SERS, XPS and DFT Study of Xanthine Adsorbed on Citrate-Stabilized Gold Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Raman Measurements
2.3. UV-Vis-NIR Absorption Measurements
2.4. XPS Measurements
2.5. Computational Details
3. Results and Discussion
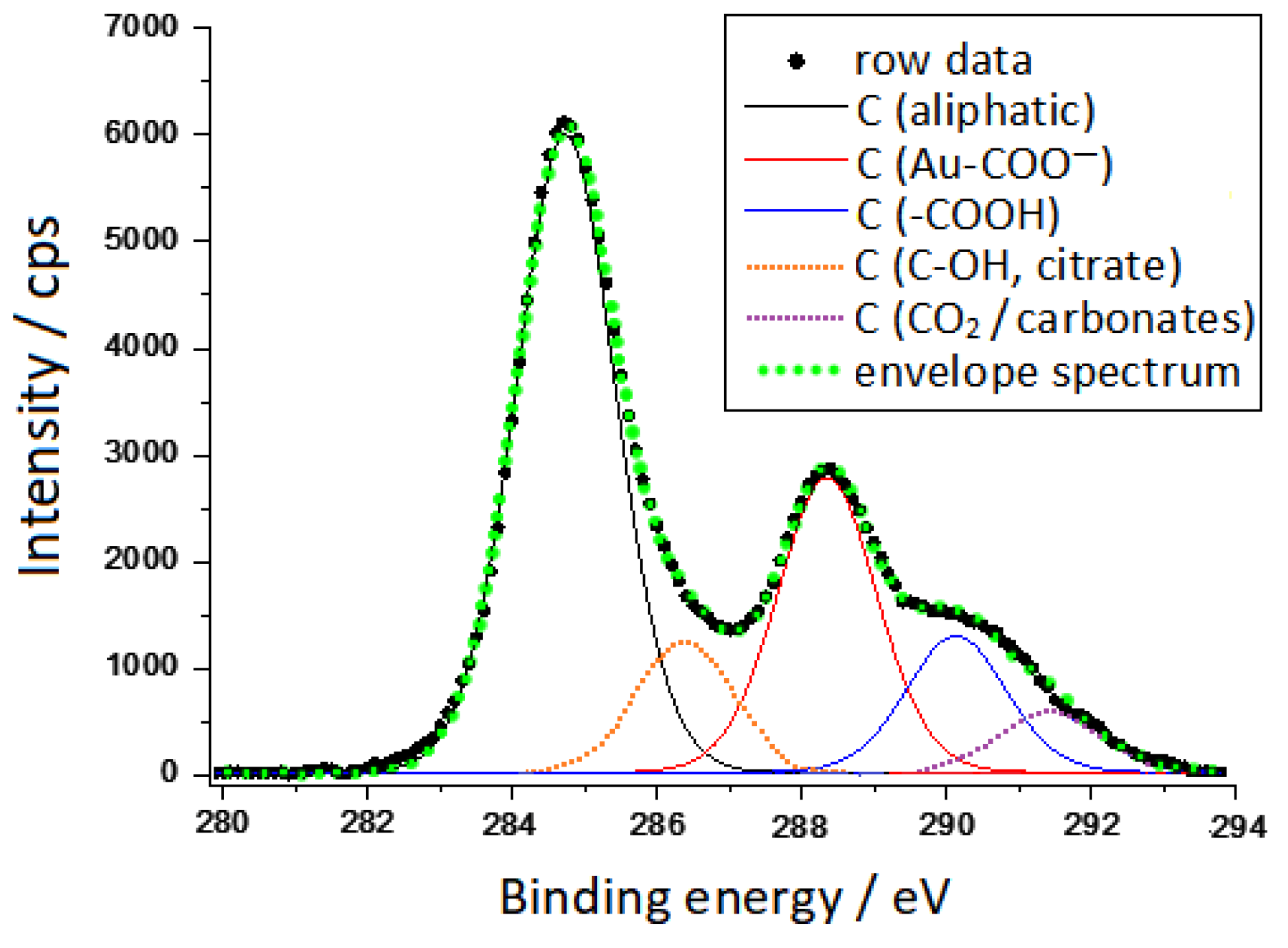
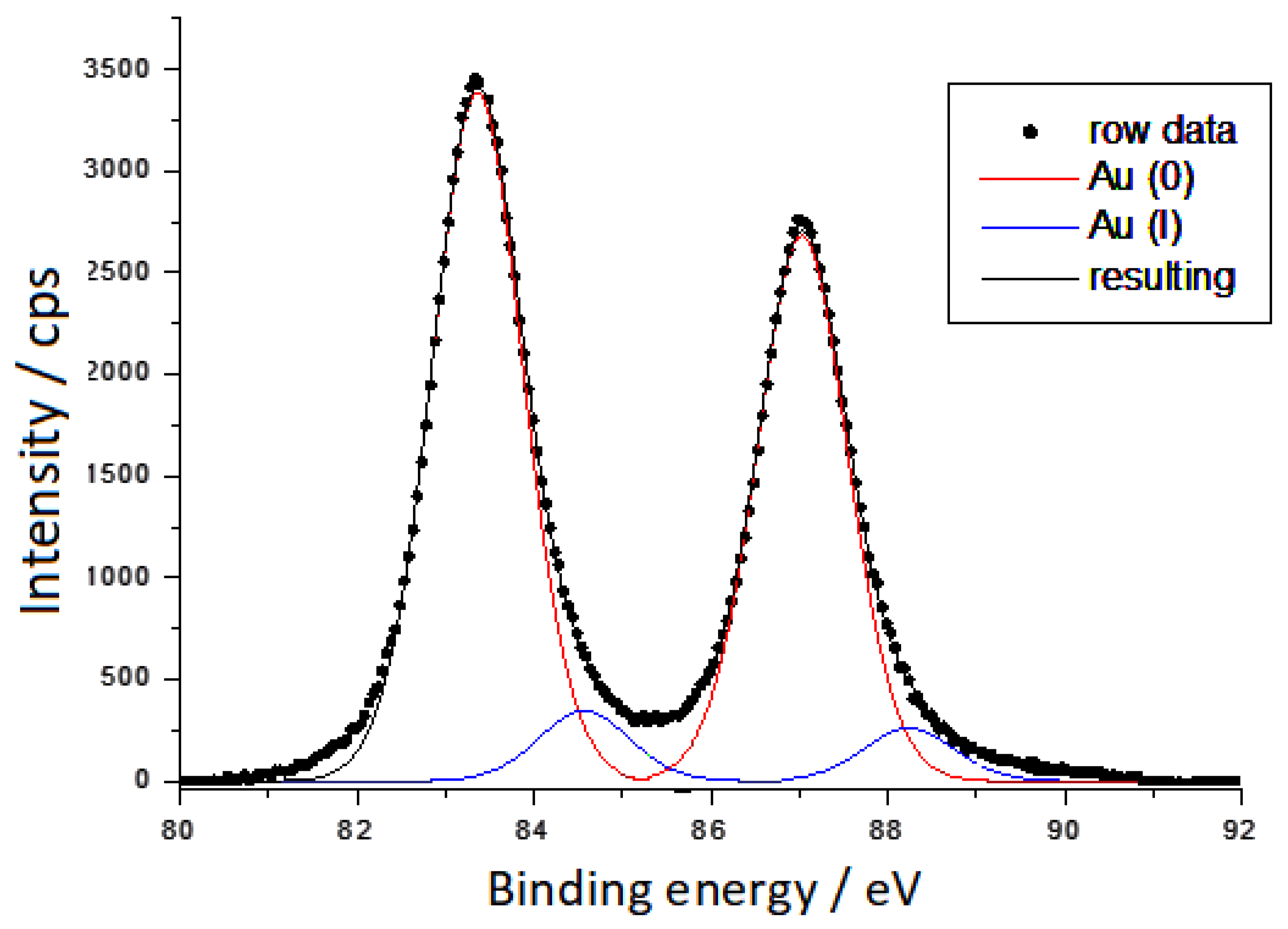
3.1. XPS Spectra
- (1)
- C in aliphatic chains (deriving from atmospheric contamination and also from the CH2 group of citrates) at binding energy (BE) = 284.8 eV;
- (2)
- C-OH of citrate at BE = 286.4 eV, according to the literature data [26];
- (3)
- COO-Au at BE = 288.3 eV, whose binding energy is a little higher than that reported at 287.6 eV by Park et al. [27];
- (4)
- COOH (non-bonded to Au) at BE = 290.2 eV, analogously to what was reported by Park et al. [27];
- (5)
- C in carbonates at BE = 291.4 eV, according to the values (291.3–291.8 eV) reported in the literature [28]. The presence of carbonates could result from the dissolution of CO2 in the aqueous solvent during the reduction process of gold ions with citrate. It is reasonable that this signal was not observed in the work of Park [27], because in that case the gold nanoparticles were washed with a thiol solution with the removal of the most soluble components.
