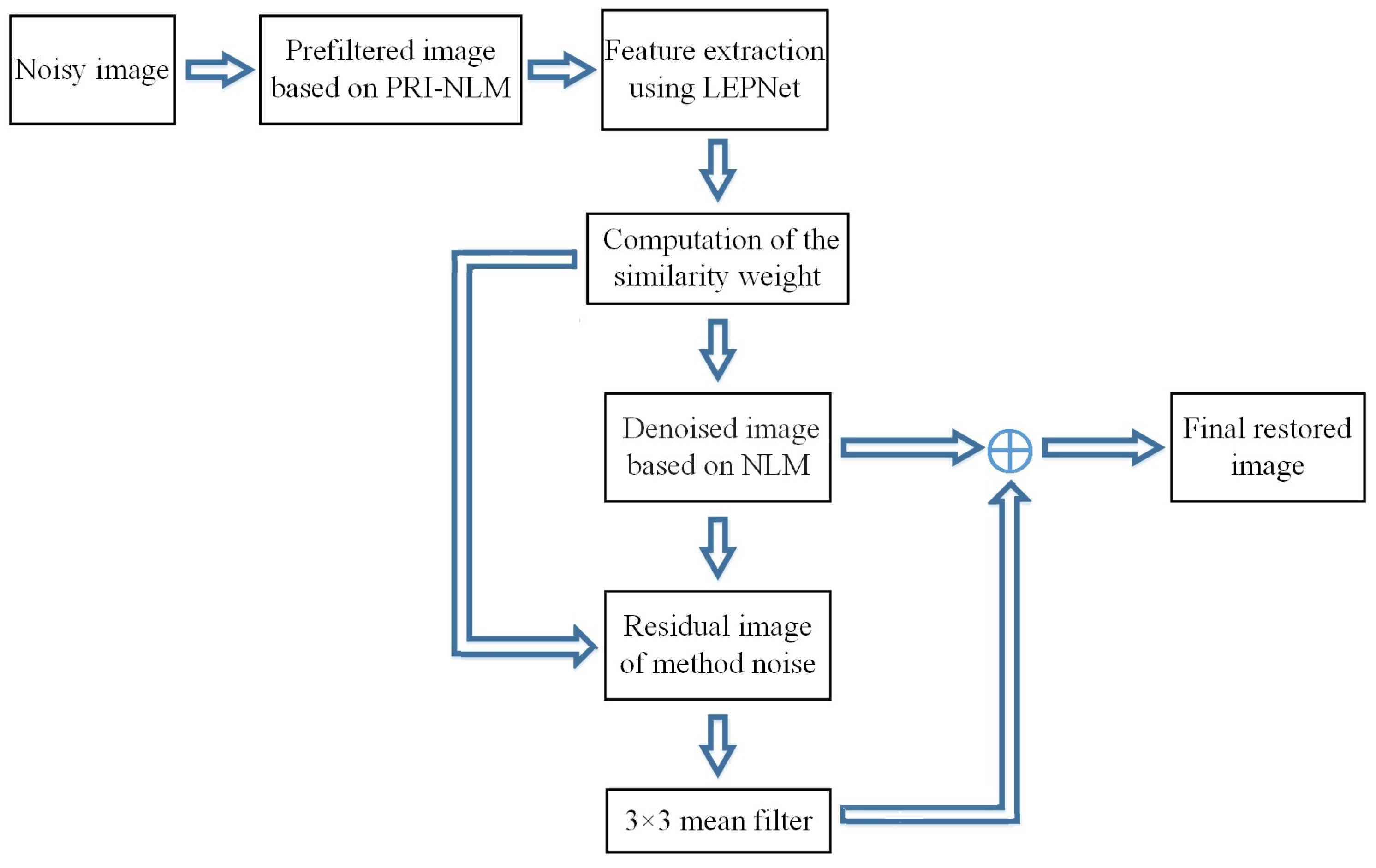
2.3. The LEPNet-Based Nonlocal Means Method
2.4. Overall Description of the Proposed Denoising Algorithm
- Step 1:
- The LEPNet model is trained using 300 PRI-NLM filtered MR images obtained from the open MRI database to learn the convolution kernels of two convolutional layers.
