Dynamic Interface Pressure Monitoring System for the Morphological Pressure Mapping of Intermittent Pneumatic Compression Therapy
Abstract
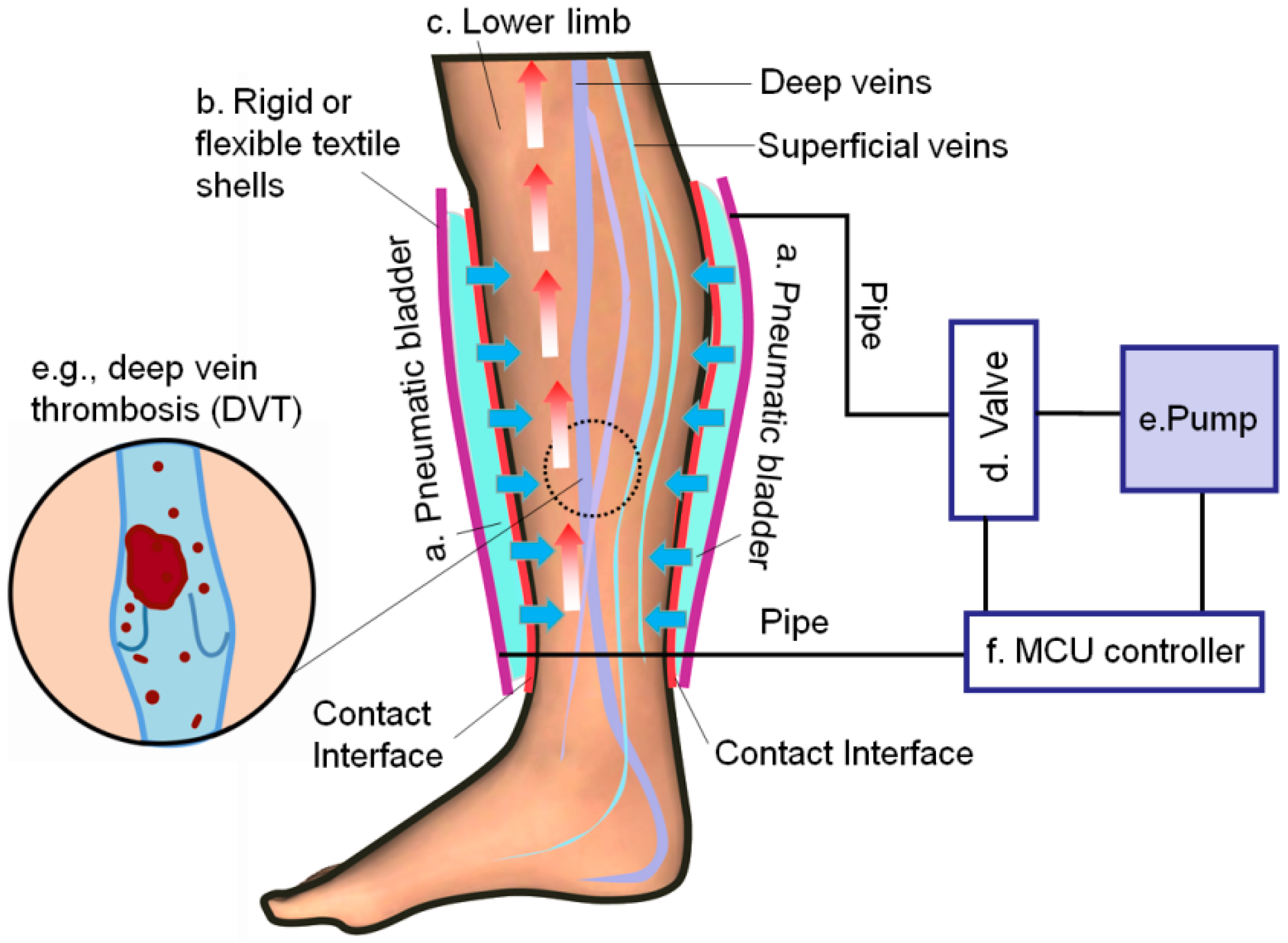
1. Introduction
2. Materials and Methods
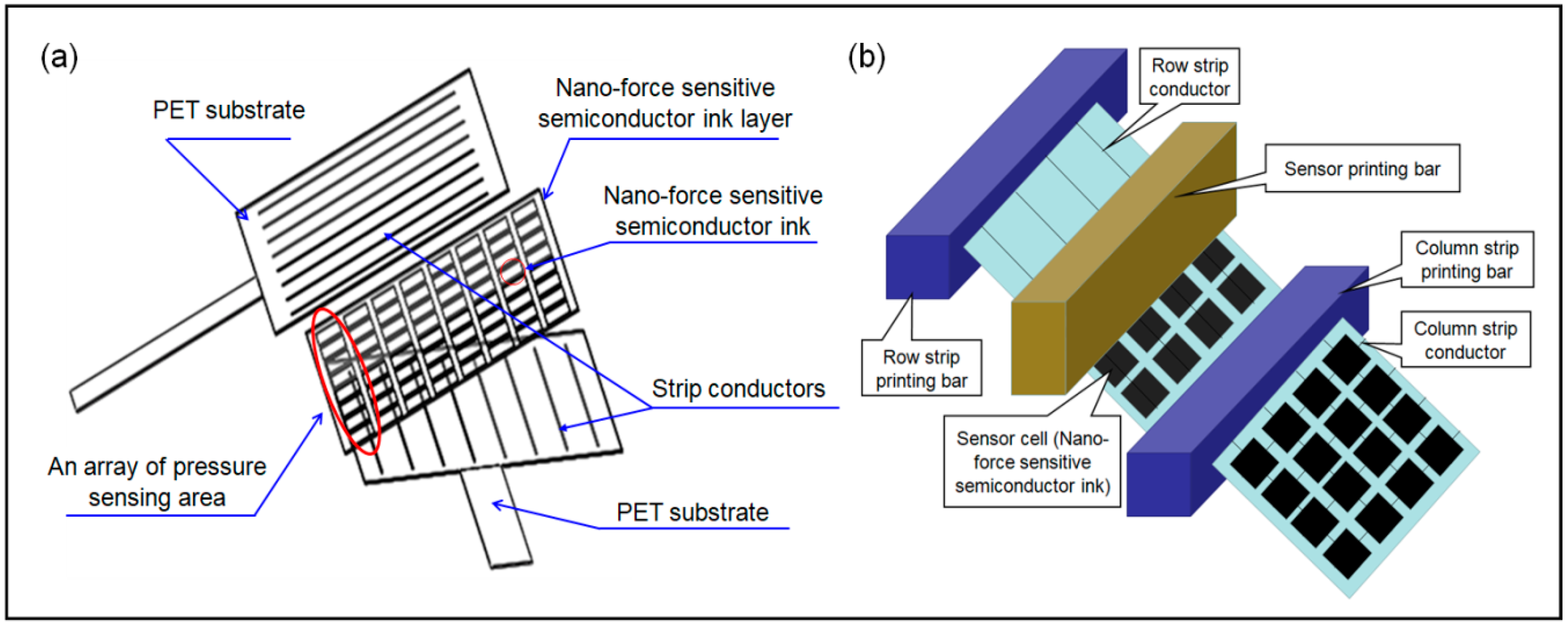
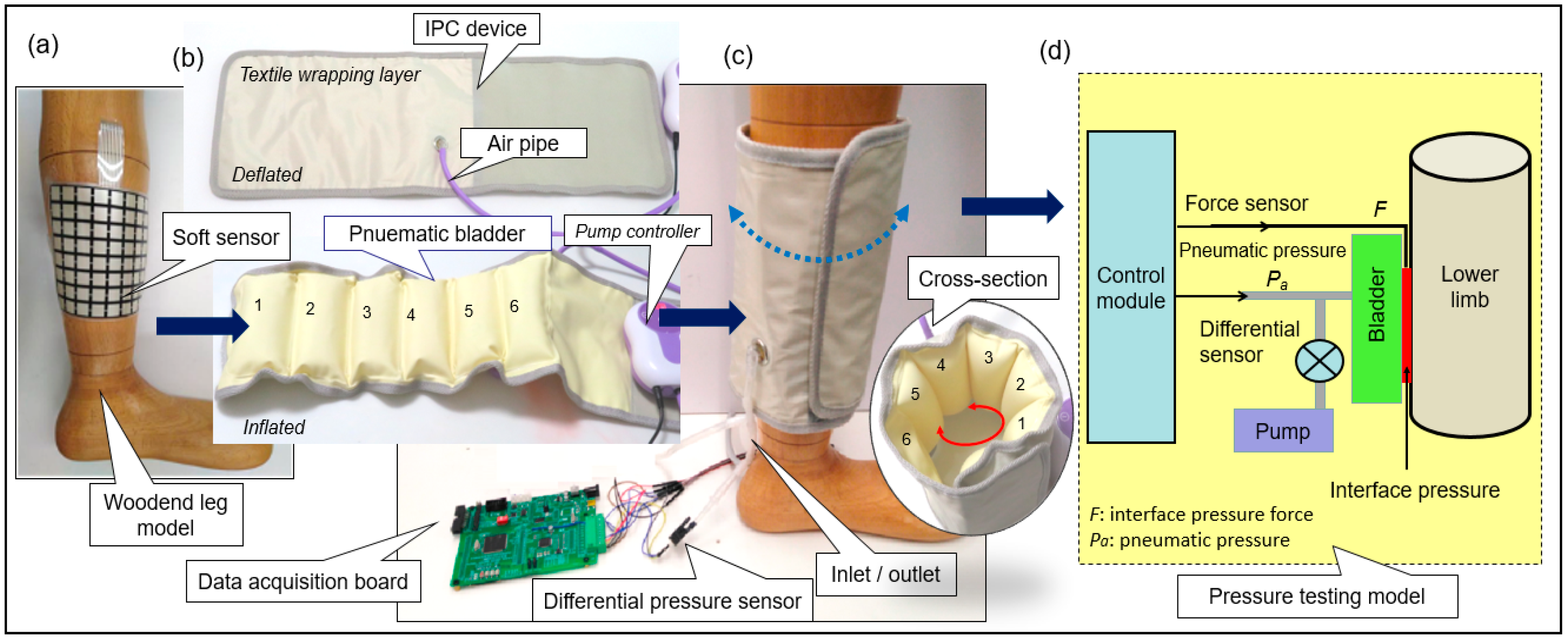
