Analysis of a Low-Cost EEG Monitoring System and Dry Electrodes toward Clinical Use in the Neonatal ICU
Abstract
1. Introduction
- Investigation and modelling of skin-electrode impedance of dry and wet electrodes on adults.
- Development of a neonatal EEG simulation test bench, using above impedance models.
- In-vivo assessment of dry versus wet electrodes on adults, and a comparison of in-vivo results versus the proposed simulation framework.
2. Materials and Methods
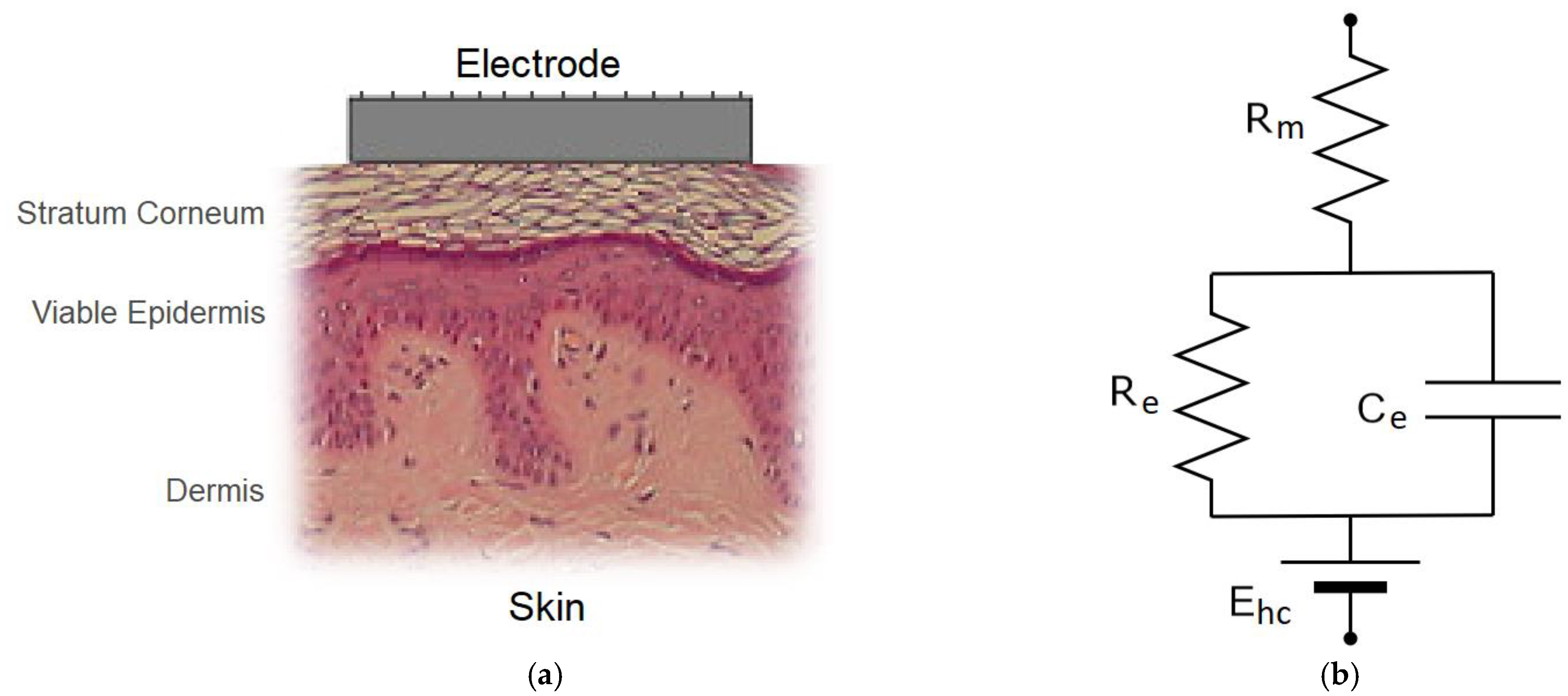
2.1. Skin-Electrode Interface
2.1.1. Skin-Electrode Impedance Modeling
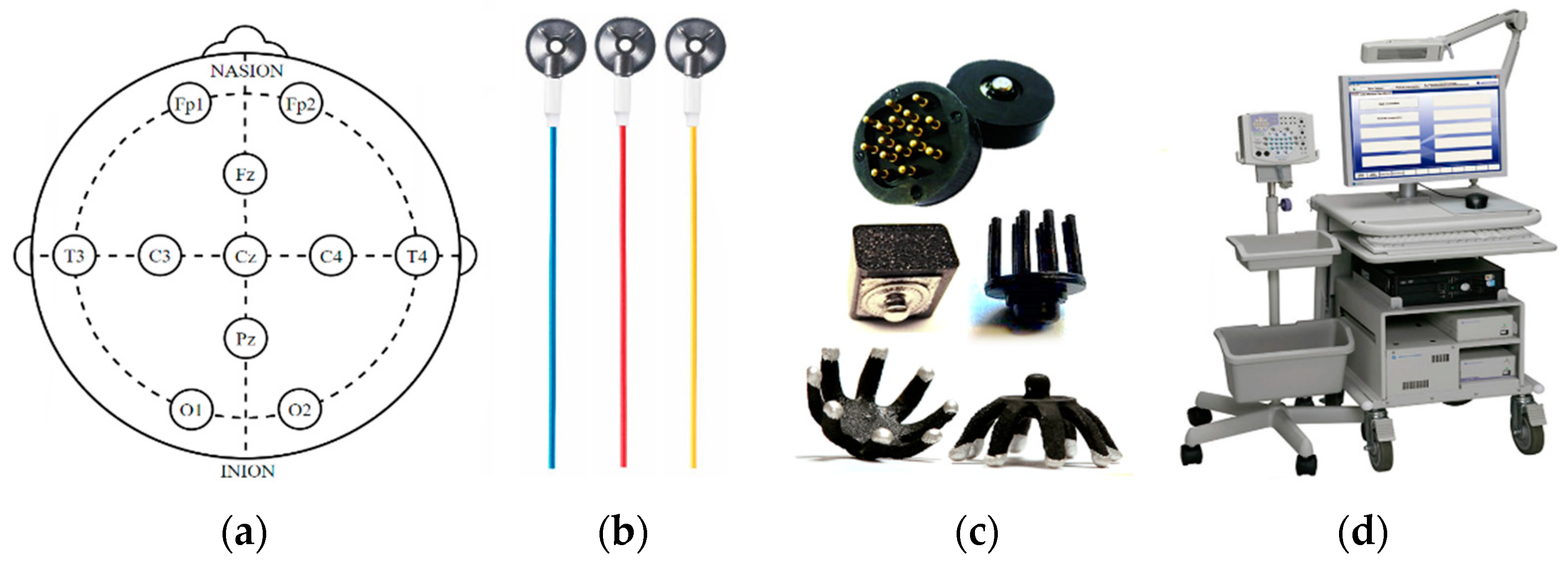