- Step 2:
- The input noisy MR image is preprocessed by the PRI-NLM filter to produce the pre-denoised image, then it is input into the trained LEPNet model to generate feature images as the output of this model.
- Step 3:
- Based on the obtained feature images, the feature vectors related to the pixels are constructed for calculating the similarity weights.
- Step 4:
- Based on the obtained similarity weights and the decay parameter, the input MR image is denoised using the NLM algorithm to produce the denoised image.
- Step 5:
- The method noise for the denoised image produced in Step 4 is processed by the NLM method and a 3 × 3 mean filter to retrieve the lost image details in the denoised image.
- Step 6:
- By combining the denoised image in Step 4 and the retrieved details in Step 5, the final restored image can be obtained.
3. Experimental Results and Discussion
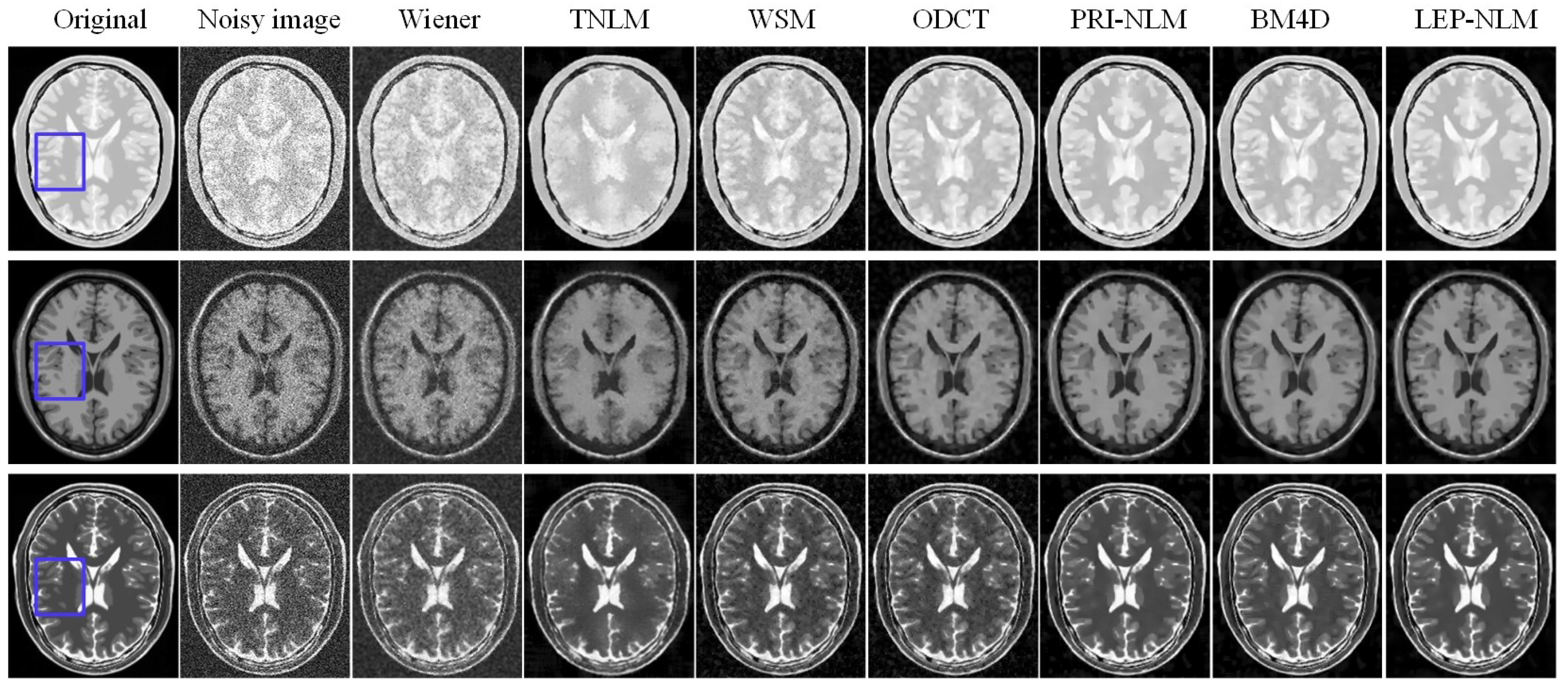
3.1. Simulated MR Images
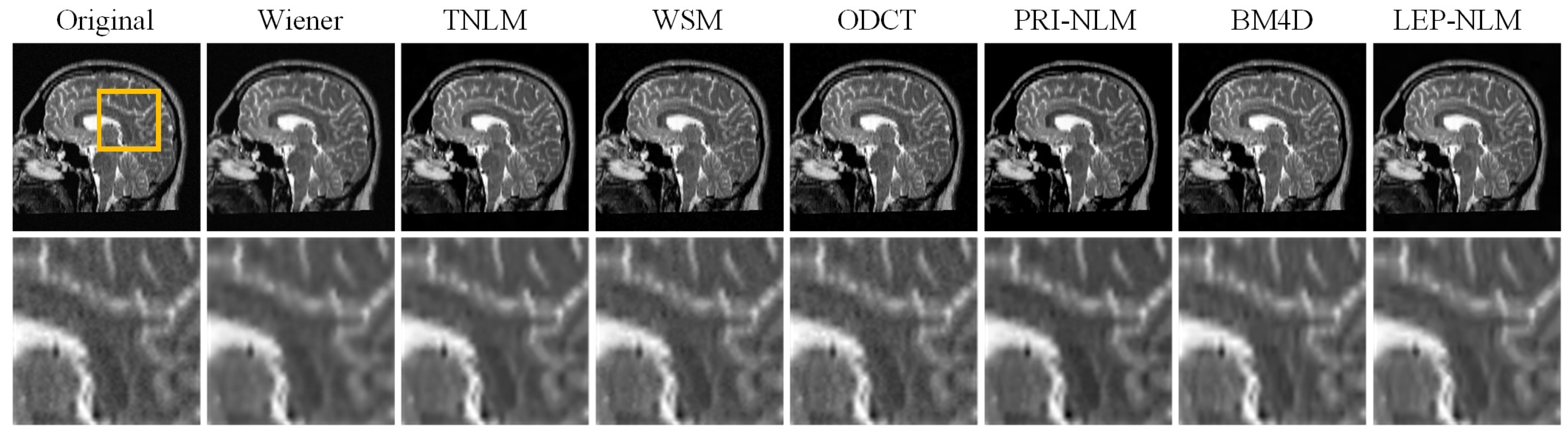
3.2. Real MR Images
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ikram, S.; Shah, J.A.; Zubair, S.; Qureshi, I.M.; Bilal, M. Improved reconstruction of MR scanned images by using a dictionary learning scheme. Sensors 2019, 19, 1918. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Chaurasia, V. A review on magnetic resonance images denoising techniques. Mach. Intell. Signal Anal. 2018, 707–715. [Google Scholar] [CrossRef]
- Murase, K.; Yamazaki, Y.; Shinohara, M.; Kawakami, K.; Kikuchi, K.; Miki, H.; Ikezoe, J. An anisotropic diffusion method for denoising dynamic susceptibility contrast-enhanced magnetic resonance images. Phys. Med. Biol. 2001, 46, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Ghassan, H.; Judith, H. Bilateral filtering of diffusion tensor magnetic resonance images. IEEE Trans. Image Process. 2007, 16, 2463–2475. [Google Scholar] [CrossRef]
- Liu, R.W.; Shi, L.; Huang, W.; Xu, J.; Yu, S.C.H.; Wang, D. Generalized total variation-based MRI Rician denoising model with spatially adaptive regularization parameters. Magn. Reson. Imaging 2014, 32, 702–720. [Google Scholar] [CrossRef] [PubMed]