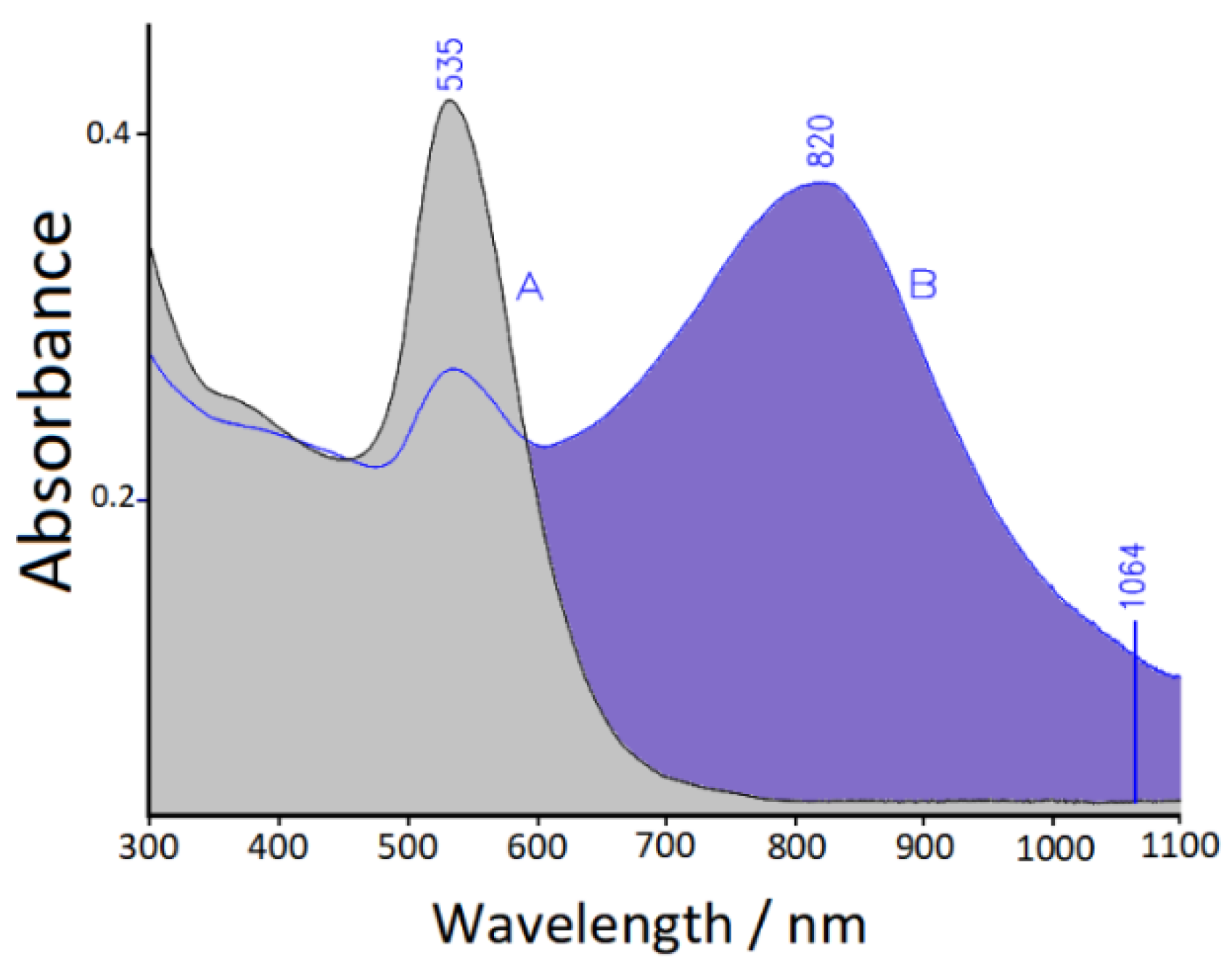
3.2. UV-Visible-NIR Absorption Spectra
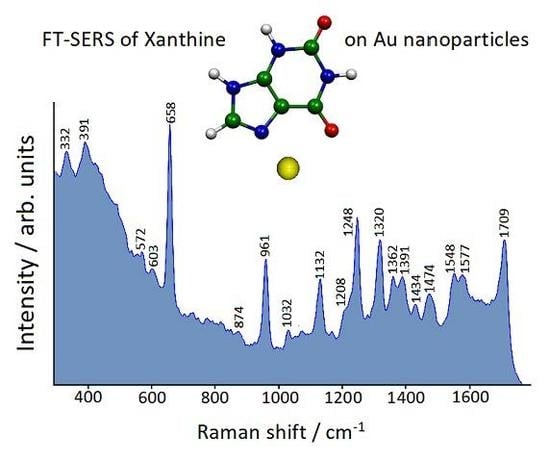
3.3. FT-SERS Spectra
3.4. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Alex, S.; Tiwari, A. Functionalized Gold Nanoparticles: Synthesis, Properties and Applications—A Review. J. Nanosci. Nanotechnol. 2015, 15, 1869–1894. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef]
- Xiong, W.; Mazid, R.; Yap, L.W.; Li, X.; Cheng, W. Plasmonic caged gold nanorods for near-infrared light controlled drug delivery. Nanoscale 2014, 6, 14388–14393. [Google Scholar] [CrossRef]
- Basu, S.; Jana, S.; Pande, S.; Pal, T. Interaction of DNA bases with silver nanoparticles: Assembly quantified through SPRS and SERS. J. Colloid Interface Sci. 2008, 321, 288–293. [Google Scholar] [CrossRef]
- Hakimian, F.; Ghourchian, H.; Hashemi, A.S.; Arastoo, M.R.; Rad, M.B. Ultrasensitive optical biosensor for detection of miRNA-155 using positively charged Au nanoparticles. Sci. Rep. 2018, 8, 2943. [Google Scholar] [CrossRef]
- Carnerero, J.M.; Sánchez-Coronilla, A.; Martín, E.I.; Jimenez-Ruiz, A.; Prado-Gotor, R. Quantification of nucleobases/gold nanoparticles interactions: Energetics of the interactions through apparent binding constants determination. Phys. Chem. Chem. Phys. 2017, 19, 22121–22128. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. (London) 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Kumar, S.; Gandhi, K.S.; Kumar, R. Modeling of Formation of Gold Nanoparticles by Citrate Method. Ind. Eng. Chem. Res. 2007, 46, 3128–3136. [Google Scholar] [CrossRef]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size Control of Gold Nanocrystals in Citrate Reduction: The Third Role of Citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef] [PubMed]
- Königsberger, E.; Wang, Z.; Seidel, J.; Wolf, G. Solubility and dissolution enthalpy of xanthine. J. Chem. Thermodyn. 2001, 33, 1–9. [Google Scholar] [CrossRef]
- Ichida, K.; Amaya, Y.; Kamatani, N.; Nishino, T.; Hosoya, T.; Sakai, O. Identification of two mutations in human xanthine dehydrogenase gene responsible for classical type I xanthinuria. J. Clin. Investig. 1997, 99, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Schlücker, S. Surface Enhanced Raman Spectroscopy: Analytical, Biophysical and Life Science Applications; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Procházka, M. Surface-Enhanced Raman Spectroscopy, Bioanalytical, Biomolecular and Medical Applications; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Keresztury, G.; Holly, S.; Besenyei, G.; Varga, J.; Wang, A.; Durig, J.R. Vibrational spectra of monothiocarbamates-II. IR and Raman spectra, vibrational assignment, conformational analysis and ab initio calculations of S-methyl-N,N-dimethylthiocarbamate. Spectrochim. Acta A 1993, 49, 2007–2026. [Google Scholar] [CrossRef]