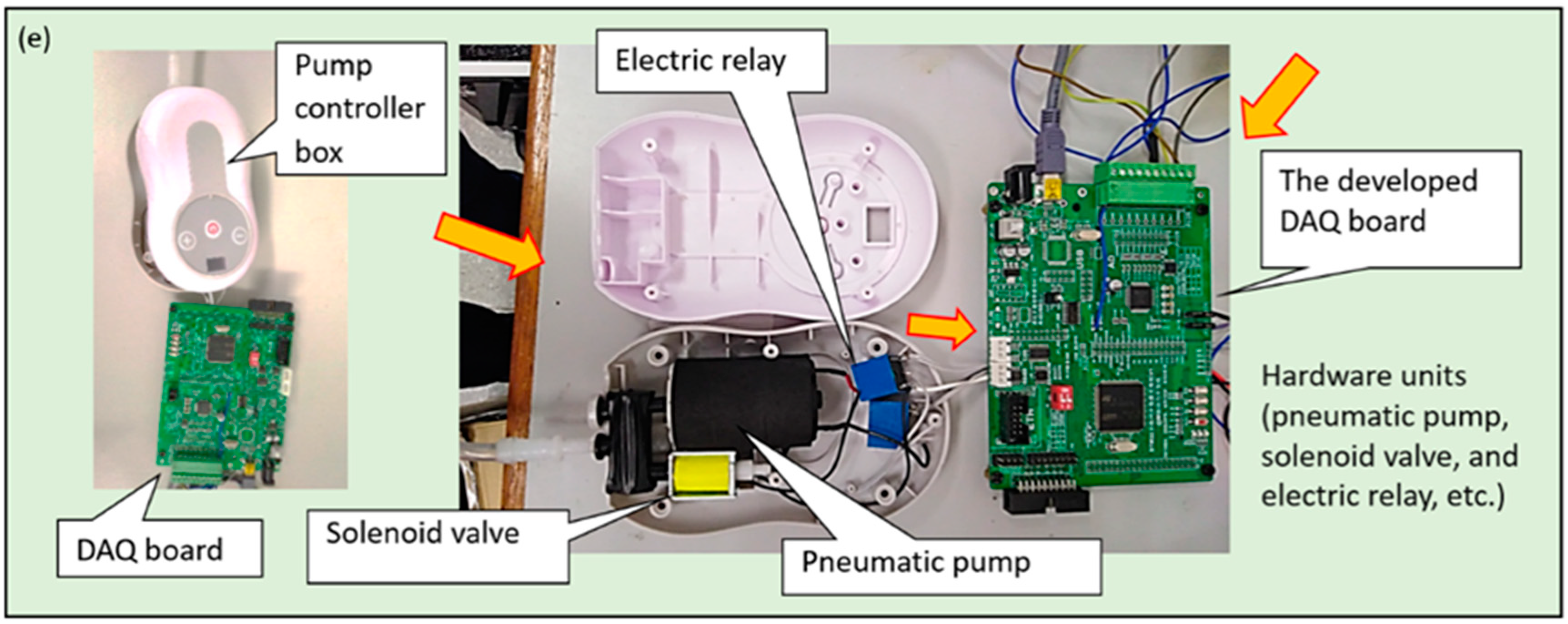
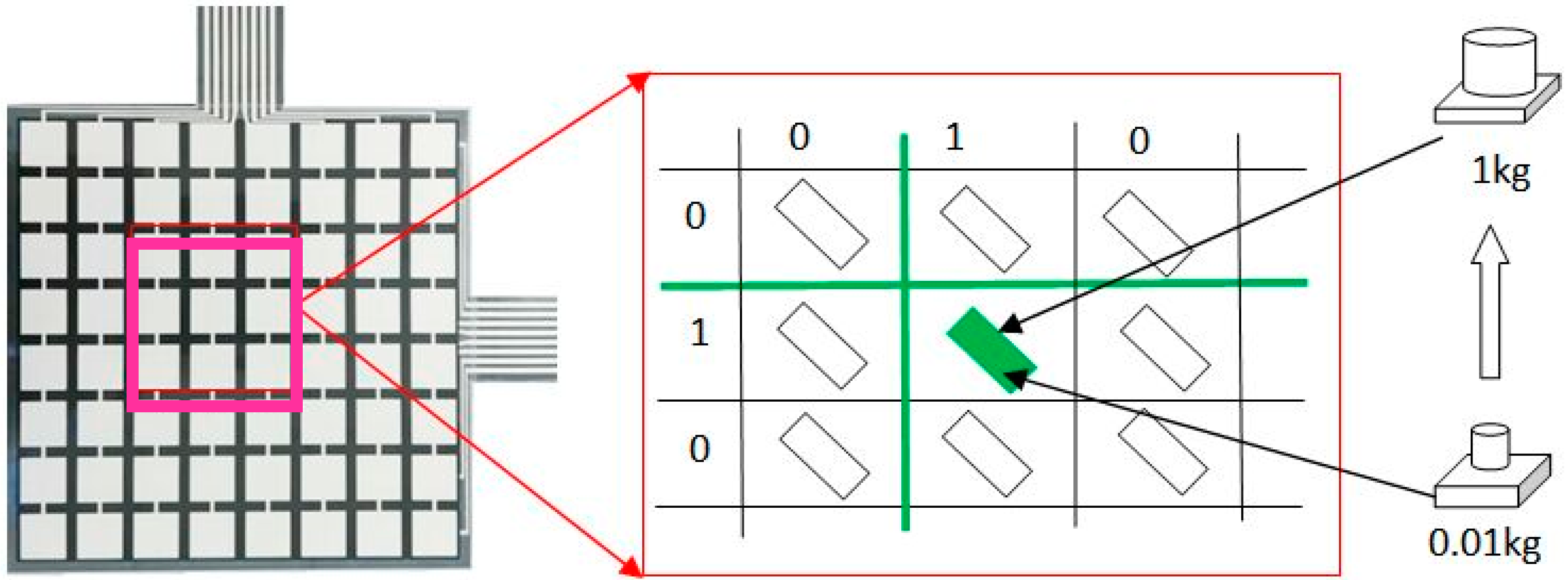
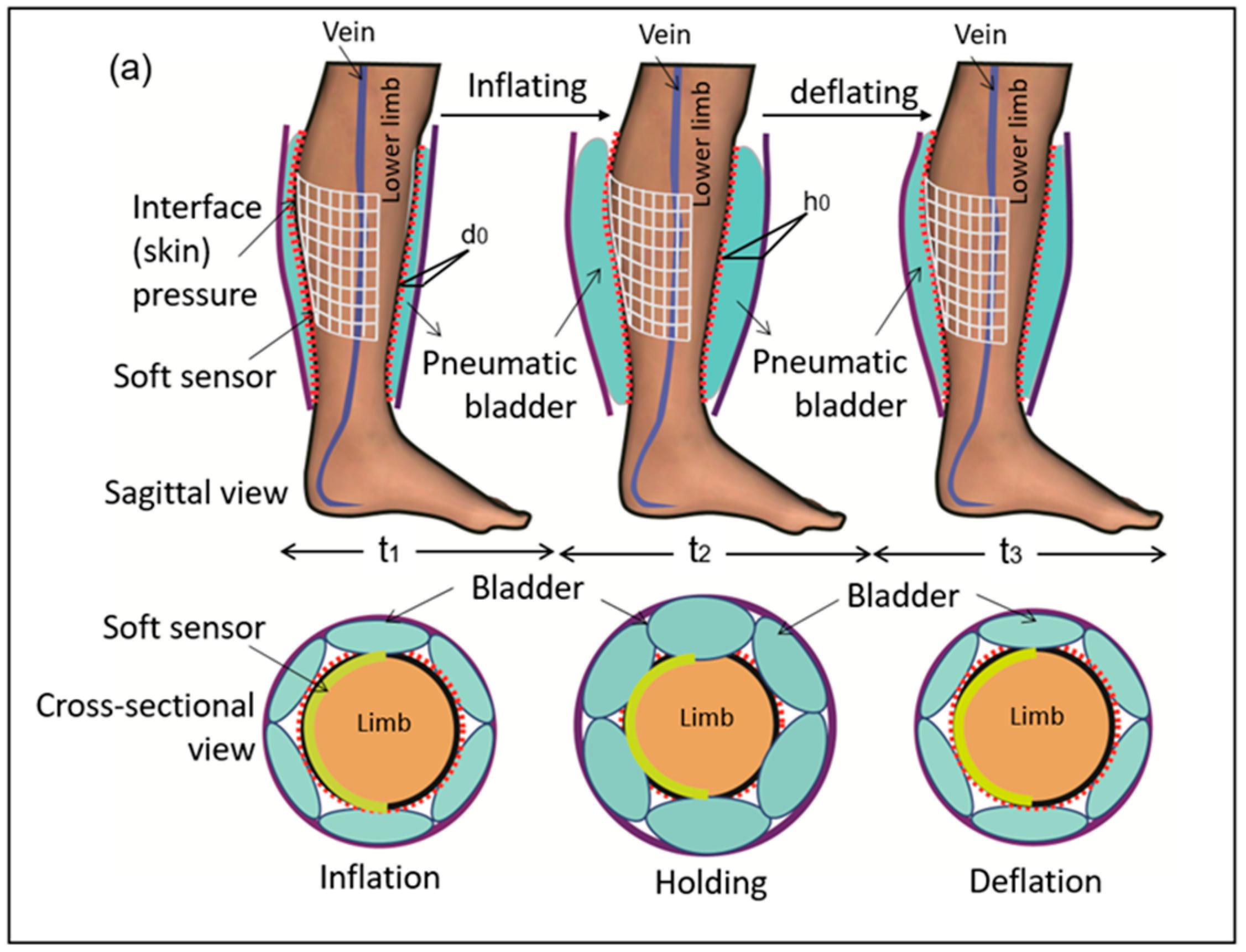
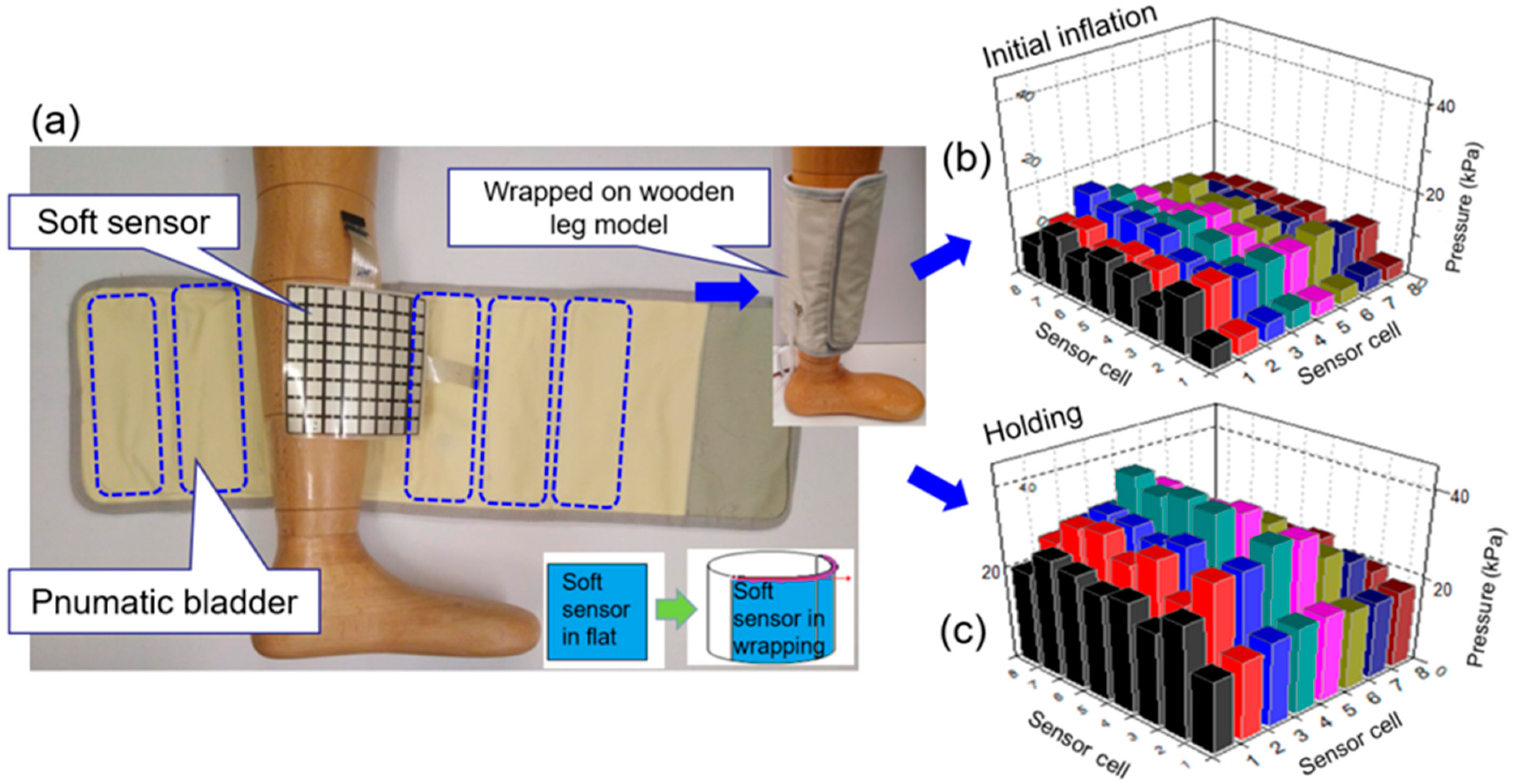
2.1. Materials and Measuring System Setup
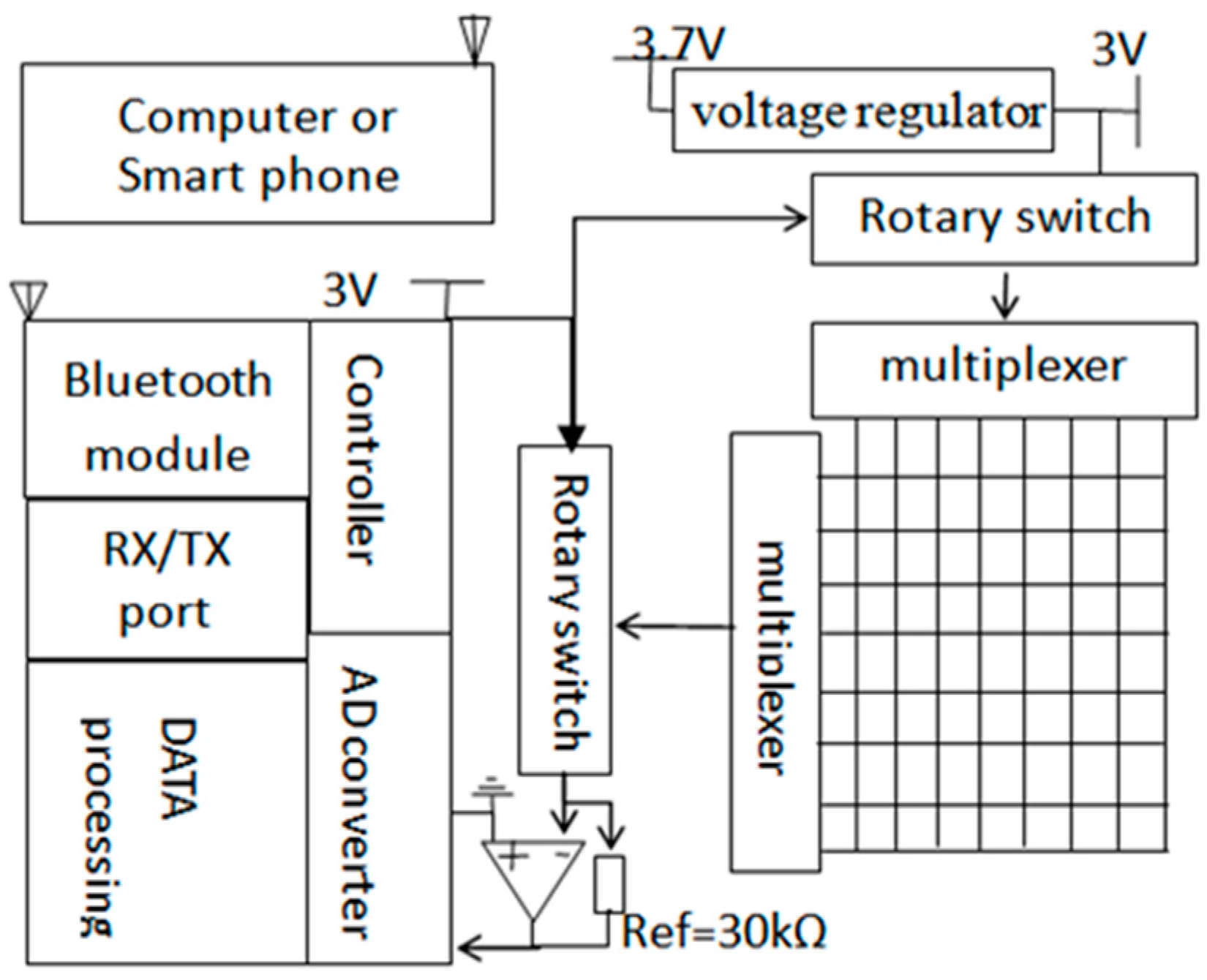
2.2. Data Acquisition (DAQ) System