- Izlian, Y.O.; Francisco, J.G.; Alfonso, A. Local complexity estimation based filtering method in wavelet domain for magnetic resonance imaging denoising. Entropy 2019, 21, 401. [Google Scholar] [CrossRef]
- Pizurica, A.; Wink, A.M.; Vansteenkiste, E.; Philips, W.; Roerdink, B.J. A review of wavelet denoising in MRI and ultrasound brain imaging. Curr. Med. Imaging Rev. 2006, 2, 247–260. [Google Scholar] [CrossRef]
- Zervakis, M.E.; Katsaggelos, A.K.; Kwon, T.M. A class of robust entropic functionals for image restoration. IEEE Trans. Image Process. 1995, 4, 752–773. [Google Scholar] [CrossRef]
- Hemalata, V.B.; Basavaraj, H.V. NLM based magnetic resonance image denoising-A review. Biomed. Signal Process. Control 2019, 47, 252–261. [Google Scholar] [CrossRef]
- Buades, A.; Coll, B.; Morel, J.M. A non-local algorithm for image denoising. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR), San Diego, CA, USA, 20–25 June 2005; pp. 60–65. [Google Scholar]
- Manjón, J.V.; Carbonell-Caballero, J.; Lull, J.J.; García-Martí, G.; Martí-Bonmatí, L.; Robles, M. MRI denoising using non-local means. Med. Image Anal. 2008, 12, 514–523. [Google Scholar] [CrossRef]
- Manjon, J.V.; Coupe, P.; Buades, A.; Collins, D.L.; Robles, M. New methods for MRI denoising based on sparseness and self-similarity. Med. Image Anal. 2012, 16, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Manjon, J.V.; Coupe, P.; Buades, A. MRI noise estimation and denoising using non-local PCA. Med. Image Anal. 2015, 22, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, M.; Katkovnik, V.; Egiazarian, K.; Foi, A. Nonlocal transform-domain filter for volumetric data denoising and reconstruction. IEEE Trans. Image Process. 2013, 22, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, L.; Liu, M.; Qian, C.; Yousuf, A.; Oto, A.; Shen, D. MRI-based prostate cancer detection with high-level representation and hierarchical classification. Med. Phys. 2017, 44, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Schonberger, J.L.; Hardmeier, H.; Sattler, T.; Pollefeys, M. Comparative evaluation of hand-crafted and learned local features. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; pp. 6959–6968. [Google Scholar]