- Keresztury, G. Handbook of Vibrational Spectroscopy; Chalmers, J.M., Griffiths, P.R., Eds.; Wiley & Sons: Chichester, UK, 2002; Volume 1, pp. 71–87. [Google Scholar]
- Tselesh, A.S. Anodic behaviour of tin in citrate solutions: The IR and XPS study on the composition of the passive layer. Thin Solid Films 2008, 516, 6253–6260. [Google Scholar] [CrossRef]
- Park, J.-W.; Shumaker-Parry, J.S. Strong Resistance of Citrate Anions on Metal Nanoparticles to Desorption under Thiol Functionalization. ACS Nano 2015, 9, 1665–1682. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1979. [Google Scholar]
- Verma, H.N.; Singh, P.; Chavan, R.M. Gold nanoparticle: Synthesis and characterization. Vet. World 2014, 7, 72–77. [Google Scholar] [CrossRef]
- Muangnapoh, T.; Sano, N.; Yusa, S.-I.; Viriya-Empikul, N.; Charinpanitkul, T. Facile strategy for stability control of gold nanoparticles synthesized by aqueous reduction method. Curr. Appl. Phys. 2010, 10, 708–714. [Google Scholar] [CrossRef]
- Polat, T.; Yıldırım, G. Investigation of solvent polarity effect on molecular structure and vibrational spectrum of xanthine with the aid of quantum chemical computations. Spectrochim. Acta Part A 2014, 123, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Kulikowska, E.; Kierdaszuk, B.; Shugar, D. Xanthine, xanthosine