2.3. Software System Setup
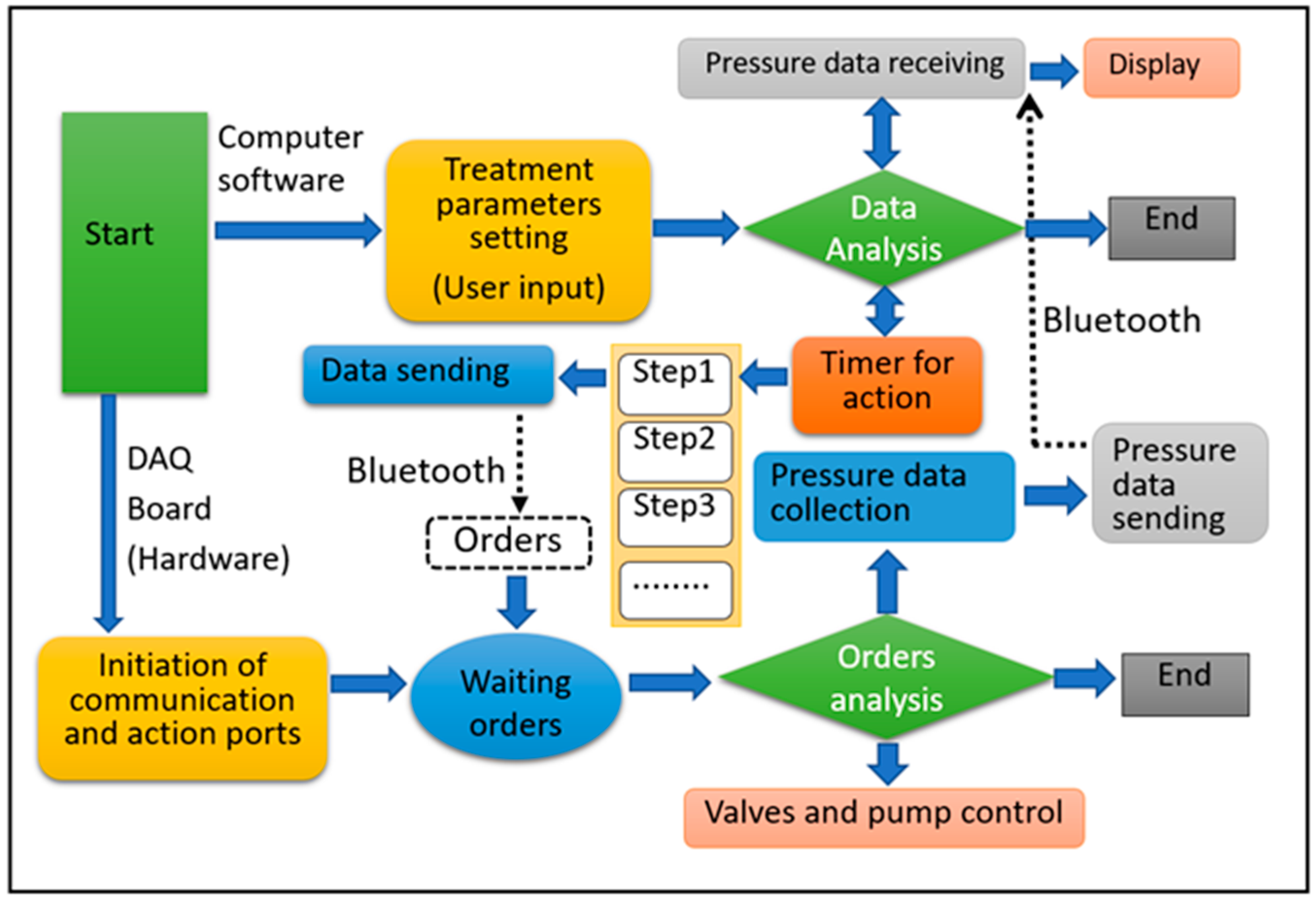
2.4. Working Modes of the IPC Device
3. Results and Discussion
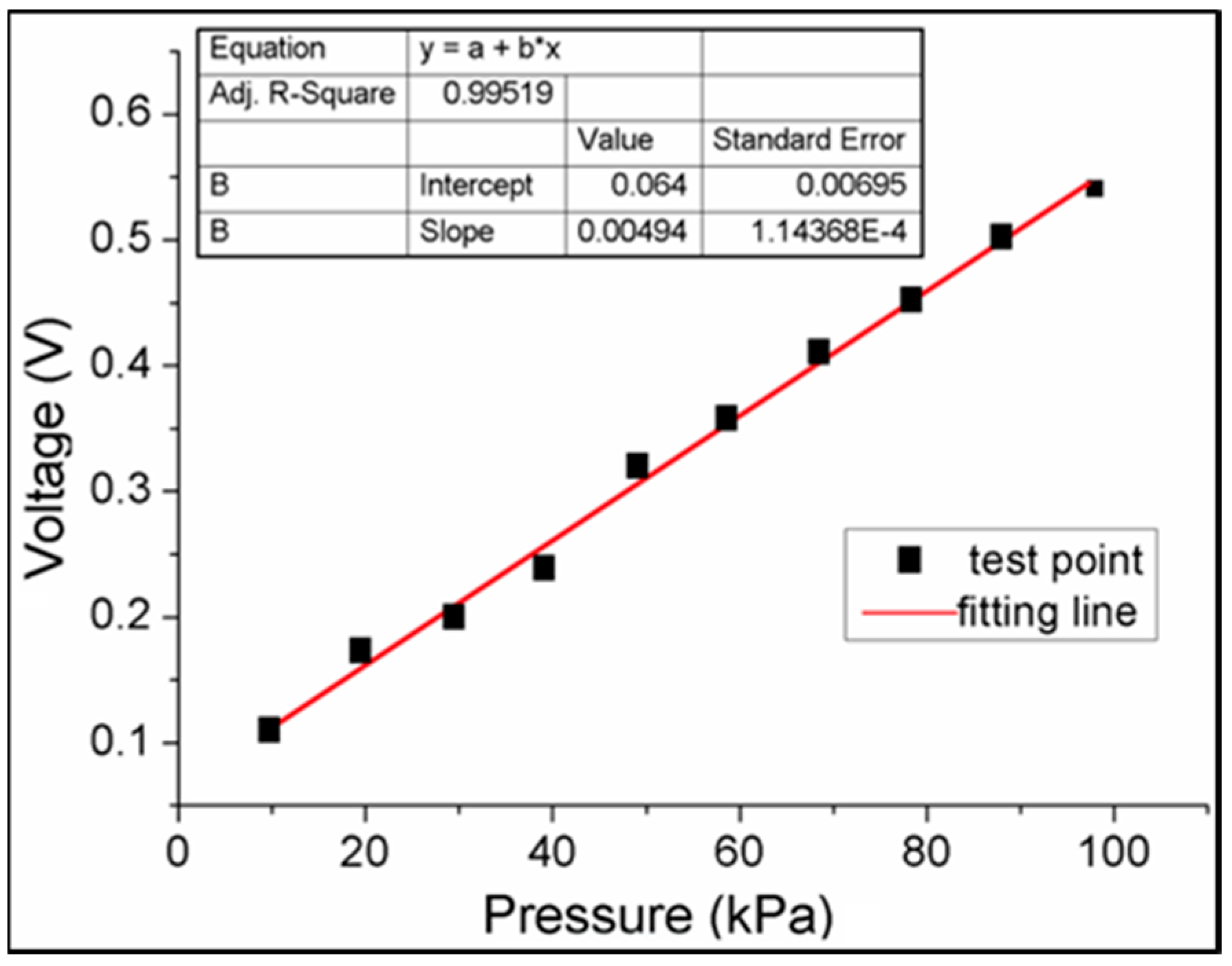
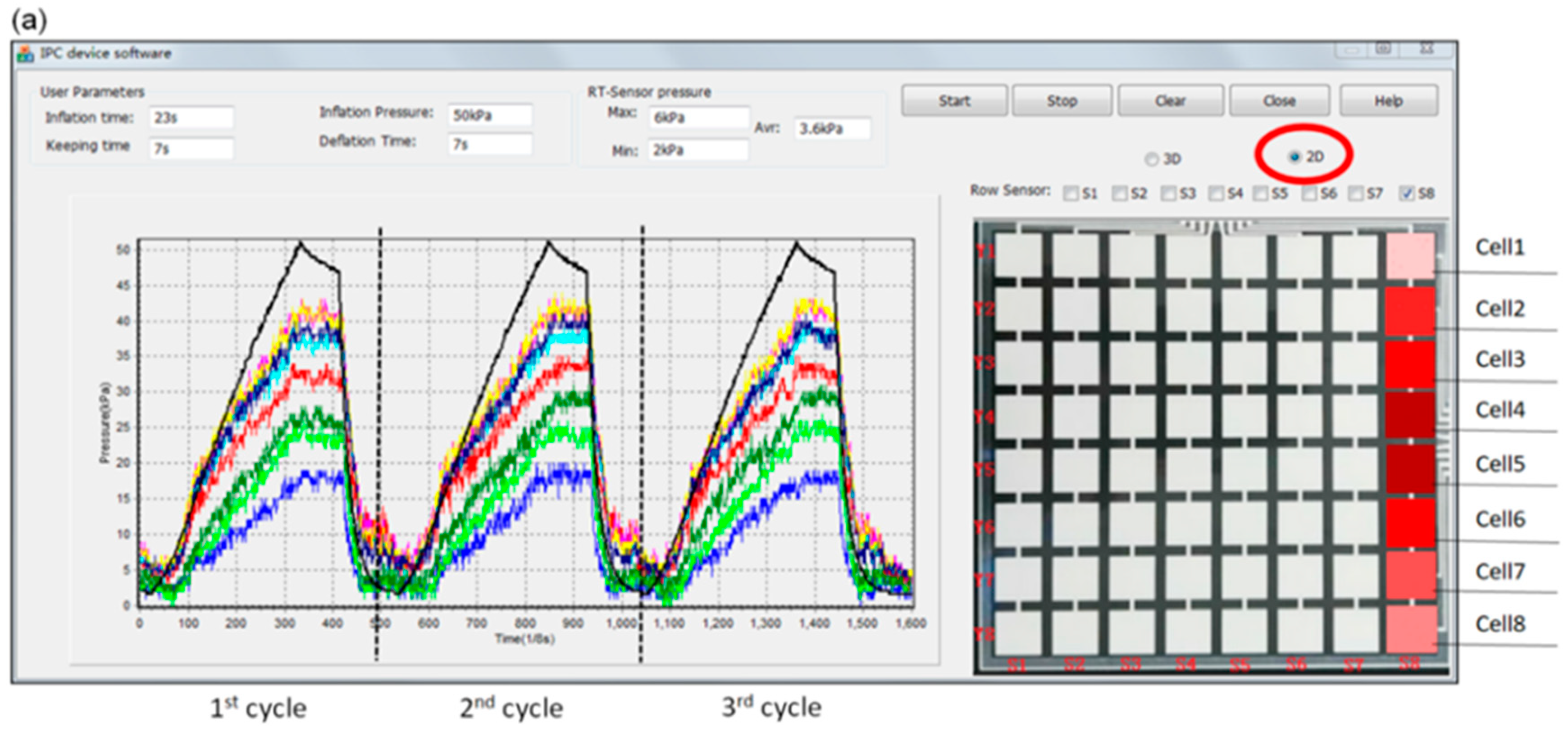
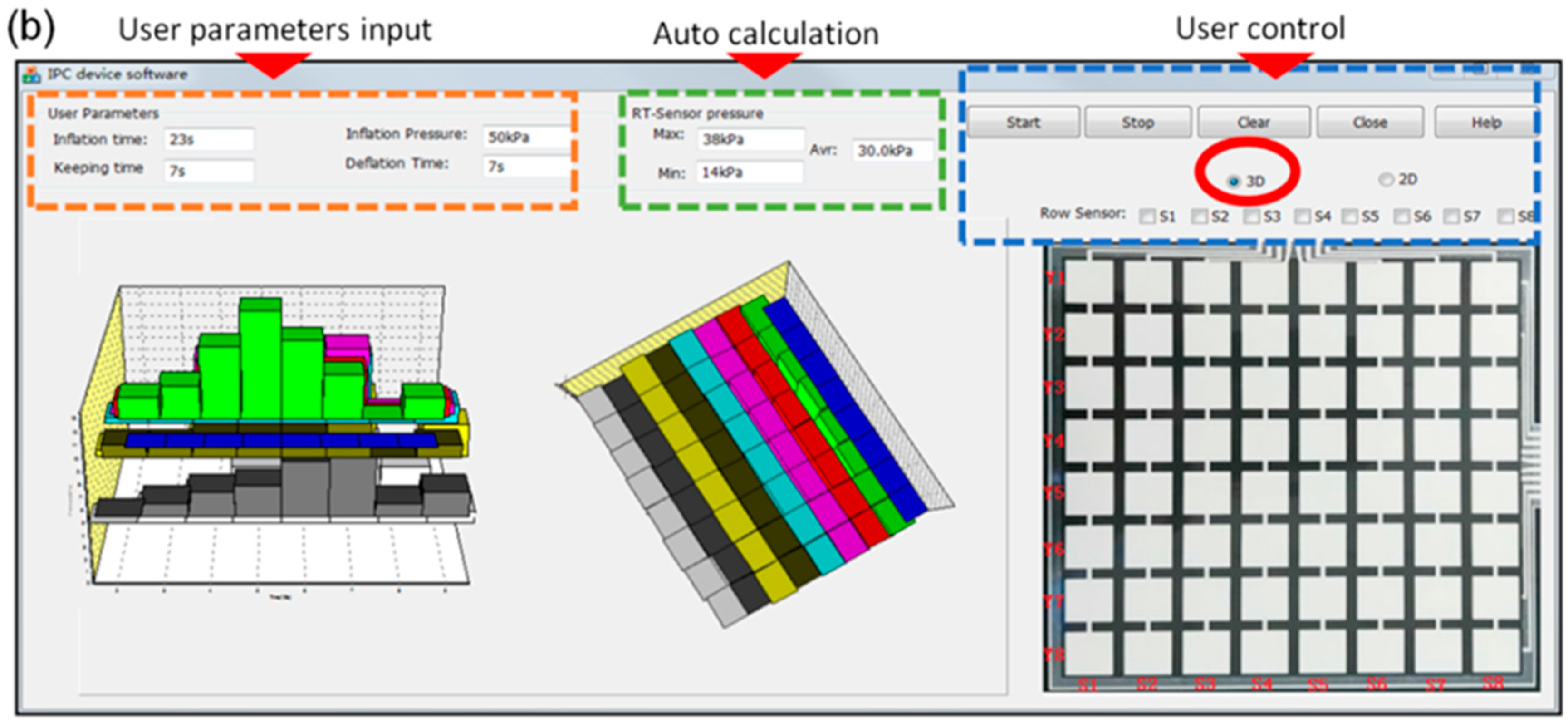