- Hinton, G.E.; Osindero, S.; Teh, Y.-W. A fast learning algorithm for deep belief nets. Neural Comput. 2006, 18, 1527–1554. [Google Scholar] [CrossRef] [PubMed]
- Bengio, Y.; Courville, A.; Vincent, P. Representation learning: A review and new perspectives. IEEE Trans. Pattern Anal. Mach. Intell. 2013, 35, 1798–1828. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Zhang, X.; Gao, H.; Yang, Y.; Wang, J. Deep belief network for spectral-spatial classification of hyperspectral remote sensor data. Sensors 2019, 19, 204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liu, Y.; Huo, K.; Zhang, S.; Zhang, Z. Radar HRRP target recognition based on stacked autoencoder and extreme learning machine. Sensors 2018, 18, 173. [Google Scholar] [CrossRef]
- Zhang, K.; Zuo, W.; Chen, Y.; Meng, D.; Zhang, L. Beyond a Gaussian denoiser: Residual learning of deep CNN for image denoising. IEEE Trans. Image Process. 2017, 26, 3142–3155. [Google Scholar] [CrossRef]
- Chan, T.H.; Jia, K.; Gao, S.; Lu, J.; Zeng, Z.; Ma, Y. PCANet: A simple deep learning baseline for image classification? IEEE Trans. Image Process. 2015, 24, 5017–5032. [Google Scholar] [CrossRef]
- Belkin, M.; Niyogi, P. Laplacian eigenmaps for dimensionality reduction and data representation. Neural Comput. 2003, 15, 1373–1396. [Google Scholar] [CrossRef]
- Van der Maaten, L.; Postma, E.; Van Den Herik, H.J. Dimensionality reduction: A comparative review. J. Mach. Learn. Res. 2009, 10, 66–71. [Google Scholar]
- Daffertshofer, A.; Lamoth, C.J.; Meijer, O.G.; Beek, P.J. PCA in studying coordination and variability: A tutorial. Clin. Biomech. 2004, 19, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Borg, I.; Groenen, P.J.F. Modern multidimensional scaling: Theory and applications. J. Educ. Meas. 2006, 40, 277–280. [Google Scholar] [CrossRef]
- Portillo-Portillo, J.; Leyva, R.; Sanchez, V.; Sanchez-Perez, G.; Perez-Meana, H.; Olivares-Mercado, J.; Nakano-Miyatake, M. Cross view gait recognition using joint-direct linear discriminant analysis. Sensors 2017, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. In Proceedings of the 25th International Conference on Neural Information Processing Systems, Lake Tahoe, NV, USA, 3–6 December 2012; pp. 1097–1105. [Google Scholar]
