A Phenotype-Based Approach for the Substrate Water Status Forecast of Greenhouse Netted Muskmelon
Abstract
1. Introduction
2. Materials and Methods
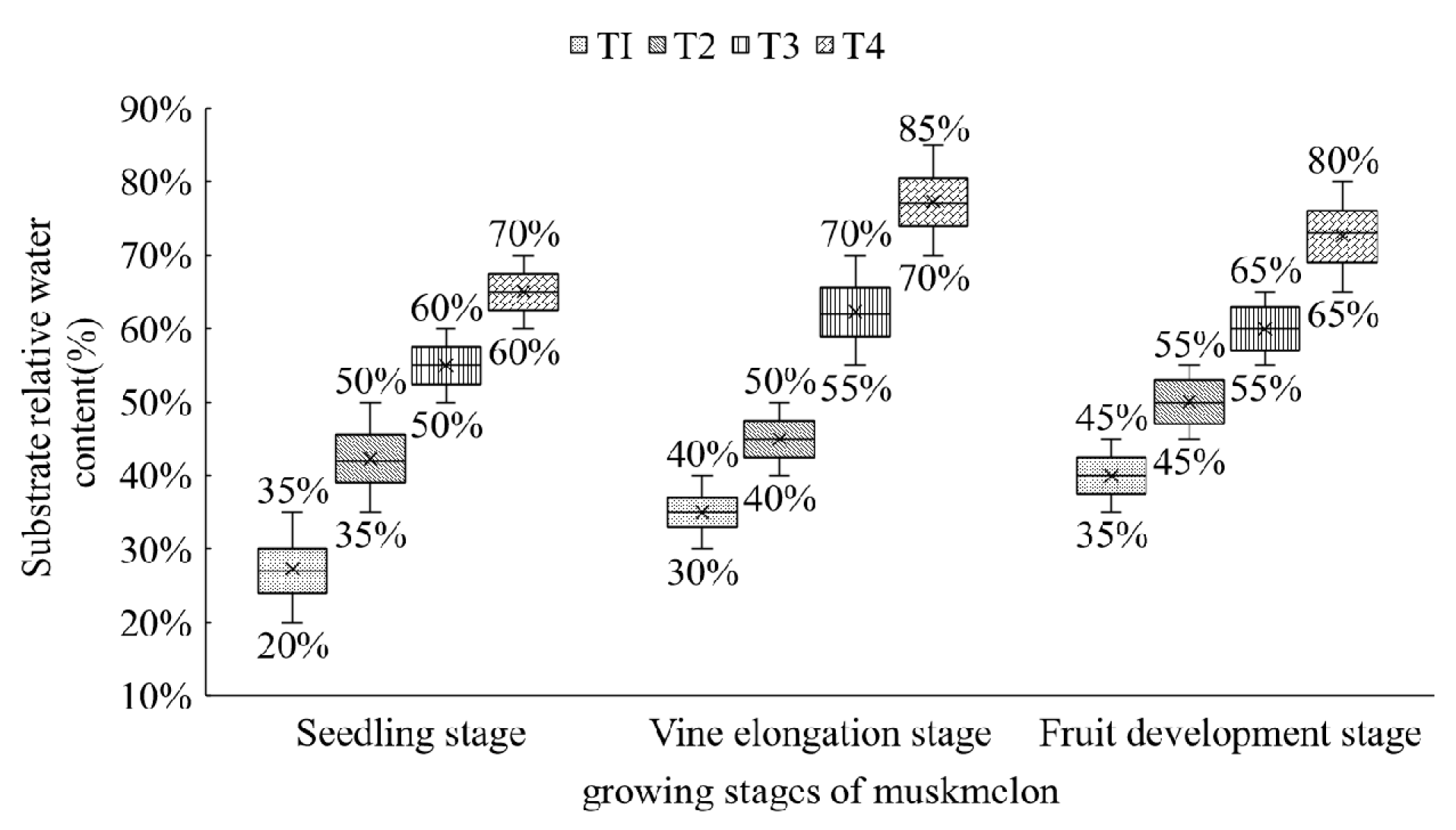
2.1. Materials and Treatments
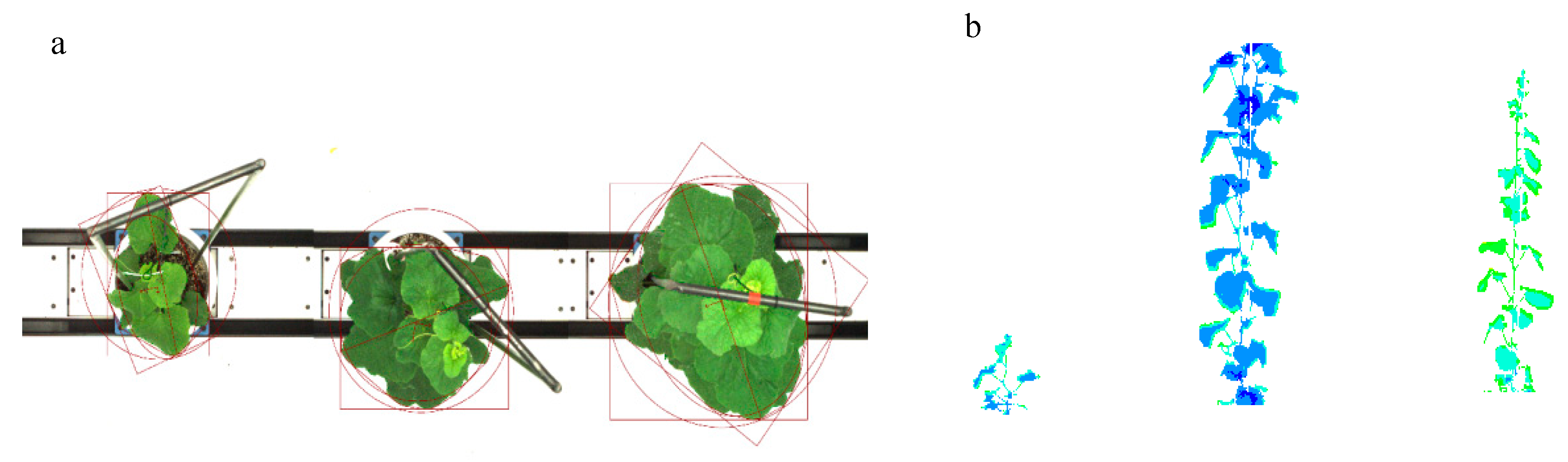
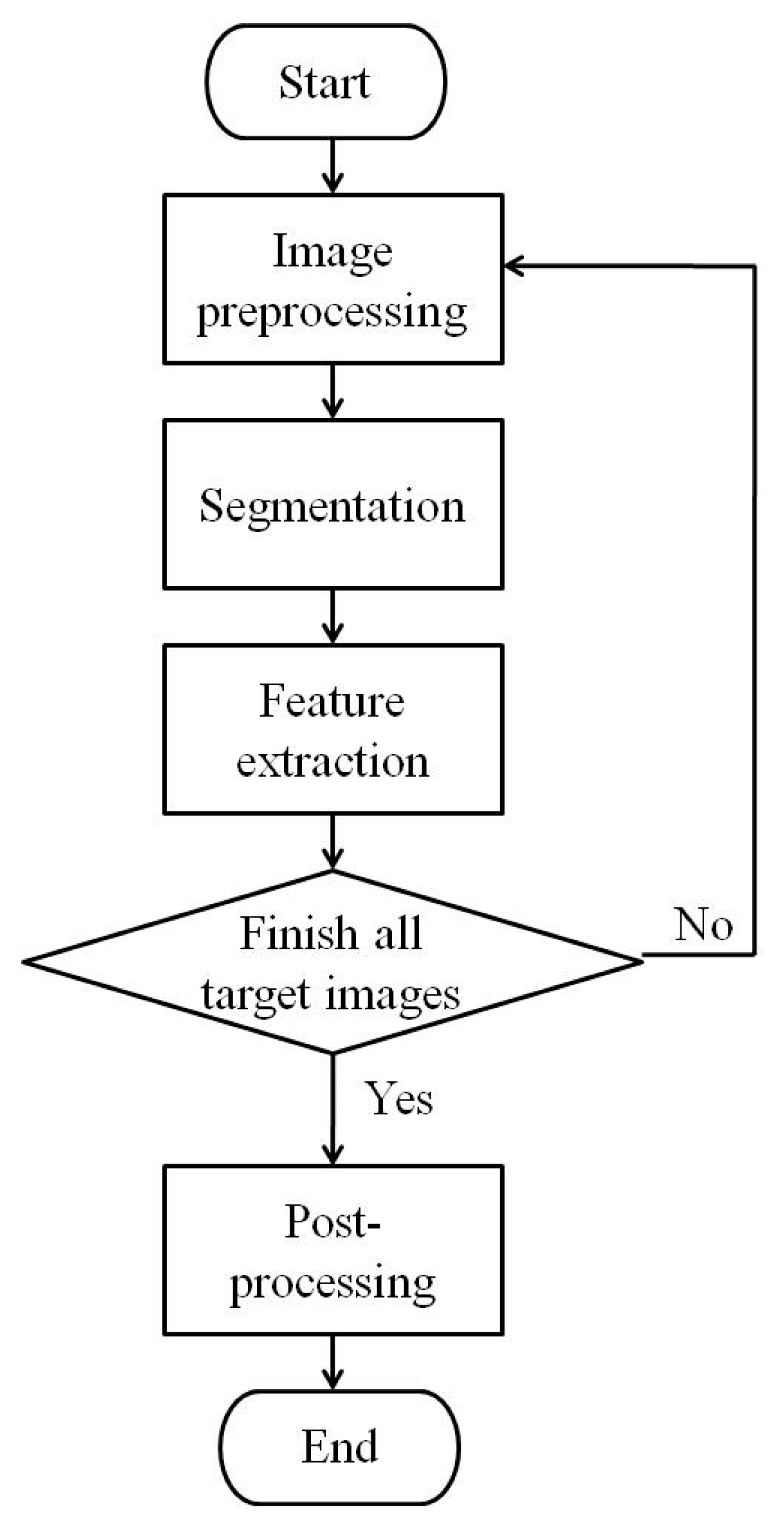
2.2. Image Acquisition and Trait Extraction
2.3. Model Development of Substrate Water Status
2.4. Data Preprocessing
2.5. Phenotypic Traits Screening
2.6. Model Development and Forecast Ability Assessment
3. Results
3.1. Subsection
3.1.1. Extraction of Phenotypic Traits
3.1.2. Analysis of Phenotypic Traits
3.1.3. Machine Learning Modeling and Forecast Ability Assessment of Phenotypic Traits
3.1.4. Trait Contribution Analysis and Model Simplification
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China (MOARAPRC). Areas and yields of vegetable, watermelon, muskmelon, strawberry, and potato in 2015, China. China Veg. 2017, 1, 18. (In Chinese). Available online: http://www.cnki.com.cn/Article/CJFDTotal-ZGSC201701004.htm (accessed on 5 June 2019).