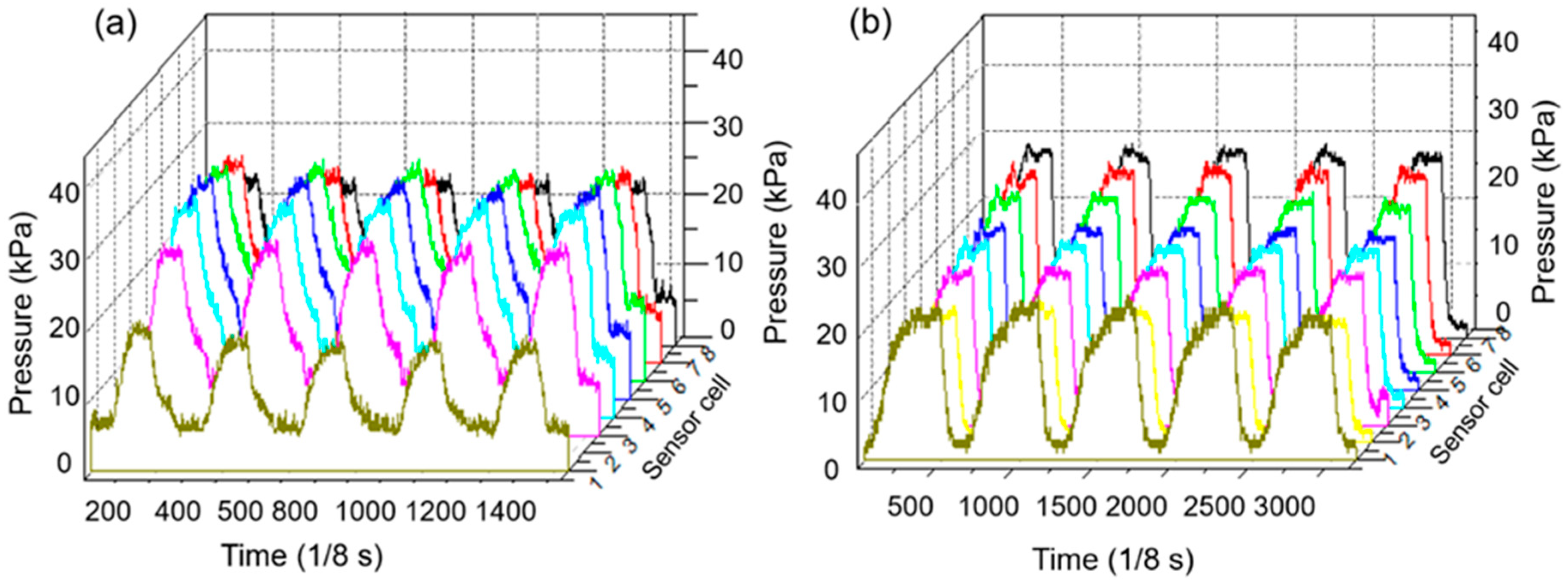
3.1. Pressure Monitoring Interface
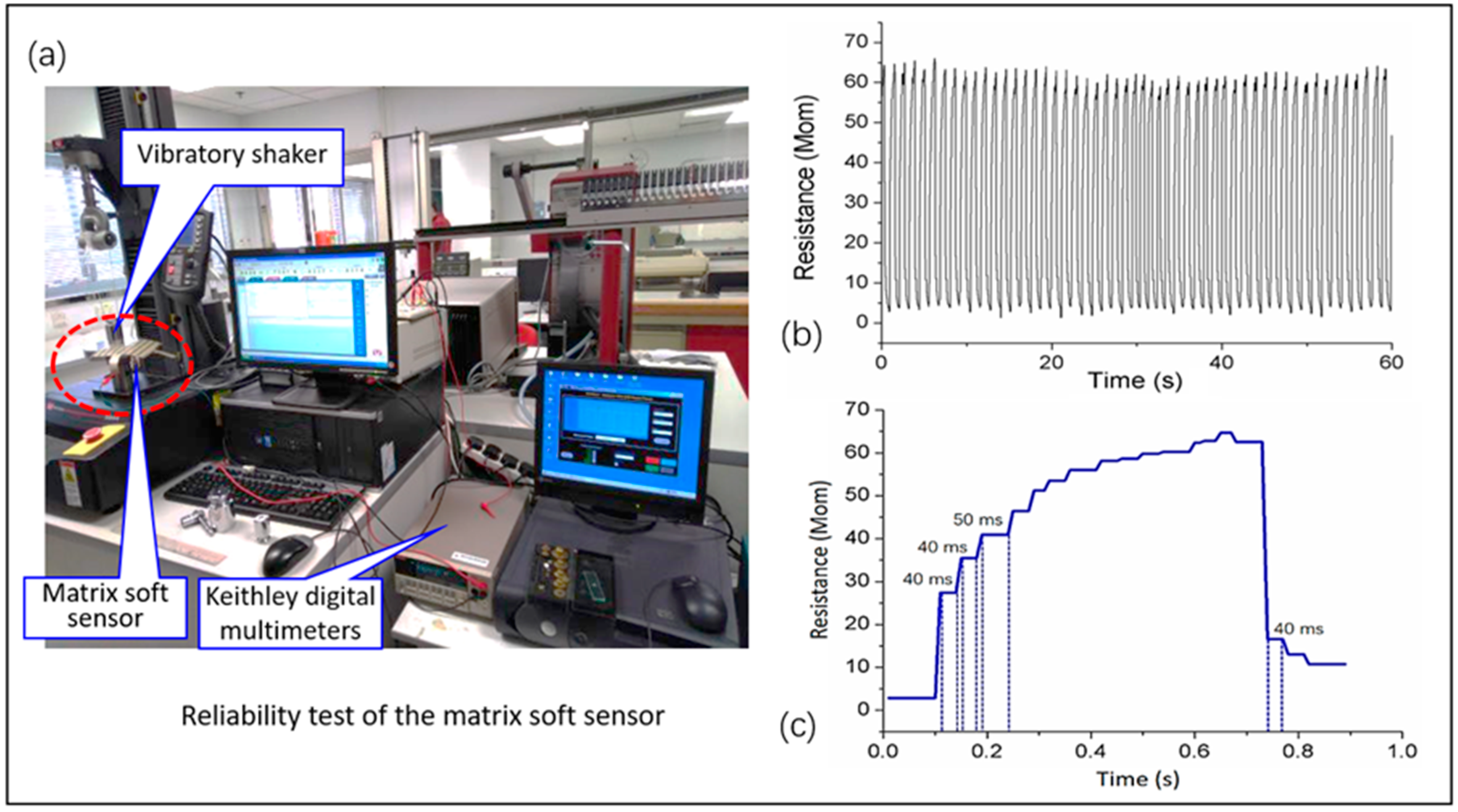
3.2. System Mechanical Analysis
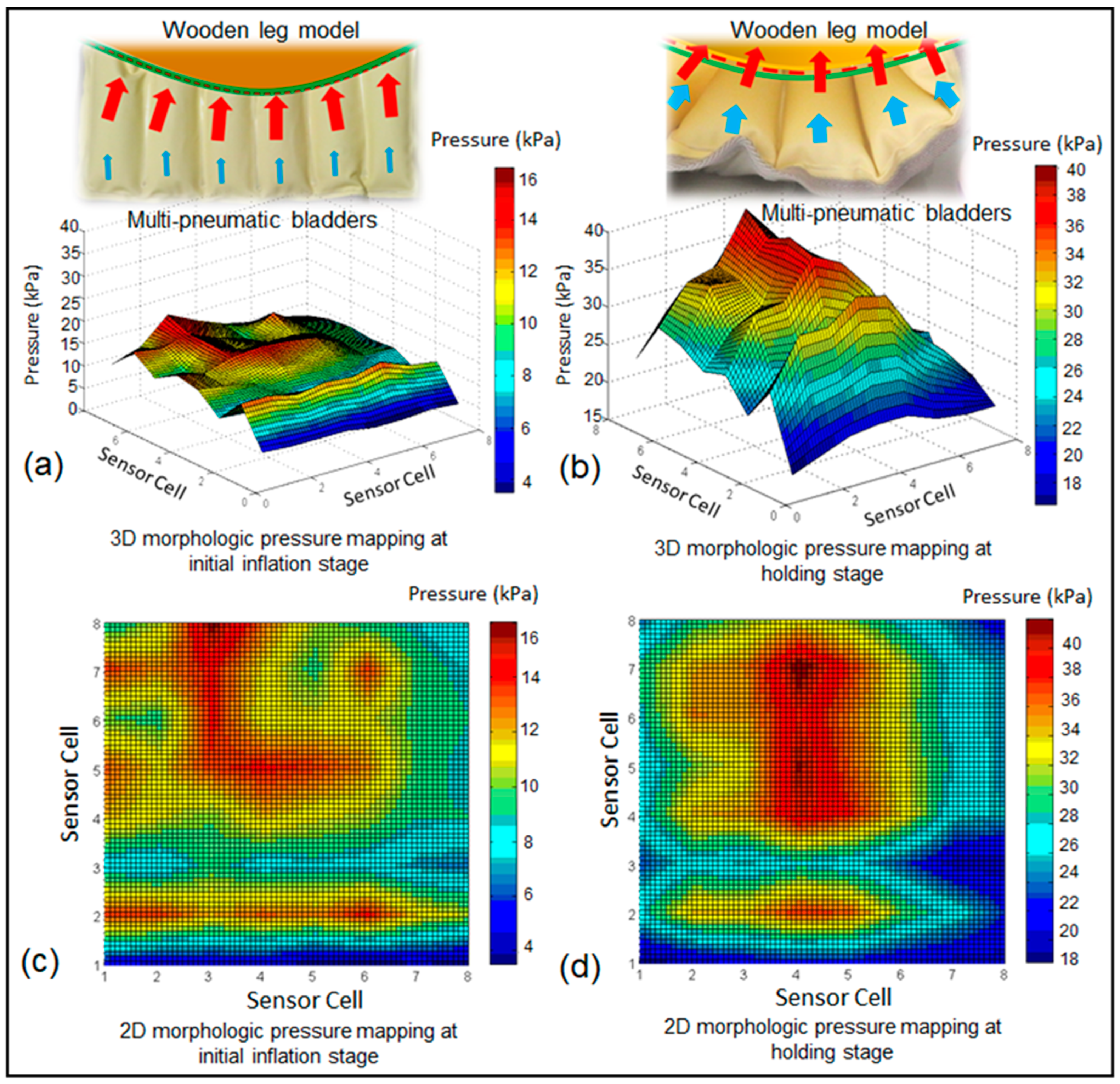
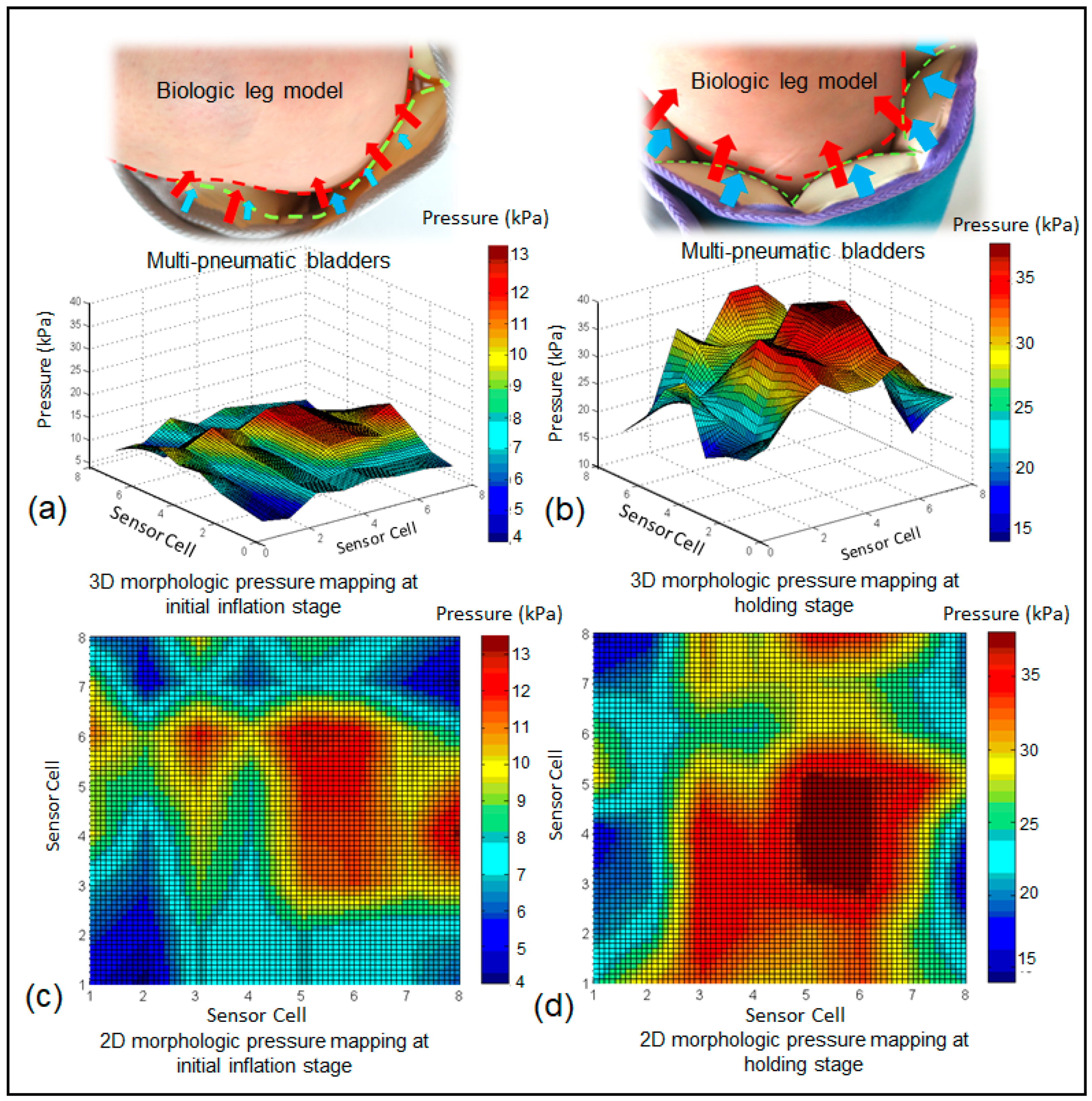
3.3. Morphological Pressure Mapping
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berliner, E.; Ozbilgin, B.; Zarin, D.A. A systematic review of pneumatic compression for treatment of chronic venous insufficiency and venous ulcers. J. Vasc. Surg. 2003, 37, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Compression Therapy Market size, Share and Trends Analysis Report by Technology (Static Compression Therapy, Dynamic Compression Therapy), by Product, by Region, and Segment Forecast, 2018–2025). Available online: https://www.grandviewresearch.com/industry-analysis/compression-therapy-market (accessed on 28 March 2019).