and its nucleotides: Solution structures of neutral and ionic forms, and relevance to substrate properties in various enzyme systems and metabolic pathways. Acta Biochim. Pol. 2004, 51, 493–531. [Google Scholar] [PubMed]
- Pagliai, M.; Muniz-Miranda, F.; Schettino, V.; Muniz-Miranda, M. Competitive Solvation and Chemisorption in Silver Colloidal Suspensions. Progr. Colloid Polym. Sci. 2011, 139, 39–44. [Google Scholar] [CrossRef][Green Version]
- Muniz-Miranda, M.; Pergolese, B.; Muniz-Miranda, F.; Caporali, S. SERS effect from Pd surfaces coated with thin films of Ag colloidal nanoparticles. J. Alloys Compd. 2015, 615, S357–S360. [Google Scholar] [CrossRef]
- Gellini, C.; Deepak, F.L.; Muniz-Miranda, M.; Caporali, S.; Muniz-Miranda, F.; Pedone, A.; Innocenti, C.; Sangregorio, C. Magneto-plasmonic colloidal nanoparticles obtained by laser ablation of nickel and silver targets in water. J. Phys. Chem. C 2017, 121, 3597–3606. [Google Scholar] [CrossRef]
- Kundu, J.; Neumann, O.; Janesko, B.G.; Zhang, D.; Lal, S.; Barhoumi, A.; Scuseria, G.E.; Halas, N.J. Adenine- and Adenosine Monophosphate (AMP)-Gold Binding Interactions Studied by Surface-Enhanced Raman and Infrared Spectroscopies. J. Phys. Chem. C 2009, 113, 14390–14397. [Google Scholar] [CrossRef]