- Contreras, J.I.; Plaza, B.M.; Lao, M.T.; Segura, M.L. Growth and nutritional response of melon to water quality and nitrogen potassium fertigation levels under greenhouse mediterranean conditions. Commun. Soil Sci. Plant Anal. 2012, 43, 434–444. [Google Scholar] [CrossRef]
- Sharma, S.P.; Leskovar, D.I.; Crosby, K.C.; Ibraim, A.M.H.; Volder, A. Differential response of muskmelon (Cucumis melo L.) cultivars to deficit irrigation. Acta Hortic. 2016, 1112, 201–208. [Google Scholar] [CrossRef]
- Sharma, S.P.; Leskovar, D.I.; Crosby, K.M.; Volder, A.; Ibrahim, A.M.H. Root growth, yield, and fruit quality responses of reticulatus and inodorus melons (Cucumis melo L.) to deficit subsurface drip irrigation. Agric. Water Manag. 2014, 136, 75–85. [Google Scholar] [CrossRef]
- Cabello, M.J.; Castellanos, M.T.; Romojaro, F.; Martinez-Madrid, C.; Ribas, F. Yield and quality of melon grown under different irrigation and nitrogen rates. Agric. Water Manag. 2009, 96, 866–874. [Google Scholar] [CrossRef]
- Yildirim, O.; Halloran, N.; Cavusoglu, S.; Sengul, N. Effects of different irrigation programs on the growth, yield, and fruit quality of drip-irrigated melon. Turk. J. Agric. For. 2009, 33, 243–255. [Google Scholar]
- Dogan, E.; Kirnak, H.; Berekatoglu, K.; Bilgel, L.; Surucu, A. Water stress imposed on muskmelon (Cucumis melo L.) with subsurface and surface drip irrigation systems under semi-arid climatic conditions. Irrig. Sci. 2008, 26, 131–138. [Google Scholar] [CrossRef]
- Afzal, A.; Duiker, S.W.; Watson, J.E. Leaf thickness to predict plant water status. Biosystems Eng. 2017, 156, 148–156. [Google Scholar] [CrossRef]
- Peterson, B.J.; Graves, W.R. Responses to root-zone water content of shrub congeners from eastern north america and mediterranean california. HortScience 2013, 48, 715–719. [Google Scholar] [CrossRef]
- Li, Y.J.; Yuan, B.Z.; Bie, Z.L.; Kang, Y. Effect of drip irrigation criteria on yield and quality of muskmelon grown in greenhouse conditions. Agric. Water Manag. 2012, 109, 30–35. [Google Scholar] [CrossRef]
- Kim, J.Y.; Glenn, D.M. Multi-modal sensor system for plant water stress assessment. Comput. Electron. Agric. 2017, 141, 27–34. [Google Scholar] [CrossRef]
- Guo, D.D.; Juan, J.X.; Chang, L.Y.; Zhang, J.J.; Huang, D.F. Discrimination of plant root zone water status in greenhouse production based on phenotyping and machine learning techniques. Sci. Rep. 2017, 7, 8303. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, Y.; Shibata, S.; Mineno, H. Multi-modal sliding window based support vector regression for predicting plant water stress. Knowl.-Based Syst. 2017, 134, 135–148. [Google Scholar] [CrossRef]
- Goldstein, A.; Fink, L.; Meitin, A.; Bohadana, S.; Lutenberg, O.; Ravid, G. Applying machine learning on sensor data for irrigation recommendations: revealing the agronomist’s tacit knowledge. Precis. Agric. 2018, 19, 421–444. [Google Scholar] [CrossRef]
- Cubero, S.; Lee, W.S.; Aleixos, N.; Albert, F.; Blasco, J. Automated systems based on machine vision for inspecting citrus fruits from the field to postharvest - A review. Food Bioprocess Technol. 2016, 9, 1623–1639. [Google Scholar] [CrossRef]
- Tang, J.; Deng, L.M.; Chen, H.; Luan, T.; Ma, W.J. Research on maize leaf recognition of characteristics from transmission image based on machine vision. Scientia Agricultura Sinica 2014, 47, 431–440. (In Chinese). Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZNYK201403004.htm (accessed on 5 June 2019).