- Partsch, H. Compression therapy: Clinical and experimental evidence. Ann. Vasc. Dis. 2012, 5, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Mosti, G.; Partsch, H. Duplex scanning to evaluate the effect of compression on venous reflux. J. Int. Union Angiol. 2010, 29, 416–420. [Google Scholar]
- Shakti, D.; Mathew, L.; Kumar, N.; Kataria, C. Effectiveness of robo-assisted lower limb rehabilitation for spastic patients: A systematic review. Biosens. Bioelectron. 2018, 117, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Pavon, J.M.; Adam, S.S.; Razouki, Z.A.; McDuffie, J.R.; Lachiewicz, P.F.; Kosinski, A.S.; Beadles, C.A.; Ortel, T.L.; Nagi, A.; Williams, J.W.; et al. Effectiveness of intermittent pneumatic compression devices for venous thromboembolism prophylaxis in high-risk surgical patients: A systematic review. J. Arthroplast. 2016, 31, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Calnan, J. Pneumatic intermittent-compression legging simulating calf-muscle pump. Lancet 1970, 296, 502–503. [Google Scholar] [CrossRef]
- Kim, H.B.; Chung, S.H.; Lee, W.; Seo, J.H.; Lee, S.H.; Kim, K.G.; Kang, H.G. Investigation of blood flow during intermittent pneumatic compression and proposal of a new compression protocol. Clin. Appl. Thromb. 2016, 24, 338–347. [Google Scholar]
- Ho, K.M. Benefit of intermittent pneumatic compression of lower limbs in reducing venous thromboembolism in hospitalised patients: Interactions between risk and effectiveness. Anaesth. Intensive Care 2014, 42, 140. [Google Scholar]
- Waller, T.; Caine, M.; Morris, R. Intermittent pneumatic compression technology for sports recovery. In The Engineering of Sport 6; Springer: Berlin, Germany, 2006; pp. 391–396. [Google Scholar]
- Rithalia, S.V.; Heath, G.H.; Gonsalkorale, M. Evaluation of intermittent pneumatic compression systems. J. Tissue Viability 2002, 12, 52–57. [Google Scholar] [CrossRef]
- Vanek, V.W. Meta-Analysis of Effectiveness of Intermittent Pneumatic Compression Devices with a Comparison of Thigh-High to Knee-High Sleeves. In Database of Abstracts of Reviews of Effects (DARE): Quality-Assessed Reviews [Internet]; Centre for Reviews and Dissemination: York, UK, 1998. [Google Scholar]
- Zhao, S.M.; Liu, R.; Guan, D. Development of an intelligent digital monitoring and biofeedback system for intermittent pneumatic compression therapy device. In Proceedings of the 2019 IEEE 8th International Conference on Fluid Power and Mechatronics (FPM), Wuhan, China, 10–13 April 2019. [Google Scholar]
- Alvarez, O.M.; Wendelken, M.; Markowitz, L.; Parker, R.; Comfort, C. Effectiveness of intermittent pneumatic compression for the treatment of venous ulcers in subjects with secondary lymphedema. Wound Rep. Regen. 2009, 17, A31. [Google Scholar]
- Feldman, J.L.; Stout, N.L.; Wanchai, A.; Stewart, B.R.; Cormier, J.N.; Armer, J.M. Intermittent pneumatic compression therapy: A systematic review. Lymphology 2012, 45, 13. [Google Scholar] [PubMed]
- Ferraresi, C.; Maffiodo, D.; Hajimirzaalian, H. A model-based method for the design of intermittent pneumatic compression systems acting on humans. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, C.; Hajimirzaalian, H.; Maffiodo, D. Identification of physical parameters in a robotized IPC device interacting with human. Trans. Tech. Publ. 2014, 490–491, 1729–1733. [Google Scholar] [CrossRef]
- Zhao, J.M.; Xiao, Z.M.; Li, T.S.; Wu, H.; Jiang, H. Different types of intermittent pneumatic compression devices for preventing venous thromboembolism in patients after total hip replacement. Cochrane Database Syst. Rev. 2012, CD009543. [Google Scholar]
- Phillips, J.J.; Gordon, S.J. Intermittent pneumatic compression dosage for adults and children with lymphedema: A Systematic review. Lymphat. Res. Boil. 2019, 17, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Mathew, L.; Syal, P.; Syal, P. Increasing trend of wearables and multimodal interface for human activity monitoring: A review. Biosens. Bioelectron. 2017, 90, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Shi, Y.; Joe, P.; Ghaffari, R.; Balooch, G.; Usgaonkar, K.; Gur, O.; Tran, P.L.; Crosby, J.R.; Meyer, M.; et al. Conformal piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nat. Mater. 2015, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Reuveny, A.; Reeder, J.; Lee, S.; Jin, H.; Liu, Q.; Yokota, T.; Sekitani, T.; Isoyama, T.; Abe, Y.; et al. A transparent bending-insensitive pressure sensor. Nat. Nanotechnol. 2016, 11, 472–478. [Google Scholar] [CrossRef]
- Mishra, S.; Norton, J.J.S.; Lee, Y.; Lee, D.S.; Agee, N.; Chen, Y.; Chun, Y.; Yeo, W.-H. Soft, conformal bioelectronics for a wireless human-wheelchair interface. Biosens. Bioelectron. 2017, 91, 796–803. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Liu, Q.; Cheng, W.; Wang, X.; Pan, L.; Xu, B.; Xu, H. A self-healable, highly stretchable, and solution processable conductive polymer composite for ultrasensitive strain and pressure sensing. Adv. Funct. Mater. 2018, 28, 1705551. [Google Scholar] [CrossRef]
- Bauer, S.; Bauer-Gogonea, S.; Graz, I.; Kaltenbrunner, M.; Keplinger, C.; Schwödiauer, R. 25th anniversary article: A soft future: From robots and sensor skin to energy harvesters. Adv. Mater. 2014, 26, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018, 3, 143–153. [Google Scholar] [CrossRef]
- Yao, S.; Zhu, Y. Nanomaterial-enabled stretchable conductors: Strategies, materials and devices. Adv. Mater. 2015, 27, 1480–1511. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D.-H. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Bonifas, A.P.; Lu, N.; Su, Y.; Li, R.; Cheng, H.; Ameen, A.; Huang, Y.; Rogers, J.A. Silicon nanomembranes for fingertip electronics. Nanotechnology 2012, 23, 344004. [Google Scholar] [CrossRef] [PubMed]
- Rausch, J.; Salun, L.; Griesheimer, S.; Ibis, M.; Werthschützky, R. Printed Resistive Strain Sensors for Monitoring of Light-Weight Structures. In Smart Sensor Phenomena, Technology, Networks, and Systems 2011; International Society for Optics and Photonics: Bellingham, WA, USA, 2011; Volume 7982, p. 79820H. [Google Scholar]
- Thompson, B.; Yoon, H.-S. Aerosol Printed Carbon Nanotube Strain Sensor. In Smart Sensor Phenomena, Technology, Networks, and Systems Integration 2012; International Society for Optics and Photonics: Bellingham, WA, USA, 2012; Volume 8346, p. 83461. [Google Scholar]
- Zhan, Z.; Lin, R.; Tran, V.T.; An, J.; Wei, Y.; Du, H.; Tran, T.; Lu, W. Paper/carbon nanotube-based wearable pressure sensor for physiological signal acquisition and soft robotic skin. ACS Appl. Mater. Interfaces 2017, 9, 37921–37928. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.-H.; Lee, Y.; Sharma, B.K.; Lee, H.-J.; Kim, J.-H.; Ahn, J.-H. Graphene-based transparent strain sensor. Carbon 2013, 51, 236–242. [Google Scholar] [CrossRef]
- Bessonov, A.; Kirikova, M.; Haque, S.; Gartseev, I.; Bailey, M.J. Highly reproducible printable graphite strain gauges for flexible devices. Sens. Actuators A Phys. 2014, 206, 75–80. [Google Scholar] [CrossRef]
- Latessa, G.; Brunetti, F.; Reale, A.; Saggio, G.; Di Carlo, A. Piezoresistive behaviour of flexible PEDOT:PSS based sensors. Sens. Actuators B Chem. 2009, 139, 304–309. [Google Scholar] [CrossRef]