- Pagliai, M.; Caporali, S.; Muniz-Miranda, M.; Pratesi, G.; Schettino, V. SERS, XPS, and DFT Study of Adenine Adsorption on Silver and Gold Surfaces. J. Phys. Chem. Lett. 2012, 3, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Pergolese, B.; Bonifacio, A.; Bigotto, A. SERS studies of the adsorption of guanine derivatives on gold colloidal nanoparticles. Phys. Chem. Chem. Phys. 2005, 7, 3610–3613. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. Raman and Computational Study on the Adsorption of Xanthine on Silver Nanocolloids. ACS Omega 2018, 3, 13530–13537. [Google Scholar] [CrossRef]
- Chase, D.B. Fourier transform Raman spectroscopy. J. Am. Chem. Soc. 1986, 108, 7485–7488. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Mancini, M.C.; Nie, S. Bioimaging: Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef]
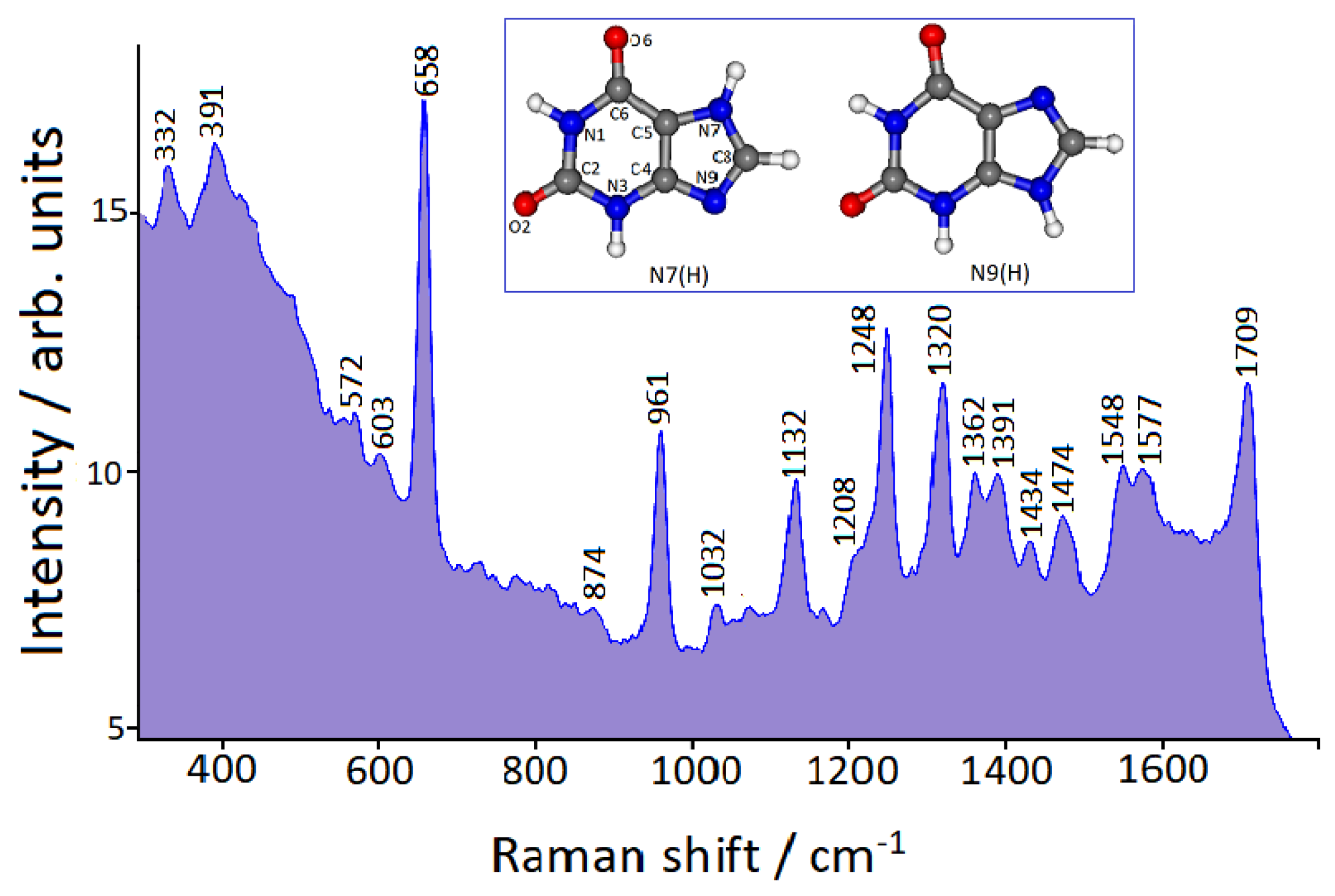
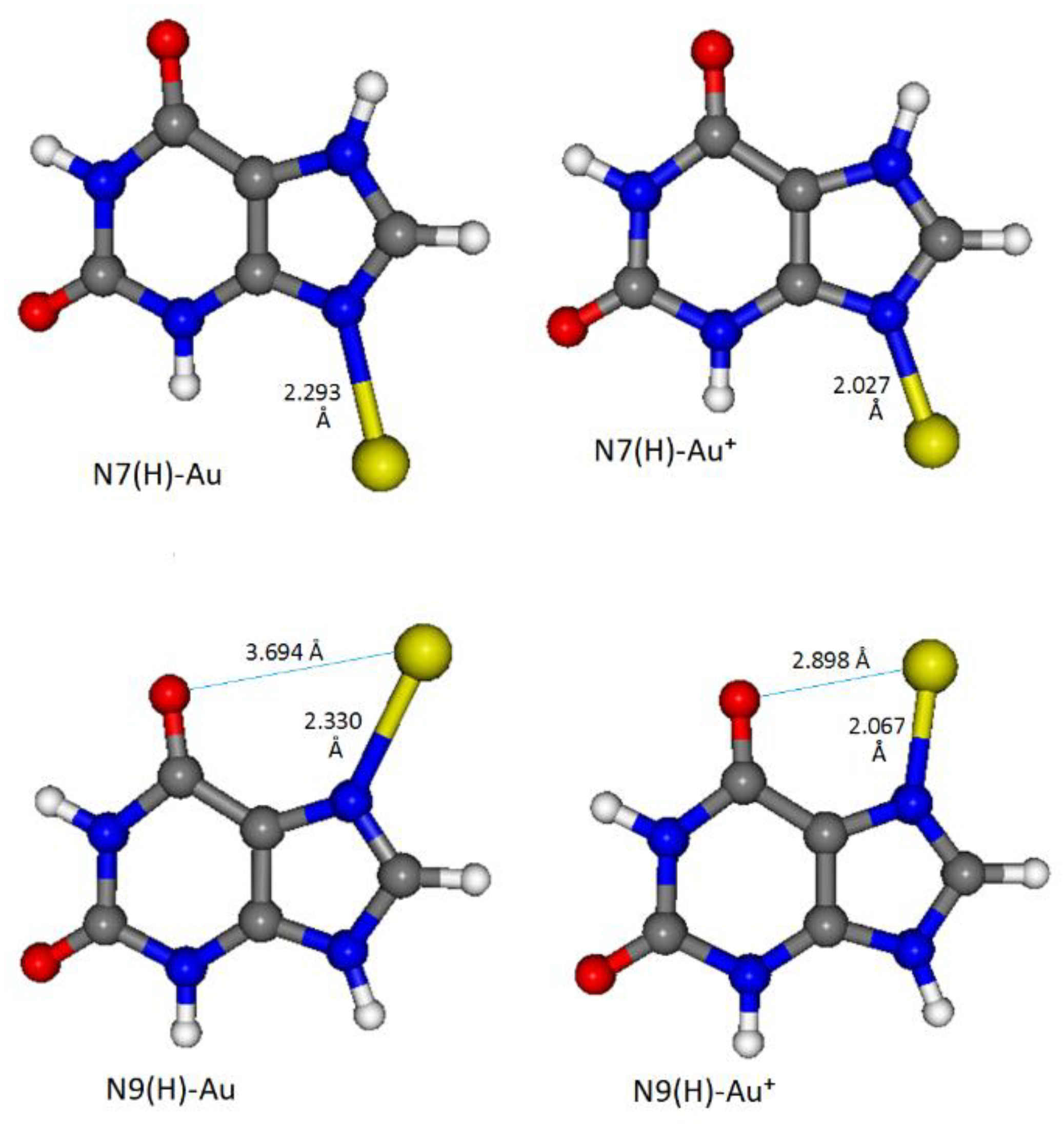
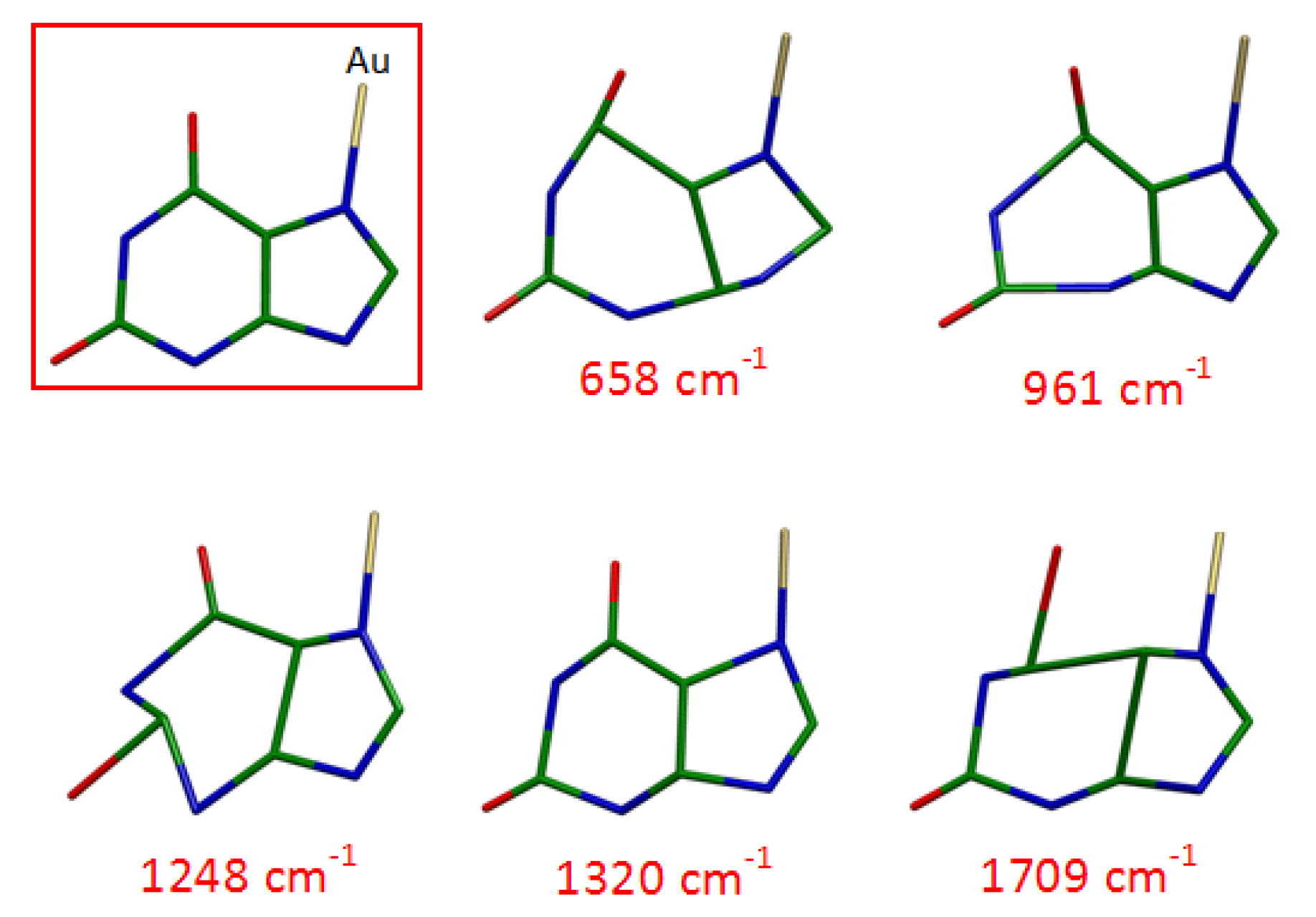
| SERS on Au Nanoparticles | Calc. N7(H)-Au° | Calc. N9(H)-Au° | Calc. N7(H)-Au+ | Calc. N9(H)-Au+ |
|---|---|---|---|---|
| 332 | 342 | 332 | 344 | 352 |
| 391 | 371 | 365 | 381 | 375 |
| 572 | 548 | 572 | 589 | |
| 603 | 613 | 611 | 619 | 617 |
| 658 | 677 | 675 | 659 | 653 |
| 874 | 873 | 841 | 855 | 857 |
| 961 | 971 | 963 | 954 | 950 |
| 1032 | 1013 | 1014 | ||
| 1132 | 1121 | 1121 | 1130 | 1132 |
| 1208 | 1207 | 1218 | 1230 | |
| 1248 | 1270 | 1281 | 1269 | 1252 |
| 1320 | 1310 | 1310 | 1334 | 1326 |