- Kacira, M.; Ling, P.P.; Short, T.H. Machine vision extracted plant movement for early detection of plant water stress. Trans. ASAE 2002, 45, 1147–1153. [Google Scholar] [CrossRef]
- Ge, Y.F.; Bai, G.; Stoerger, V.; Schnable, J.C. Temporal dynamics of maize plant growth, water use, and leaf water content using automated high throughput RGB and hyperspectral imaging. Comput. Electron. Agric. 2016, 127, 625–632. [Google Scholar] [CrossRef]
- Neilson, E.H.; Edwards, A.M.; Blomstedt, C.K.; Berger, B.; Moller, B.L.; Gleadow, R.M. Utilization of a high-throughput shoot imaging system to examine the dynamic phenotypic responses of a C4 cereal crop plant to nitrogen and water deficiency over time. J. Exp. Bot. 2015, 66, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Fahlgren, N.; Feldman, M.; Gehan, M.A.; Wilson, M.S.; Shyu, C.; Bryant, D.W.; Hill, S.T.; McEntee, C.J.; Warnasooriya, S.N.; Kumar, I. A versatile phenotyping system and analytics platform reveals diverse temporal responses to water availability in Setaria. Mol. Plant 2015, 8, 1520–1535. [Google Scholar] [CrossRef]
- Story, D.; Kacira, M. Design and implementation of a computer vision-guided greenhouse crop diagnostics system. Mach. Vision Appl. 2015, 26, 495–506. [Google Scholar] [CrossRef]
- Duro, D.C.; Franklin, S.E.; Dube, M.G. A comparison of pixel-based and object-based image analysis with selected machine learning algorithms for the classification of agricultural landscapes using SPOT-5 HRG imagery. Remote Sens. Environ. 2012, 118, 259–272. [Google Scholar] [CrossRef]
- Pantazi, X.E.; Moshou, D.; Tamouridou, A.A. Automated leaf disease detection in different crop species through image features analysis and One Class Classifiers. Comput. Electron. Agric. 2019, 156, 96–104. [Google Scholar] [CrossRef]
- Elavarasan, D.; Vincent, D.R.; Sharma, V.; Zomaya, A.Y.; Srinivasan, K. Forecasting yield by integrating agrarian factors and machine learning models: A survey. Comput. Electron. Agric. 2018, 155, 257–282. [Google Scholar] [CrossRef]
- Raza, S.E.A.; Prince, G.; Clarkson, J.P.; Rajpoot, N.M. Automatic detection of diseased tomato plants using thermal and stereo visible light images. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.M.; Chen, D.J.; Gillani, Z.; Klukas, C.; Chen, M. Advanced phenotyping and phenotype data analysis for the study of plant growth and development. Front. Plant Sci. 2015, 6, 619. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Möller, M.; Alchanatis, V.; Cohen, Y.; Meron, M.; Tsipris, J.; Naor, A.; Ostrovsky, V. Use of thermal and visible imagery for estimating crop water status of irrigated grapevine. J. Exp. Bot. 2007, 58, 827–838. [Google Scholar]
- Chen, D.J.; Neumann, K.; Friedel, S.; Kilian, B.; Chen, M.; Altmann, T.; Klukas, C. Dissecting the phenotypic components of crop plant growth and drought responses based on high-throughput image analysis. Plant Cell 2014, 26, 4636–4655. [Google Scholar] [CrossRef]
- Yang, G.; Lu, W.; Lin, Y.; Luo, J.; Wang, C.; Meder, R.; Warburton, P.; Arnold, R. Monitoring water potential and relative water content in eucalyptus camaldulensis using near infrared spectroscopy. J. Trop. For. Sci. 2017, 29, 121–128. [Google Scholar]
- Buddenbaum, H.; Stern, O.; Paschmionka, B.; Hass, E.; Gattung, T.; Stoffels, J.; Hill, J.; Werner, W. Using VNIR and SWIR field imaging spectroscopy for drought stress monitoring of beech seedlings. Int. J. Remote Sens. 2015, 36, 4590–4605. [Google Scholar] [CrossRef]
- Veysi, S.; Naseri, A.; Hamzeh, S.; Bartholomeus, H. A satellite based crop water stress index for irrigation scheduling in sugarcane fields. Agric. Water Manag. 2017, 189, 70–86. [Google Scholar] [CrossRef]
- Shivers, S.W.; Roberts, D.A.; McFadden, J.P. Using paired thermal and hyperspectral aerial imagery to quantify land surface temperature variability and assess crop stress within California orchards. Remote Sens. Environ. 2019, 222, 215–231. [Google Scholar] [CrossRef]
- Chen, H.B.; Li, J.H.; Liu, C.H.; Liu, D.C. Sugarcane leaf water potential model based on canopy spectral signature. J. Irrig. & Drain. 2014, 33, 92–96. (In Chinese). Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-GGPS201403020.htm (accessed on 5 June 2019).