- Andrew, L.M.; Awni, Y.H.; Andrew, Y.N. Rectifier nonlinearities improve neural network acoustic models. In Proceedings of the 30th International Conference on Machine Learning, Atlanta, GA, USA, 30 March 2013. [Google Scholar]
- Basu, S.; Fletcher, T.; Whitaker, R. Rician noise removal in diffusion tensor MRI. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Copenhagen, Denmark, 1–6 October 2006; pp. 117–125. [Google Scholar]
- Nowak, R.D. Wavelet-based rician noise removal for magnetic resonance imaging. IEEE Trans. Image Process. 1999, 8, 1408–1419. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–69. [Google Scholar] [CrossRef]
- The Cancer Imaging Archive. Available online: https://wiki.cancerimagingarchive.net/display/Public/SPIE-AAPM-NCI+PROSTATEx+Challenges (accessed on 10 September 2018).
- Litjens, G.; Debats, O.; Barentsz, J.; Karssemeijer, N.; Huisman, H. ProstateX challenge data. The Cancer Imaging Archive. Available online: https://doi.org/10.7937/K9TCIA.2017.MURS5CL (accessed on 10 September 2018).
- Litjens, G.; Debats, O.; Barentsz, J.; Karssemeijer, N.; Huisman, H. Computer-aided detection of prostate cancer in MRI. IEEE Trans. Med. Imaging 2014, 33, 1083–1092. [Google Scholar] [CrossRef]
- Clark, K.; Vendt, B.; Smith, K.; Freymann, J.; Kirby, J.; Koppel, P.; Tarbox, L. The cancer imaging archive (TCIA): Maintaining and operating a public information repository. J. Digit. Imaging 2013, 26, 1045–1057. [Google Scholar] [CrossRef]
- Kervrann, C.; Boulanger, J. Local adaptivity to variable smoothness for exemplar-based image regularization and representation. Int. J. Comput. Vis. 2008, 79, 45–69. [Google Scholar] [CrossRef]
- Zhong, H.; Yang, C.; Zhang, X. A new weight for nonlocal means denoising using method noise. IEEE Signal Process. Lett. 2012, 19, 535–538. [Google Scholar] [CrossRef]
- BrainWeb: Simulated Brain Database. Available online: http://brainweb.bic.mni.mcgill.ca/brainweb/ (accessed on 15 October 2018).
- The Whole Brain Atlas. Available online: http://www.med.harvard.edu/aanlib/home.html (accessed on 15 October 2018).