| 1362 | 1322 | 1379 | 1346 | |
| 1391 | 1408 | 1421 | 1415 | 1415 |
| 1434 | 1422 | 1435 | 1433 | 1428 |
| 1474 | 1464 | 1464 | 1458 | |
| 1548 | 1502 | 1515 | ||
| 1577 | 1584 | 1573 | 1591 | 1588 |
| 1709 | 1712 | 1720 | 1720 | 1702 |
| energy (a.u.) | −697.8012 | −697.7842 | −697.5511 | −697.5584 |
| charge-transfer 1 | −0.1910 e | −0.1763 e | −0.3279 e | −0.3544 e |
| charge-transfer 2 | −0.1938 e | −0.1916 e | −0.5308 e | −0.5361 e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caporali, S.; Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. SERS, XPS and DFT Study of Xanthine Adsorbed on Citrate-Stabilized Gold Nanoparticles. Sensors 2019, 19, 2700. https://doi.org/10.3390/s19122700
Caporali S, Muniz-Miranda F, Pedone A, Muniz-Miranda M. SERS, XPS and DFT Study of Xanthine Adsorbed on Citrate-Stabilized Gold Nanoparticles. Sensors. 2019; 19(12):2700. https://doi.org/10.3390/s19122700
Chicago/Turabian StyleCaporali, Stefano, Francesco Muniz-Miranda, Alfonso Pedone, and Maurizio Muniz-Miranda. 2019. "SERS, XPS and DFT Study of Xanthine Adsorbed on Citrate-Stabilized Gold Nanoparticles" Sensors 19, no. 12: 2700. https://doi.org/10.3390/s19122700
APA StyleCaporali, S., Muniz-Miranda, F., Pedone, A., & Muniz-Miranda, M. (2019). SERS, XPS and DFT Study of Xanthine Adsorbed on Citrate-Stabilized Gold Nanoparticles. Sensors, 19(12), 2700. https://doi.org/10.3390/s19122700