- King, M.; Baragwanath, A.; Rosamond, M.; Wood, D.; Gallant, A. Porous PDMS force sensitive resistors. Procedia Chem. 2009, 1, 568–571. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Fan, D. 3D Graphite-polymer flexible strain sensors with ultrasensitivity and durability for real-time human vital sign monitoring and musical instrument education. Adv. Mater. Technol. 2017, 2, 1700070. [Google Scholar] [CrossRef]
- Yin, G.; Hu, N.; Karube, Y.; Liu, Y.; Li, Y.; Fukunaga, H. A carbon nanotube/polymer strain sensor with linear and anti-symmetric piezoresistivity. J. Compos. Mater. 2011, 45, 1315–1323. [Google Scholar]
- Ramalingame, R.; Hu, Z.; Gerlach, C.; Rajendran, D.; Zubkova, T.; Baumann, R.; Kanoun, O. Flexible piezoresistive sensor matrix based on a carbon nanotube PDMS composite for dynamic pressure distribution measurement. J. Sens. Sens. Syst. 2019, 8, 1–7. [Google Scholar] [CrossRef]
- Chun, K.-Y.; Oh, Y.; Rho, J.; Ahn, J.-H.; Kim, Y.-J.; Choi, H.R.; Baik, S. Highly conductive, printable and stretchable composite films of carbon nanotubes and silver. Nat. Nanotechnol. 2010, 5, 853–857. [Google Scholar] [CrossRef]
- Hu, S.L.; Zhao, S.M.; Zhou, L.; Lai, D.H.; Jiang, H.Z.; Zhang, L. Research on rapid detection system and separation of filter rod pressure drop. Transducer Microsyst. Technol. 2017, 36, 86–89. [Google Scholar]
- Liu, R.; Kwok, Y.L.; Li, Y.; Lao, T.T.H.; Zhang, X.; Dai, X.Q. Objective evaluation of skin pressure distribution of graduated elastic compression stockings. Dermatol. Surg. 2005, 31, 615–624. [Google Scholar] [CrossRef]
- Liu, R.; Kwok, Y.L.; Li, Y.; Lao, T.T.; Zhang, X. The Effects of graduated compression stockings (GCSs) on cutaneous surface pressure along the path of main superficial veins of lower limbs. Wounds Compend. Clin. Res. Pract. 2006, 18, 150–157. [Google Scholar]
- Chen, D.; Pei, Q. Electronic muscles and skins: A review of soft sensors and actuators. Chem. Rev. 2017, 117, 11239–11268. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Deng, J.; Liu, R.; Hsiao, Y.-C.; Zhang, S.L.; Peng, W.; Wu, H.-M.; Wang, X.; Wang, Z.L. actively perceiving and responsive soft robots enabled by self-powered, highly extensible, and highly sensitive triboelectric proximity- and pressure-sensing skins. Adv. Mater. 2018, 30, 1801114. [Google Scholar] [CrossRef]
- Lau, P.H.; Takei, K.; Wang, C.; Ju, Y.; Kim, J.; Yu, Z.; Takahashi, T.; Cho, G.; Javey, A. Fully printed, high performance carbon nanotube thin-film transistors on flexible substrates. Nano Lett. 2013, 13, 3864–3869. [Google Scholar] [CrossRef]
- Rim, Y.S.; Bae, S.-H.; Chen, H.; De Marco, N.; Yang, Y.; Bae, S. Recent progress in materials and devices toward printable and flexible sensors. Adv. Mater. 2016, 28, 4415–4440. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, M.; Dargahi, J.; Kövecses, J.; Mardasi, M.G.; Nouri, S. A new approach for modeling piezoresistive force sensors based on semiconductive polymer composites. IEEE ASME Trans. Mechatron. 2012, 17, 572–581. [Google Scholar] [CrossRef]
- Holm, R. Electric Contacts: Theory and Application; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Zhang, X.W.; Pan, Y.; Zheng, Q.; Yi, X.S. Time dependence of piezoresistance for the conductor-filled polymer composites. J. Polym. Sci. Part B: Polym. Phys. 2000, 38, 2739–2749. [Google Scholar] [CrossRef]
- Brinson, H.F.; Brinson, L.C. Polymer Engineering Science and Viscoelasticity: An Introduction; Springer: Berlin, Germany, 2008; Volume 4, p. 46. [Google Scholar]
- Wang, X.; Gu, Y.; Xiong, Z.; Cui, Z.; Zhang, T. Silk-molded flexible, ultrasensitive, and highly stable electronic skin for monitoring human physiological signals. Adv. Mater. 2014, 26, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Joe, D.J.; Kim, D.H.; Park, H.; Han, J.H.; Jeong, C.K.; Park, H.; Park, J.G.; Joung, B.; Lee, K.J. Self-powered real-time arterial pulse monitoring using ultrathin epidermal piezoelectric sensors. Adv. Mater. 2017, 29, 1702308. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zeng, S.; Zhang, Z. The design of wireless data acquisition system based on STM32 and virtual instrument. In Proceedings of the International Conference on Wireless Communications, Shanghai, China, 21–23 September 2012. [Google Scholar]
- Zhang, H.-F.; Kang, W. Design of the data acquisition system based on STM32. Procedia Comput. Sci. 2013, 17, 222–228. [Google Scholar] [CrossRef]
- Behrens, I.; Doering, L.; Peiner, E. Piezoresistive cantilever as portable micro force calibration standard. J. Micromech. Microeng. 2003, 13, S171–S177. [Google Scholar] [CrossRef]
- Brimacombe, J.M.; Wilson, D.R.; Hodgson, A.J.; Ho, K.C.T.; Anglin, C. Effect of calibration method on tekscan sensor accuracy. J. Biomech. Eng. 2009, 131, 034503. [Google Scholar] [CrossRef] [PubMed]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Rabee, F.; Liao, Y.; Yang, M. Minimizing multiple-priority inversion protocol in hard real time system. In Proceedings of the IEEE International Conference on Dependable (IEEE), Chengdu, China, 21–22 December 2013. [Google Scholar]
- Halbwachs, N.; Proy, Y.E.; Roumanoff, P. Verification of systems in real time through analysis of linear relationships. Form. Methods Syst. Des. 1997, 11, 157–185. [Google Scholar] [CrossRef]
- Shin, K.; Kim, H. Hard deadlines in real-time control systems. Control Eng. Pr. 1993, 1, 623–628. [Google Scholar] [CrossRef]
- Malone, M.D.; Cisek, P.L.; Holland, B.; Eid, I.G.; Comerota, A.J. High-pressure, rapid-inflation pneumatic compression improves venous hemodynamics in healthy volunteers and patients who are post-thrombotic. J. Vasc. Surg. 1999, 29, 593–599. [Google Scholar] [CrossRef]
- Eisele, R.; Kinzl, L.; Koelsch, T. Rapid-inflation intermittent pneumatic compression for prevention of deep venous thrombosis. J. Bone Jt. Surg. Am. Vol. 2007, 89, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Kordzadeh, A.; Jónás, A.; Panayiotopoulos, Y.P. Intermittent pneumatic compression in treatment of chronic venous leg ulcers: A case report and review of literature. Case Rep. Clin. Med. 2014, 3, 513–517. [Google Scholar] [CrossRef]
- Olszewski, W.L.; Jain, P.; Ambujam, G.; Zaleska, M.; Cakala, M.; Gradalski, T. Tissue fluid pressure and flow during pneumatic compression in lymphedema o lower limbs. Lymph. Res. Biol. 2011, 9, 77–83. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Liu, R.; Fei, C.; Guan, D. Dynamic Interface Pressure Monitoring System for the Morphological Pressure Mapping of Intermittent Pneumatic Compression Therapy. Sensors 2019, 19, 2881. https://doi.org/10.3390/s19132881
Zhao S, Liu R, Fei C, Guan D. Dynamic Interface Pressure Monitoring System for the Morphological Pressure Mapping of Intermittent Pneumatic Compression Therapy. Sensors. 2019; 19(13):2881. https://doi.org/10.3390/s19132881
Chicago/Turabian StyleZhao, Shumi, Rong Liu, Chengwei Fei, and Dong Guan. 2019. "Dynamic Interface Pressure Monitoring System for the Morphological Pressure Mapping of Intermittent Pneumatic Compression Therapy" Sensors 19, no. 13: 2881. https://doi.org/10.3390/s19132881
APA StyleZhao, S., Liu, R., Fei, C., & Guan, D. (2019). Dynamic Interface Pressure Monitoring System for the Morphological Pressure Mapping of Intermittent Pneumatic Compression Therapy. Sensors, 19(13), 2881. https://doi.org/10.3390/s19132881