- Chen, Z.F.; Song, N.; Wang, J.L.; Sun, J.S. Leaf water potential estimating models of winter wheat based on hyperspectral remote sensing. Scientia Agricultura Sinica 2017, 50, 871–880. (In Chinese). Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZNYK201705010.htm (accessed on 5 June 2019).



| Trait | No. | Description | Difference among Four Water Treatments 1 | ||
|---|---|---|---|---|---|
| Seedling Stage | Vine Elongation Stage | Fruit Development Stage | |||
| Morphology | |||||
| Projection area | 1 | Area of plant projection (mm2) | * | * | * |
| Object Extent X | 2 | Minimum width of plant projection (mm) | * | * | * |
| Object Extent Y | 3 | Minimum height of plant projection (mm) | * | * | * |
| DBX area | 4 | Minimum external polygonal area of plant projection (mm2) | * | * | * |
| Convex hull circumference | 5 | mm | * | * | * |
| Diameter | 6 | Circumscribed circle diameter of plant projection (mm) | NS | * | * |
| Min rectangle area | 7 | Minimum external rectangle area of plant projection (mm2) | * | * | * |
| Compactness | 8 | Square of the object perimeter to object area | NS | * | * |
| Eccentricity | 9 | The ratio of the distance between the foci to the length of the major axis | NS | * | * |
| Invisible light | |||||
| Mean blue index | 10 | Mean blue index of muskmelon image within RGB color space | NS | * | * |
| Variance of mean blue index | 11 | Variance of mean blue index of muskmelon image within RGB color space | NS | * | * |
| Mean green index | 12 | Mean green index of muskmelon image within RGB color space | * | * | NS |
| Variance of mean blue index | 13 | Variance of mean green index of muskmelon image within RGB color space | * | * | * |
| Mean red index | 14 | Mean red index of muskmelon image within RGB color space | * | * | * |
| Variance of mean red index | 15 | Variance of mean red index of muskmelon image within RGB color space | NS | * | * |
| Near-infrared (NIR) | |||||
| A1 | 16 | Number of plant pixels with NIR intensity in 122–138 | NS | * | * |
| R1 | 17 | Ratio of plant pixels with NIR intensity in 122–138 | NS | * | * |
| A2 | 18 | Number of plant pixels with NIR intensity in 154–170 | * | * | * |
| R2 | 19 | Ratio of plant pixels with NIR intensity in 154–170 | * | * | * |
| A3 | 20 | Number of plant pixels with NIR intensity in 170–186 | * | * | * |
| R3 | 21 | Ratio of plant pixels with NIR intensity in 170–186 | NS | * | * |
| A4 | 22 | Number of plant pixels with NIR intensity in 186–202 | * | * | * |
| R4 | 23 | Ratio of plant pixels with NIR intensity in 186–202 | * | * | * |
| A5 | 24 | Number of plant pixels with NIR intensity in 202–218 | * | * | * |
| R5 | 25 | Ratio of plant pixels with NIR intensity in 202–218 | * | * | * |