- Coupe, P.; Hellier, P.; Prima, S.; Kervrann, C.; Barillot, C. 3D Wavelet Sub-Bands Mixing for Image Denoising. Int. J. Biomed. Imaging 2008, 1, 590183. [Google Scholar] [CrossRef]
- Kala, R.; Deepa, P. Adaptive hexagonal fuzzy hybrid filter for Rician noise removal in MRI images. Neural Comput. Appl. 2018, 29, 237–249. [Google Scholar] [CrossRef]



| Methods | Noisy | Wiener | TNLM | WSM | ODCT | PRI-NLM | BM4D | LEP-NLMw | LEP-NLM | |
|---|---|---|---|---|---|---|---|---|---|---|
| PSNR | 2% | 33.47 | 33.69 | 39.47 | 38.59 | 42.58 | 43.49 | 43.68 | 43.53 | 43.58 |
| 5% | 25.40 | 27.76 | 33.80 | 34.03 | 36.60 | 36.98 | 37.31 | 37.83 | 37.91 | |
| 10% | 19.46 | 22.34 | 29.07 | 29.25 | 31.69 | 31.90 | 32.49 | 32.62 | 32.85 | |
| 15% | 16.12 | 18.93 | 26.38 | 26.07 | 28.71 | 28.94 | 29.61 | 29.66 | 30.00 | |
| 20% | 13.94 | 16.51 | 24.00 | 23.63 | 26.55 | 26.94 | 27.52 | 27.61 | 28.03 | |
| 25% | 12.19 | 14.65 | 22.38 | 21.68 | 24.72 | 25.28 | 25.85 | 25.96 | 26.44 | |
| 30% | 10.88 | 13.12 | 20.42 | 20.07 | 23.14 | 23.92 | 24.38 | 24.63 | 25.15 | |
| SSIM | 2% | 0.780 | 0.868 | 0.976 | 0.969 | 0.977 | 0.984 | 0.982 | 0.985 | 0.985 |
| 5% | 0.558 | 0.786 | 0.936 | 0.904 | 0.945 | 0.950 | 0.946 | 0.952 | 0.960 | |
| 10% | 0.377 | 0.660 | 0.842 | 0.810 | 0.876 | 0.898 | 0.896 | 0.887 | 0.915 | |
| 15% | 0.285 | 0.544 | 0.761 | 0.718 | 0.817 | 0.848 | 0.851 | 0.832 | 0.870 | |
| 20% | 0.233 | 0.444 | 0.694 | 0.630 | 0.768 | 0.801 | 0.809 | 0.780 | 0.823 | |
| 25% | 0.191 | 0.377 | 0.624 | 0.552 | 0.726 | 0.761 | 0.766 | 0.738 | 0.781 | |
| 30% | 0.157 | 0.331 | 0.559 | 0.483 | 0.683 | 0.726 | 0.727 | 0.703 | 0.745 |
| Methods | Noisy | Wiener | TNLM | WSM | ODCT | PRI-NLM | BM4D | LEP-NLMw | LEP-NLM | |
|---|---|---|---|---|---|---|---|---|---|---|
| PSNR | 2% | 33.57 | 34.13 | 40.24 | 41.07 | 43.30 | 44.05 | 44.21 | 44.64 | 44.82 |
| 5% | 25.66 | 28.19 | 34.56 | 34.76 | 37.16 | 37.84 | 38.01 | 38.38 | 38.64 | |
| 10% | 19.63 | 22.42 | 29.45 | 29.34 | 32.11 | 32.82 | 33.10 | 33.42 | 33.82 | |
| 15% | 16.10 | 18.77 | 25.95 | 26.06 | 29.00 | 29.69 | 29.93 | 30.36 | 30.85 | |
| 20% | 13.59 | 16.17 | 23.63 | 23.68 | 26.80 | 27.28 | 27.64 | 28.02 | 28.58 | |
| 25% | 11.72 | 14.13 | 21.34 | 21.79 | 24.97 | 25.41 | 25.89 | 26.17 | 26.73 | |
| 30% | 10.25 | 12.53 | 19.84 | 20.19 | 23.51 | 24.00 | 24.48 | 24.67 | 25.25 | |
| SSIM | 2% | 0.746 | 0.832 | 0.972 | 0.959 | 0.985 | 0.985 | 0.983 | 0.977 | 0.987 |
| 5% | 0.568 | 0.762 | 0.912 | 0.881 | 0.931 | 0.951 | 0.949 | 0.927 | 0.957 | |