| A6 | 26 | Number of plant pixels with NIR intensity in 218–234 | * | * | * |
| R6 | 27 | Ratio of plant pixels with NIR intensity in 218–234 | * | * | * |
| A7 | 28 | Number of plant pixels with NIR intensity in 234–250 | * | * | * |
| R7 | 29 | Ratio of plant pixels with NIR intensity in 234–250 | * | * | NS |
| Stage | Random Forest Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Original | Simplified | |||||||
| Mtry | Accuracy (%) | Kappa | Time-Consuming (s) | Mtry | Accuracy (%) | Kappa | Time-Consuming (s) | |
| Seedling stage | 2 | 78.50 | 0.710 | 31.08 | 2 | 77.60 | 0.698 | 12.88 |
| Vine elongation stage | 5 | 95.70 | 0.941 | 136.16 | 5 | 94.40 | 0.922 | 17.44 |
| Fruit development stage | 5 | 99.50 | 0.993 | 30.46 | 1 | 90.00 | 0.846 | 11.14 |
| Stage | Random Forest Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Original | Simplified | |||||||
| Seedling stage | ||||||||
| T1 | 20.5 | 1.2 | 0.6 | 1.2 | 19 | 1.5 | 1.8 | 2.7 |
| T2 | 2.1 | 16.4 | 2.4 | 1.2 | 1.5 | 15.8 | 2.7 | 2.4 |
| T3 | 2.4 | 0.3 | 25.9 | 4.2 | 2.4 | 0.3 | 25.9 | 4.2 |
| T4 | 0.0 | 2.7 | 3.3 | 15.8 | 0.0 | 2.7 | 3.3 | 15.8 |
| Mean | 0.785 | 0.776 | ||||||
| Vine elongation stage | ||||||||
| T1 | 9.8 | 0.8 | 0.0 | 0.0 | 9.8 | 1.0 | 0.0 | 0.0 |
| T2 | 0.3 | 24.6 | 0.4 | 0.0 | 0.3 | 24.0 | 1.1 | 0.2 |
| T3 | 0.7 | 0.7 | 29.6 | 0.5 | 0.7 | 0.7 | 28.8 | 0.5 |
| T4 | 0.0 | 0.5 | 0.3 | 31.8 | 0.0 | 0.7 | 0.5 | 31.6 |
| Mean | 0.957 | 0.944 | ||||||
| Fruit development stage | ||||||||
| T1 | - | - | - | - | - | - | - | - |
| T2 | - | 24.0 | 0.5 | 0.0 | - | 17.6 | 3.7 | 0.0 |
| T3 | - | 0.0 | 40.6 | 0.0 | - | 6.4 | 37.4 | 0.0 |
| T4 | - | 0.0 | 0.0 | 34.9 | - | 0.0 | 0.0 | 34.9 |
| Mean | 0.995 | 0.900 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, L.; Yin, Y.; Xiang, J.; Liu, Q.; Li, D.; Huang, D. A Phenotype-Based Approach for the Substrate Water Status Forecast of Greenhouse Netted Muskmelon. Sensors 2019, 19, 2673. https://doi.org/10.3390/s19122673
Chang L, Yin Y, Xiang J, Liu Q, Li D, Huang D. A Phenotype-Based Approach for the Substrate Water Status Forecast of Greenhouse Netted Muskmelon. Sensors. 2019; 19(12):2673. https://doi.org/10.3390/s19122673
Chicago/Turabian StyleChang, Liying, Yilu Yin, Jialin Xiang, Qian Liu, Daren Li, and Danfeng Huang. 2019. "A Phenotype-Based Approach for the Substrate Water Status Forecast of Greenhouse Netted Muskmelon" Sensors 19, no. 12: 2673. https://doi.org/10.3390/s19122673
APA StyleChang, L., Yin, Y., Xiang, J., Liu, Q., Li, D., & Huang, D. (2019). A Phenotype-Based Approach for the Substrate Water Status Forecast of Greenhouse Netted Muskmelon. Sensors, 19(12), 2673. https://doi.org/10.3390/s19122673