| 10% | 0.378 | 0.625 | 0.825 | 0.778 | 0.851 | 0.898 | 0.901 | 0.854 | 0.903 | |
| 15% | 0.259 | 0.497 | 0.727 | 0.682 | 0.782 | 0.844 | 0.855 | 0.798 | 0.858 | |
| 20% | 0.182 | 0.406 | 0.645 | 0.595 | 0.727 | 0.787 | 0.809 | 0.745 | 0.798 | |
| 25% | 0.133 | 0.327 | 0.545 | 0.516 | 0.674 | 0.729 | 0.760 | 0.694 | 0.744 | |
| 30% | 0.101 | 0.268 | 0.444 | 0.443 | 0.629 | 0.684 | 0.711 | 0.647 | 0.694 |
| Methods | Noisy | Wiener | TNLM | WSM | ODCT | PRI-NLM | BM4D | LEP-NLMw | LEP-NLM | |
|---|---|---|---|---|---|---|---|---|---|---|
| PSNR | 2% | 33.46 | 35.07 | 37.99 | 38.42 | 42.07 | 42.82 | 43.03 | 43.59 | 43.59 |
| 5% | 25.42 | 26.53 | 32.07 | 33.35 | 35.58 | 36.10 | 36.25 | 36.93 | 37.00 | |
| 10% | 19.30 | 21.64 | 26.70 | 28.13 | 30.54 | 31.06 | 31.28 | 31.79 | 31.97 | |
| 15% | 15.91 | 18.32 | 23.76 | 24.77 | 27.52 | 27.96 | 28.24 | 28.75 | 29.01 | |
| 20% | 13.54 | 15.73 | 21.94 | 22.23 | 25.14 | 25.57 | 26.08 | 26.40 | 26.70 | |
| 25% | 11.64 | 13.78 | 19.97 | 20.21 | 22.97 | 23.46 | 24.32 | 24.39 | 24.70 | |
| 30% | 10.10 | 12.05 | 18.43 | 18.59 | 21.11 | 21.66 | 22.83 | 22.66 | 22.96 | |
| SSIM | 2% | 0.797 | 0.867 | 0.978 | 0.972 | 0.979 | 0.986 | 0.983 | 0.988 | 0.989 |
| 5% | 0.620 | 0.792 | 0.938 | 0.911 | 0.952 | 0.956 | 0.952 | 0.957 | 0.966 | |
| 10% | 0.468 | 0.691 | 0.847 | 0.811 | 0.888 | 0.913 | 0.907 | 0.901 | 0.928 | |
| 15% | 0.375 | 0.592 | 0.755 | 0.718 | 0.830 | 0.867 | 0.854 | 0.851 | 0.888 | |
| 20% | 0.309 | 0.494 | 0.709 | 0.632 | 0.773 | 0.820 | 0.808 | 0.804 | 0.843 | |
| 25% | 0.247 | 0.415 | 0.604 | 0.552 | 0.708 | 0.767 | 0.764 | 0.760 | 0.799 | |
| 30% | 0.199 | 0.347 | 0.526 | 0.484 | 0.632 | 0.700 | 0.719 | 0.711 | 0.749 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Ding, M.; Zhang, X. Laplacian Eigenmaps Network-Based Nonlocal Means Method for MR Image Denoising. Sensors 2019, 19, 2918. https://doi.org/10.3390/s19132918
Yu H, Ding M, Zhang X. Laplacian Eigenmaps Network-Based Nonlocal Means Method for MR Image Denoising. Sensors. 2019; 19(13):2918. https://doi.org/10.3390/s19132918
Chicago/Turabian StyleYu, Houqiang, Mingyue Ding, and Xuming Zhang. 2019. "Laplacian Eigenmaps Network-Based Nonlocal Means Method for MR Image Denoising" Sensors 19, no. 13: 2918. https://doi.org/10.3390/s19132918
APA StyleYu, H., Ding, M., & Zhang, X. (2019). Laplacian Eigenmaps Network-Based Nonlocal Means Method for MR Image Denoising. Sensors, 19(13), 2918. https://doi.org/10.3390/s19132918