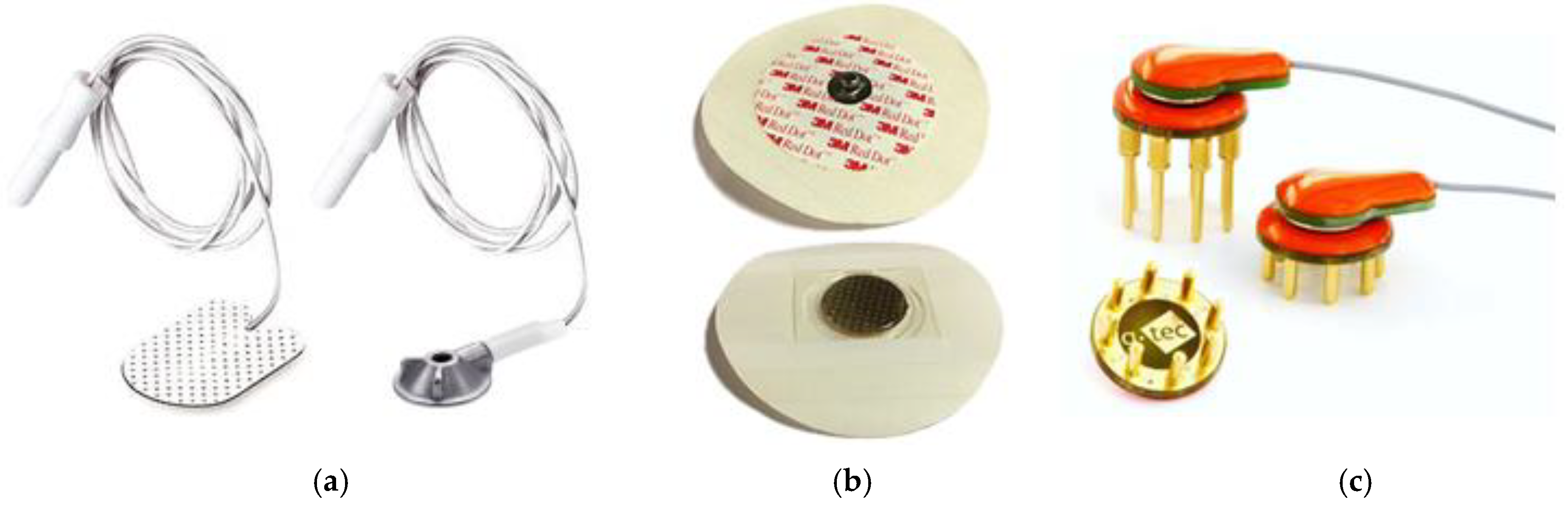
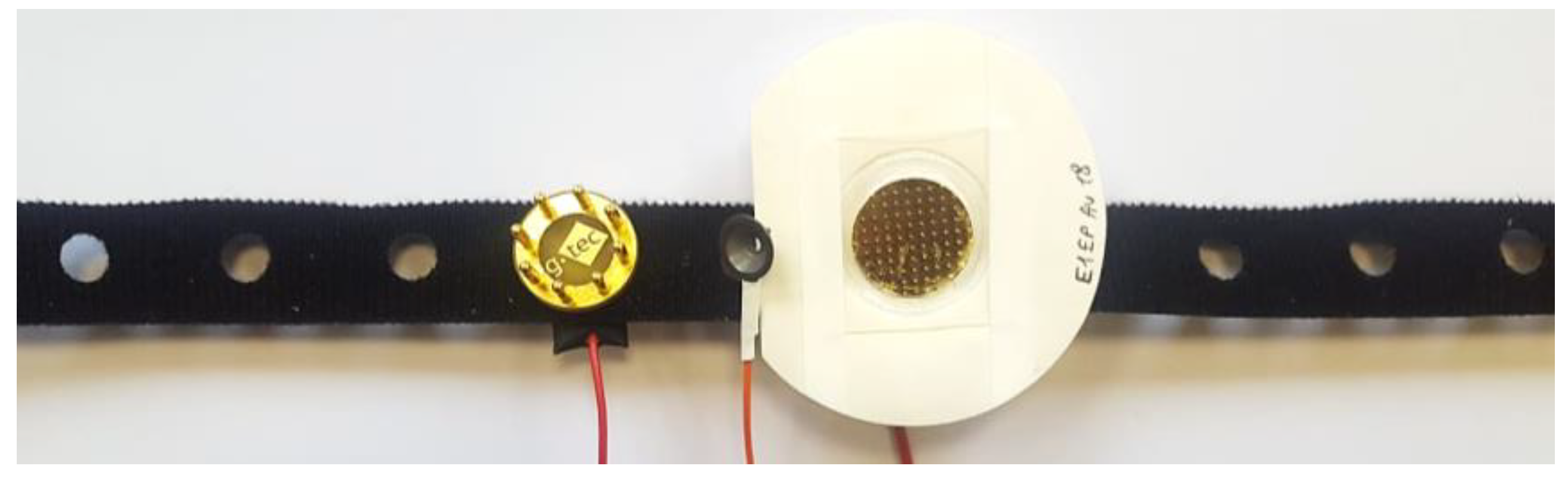
2.1.2. EEG Electrodes
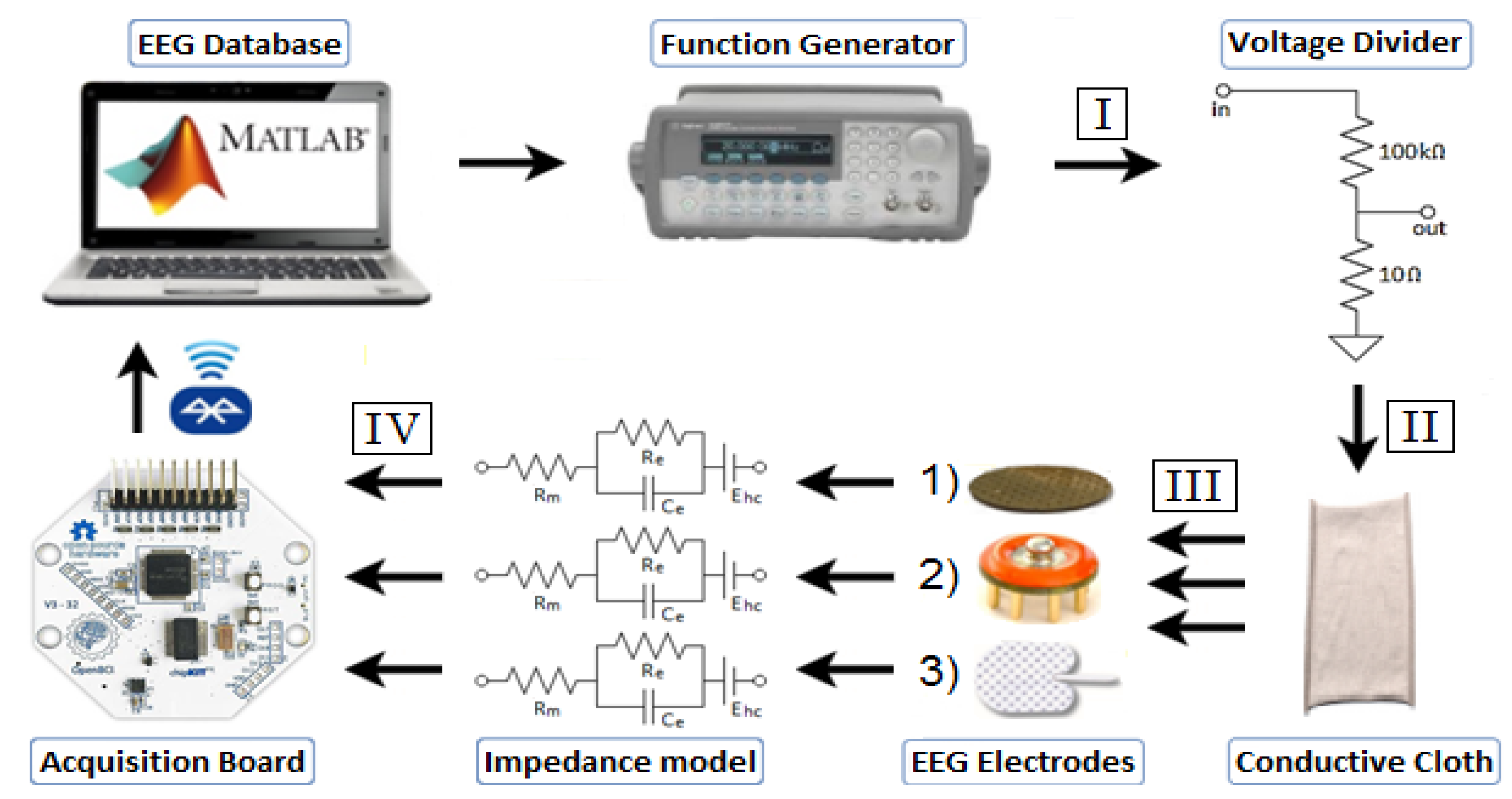
2.1.3. Impedance Testing
2.2. System Framework
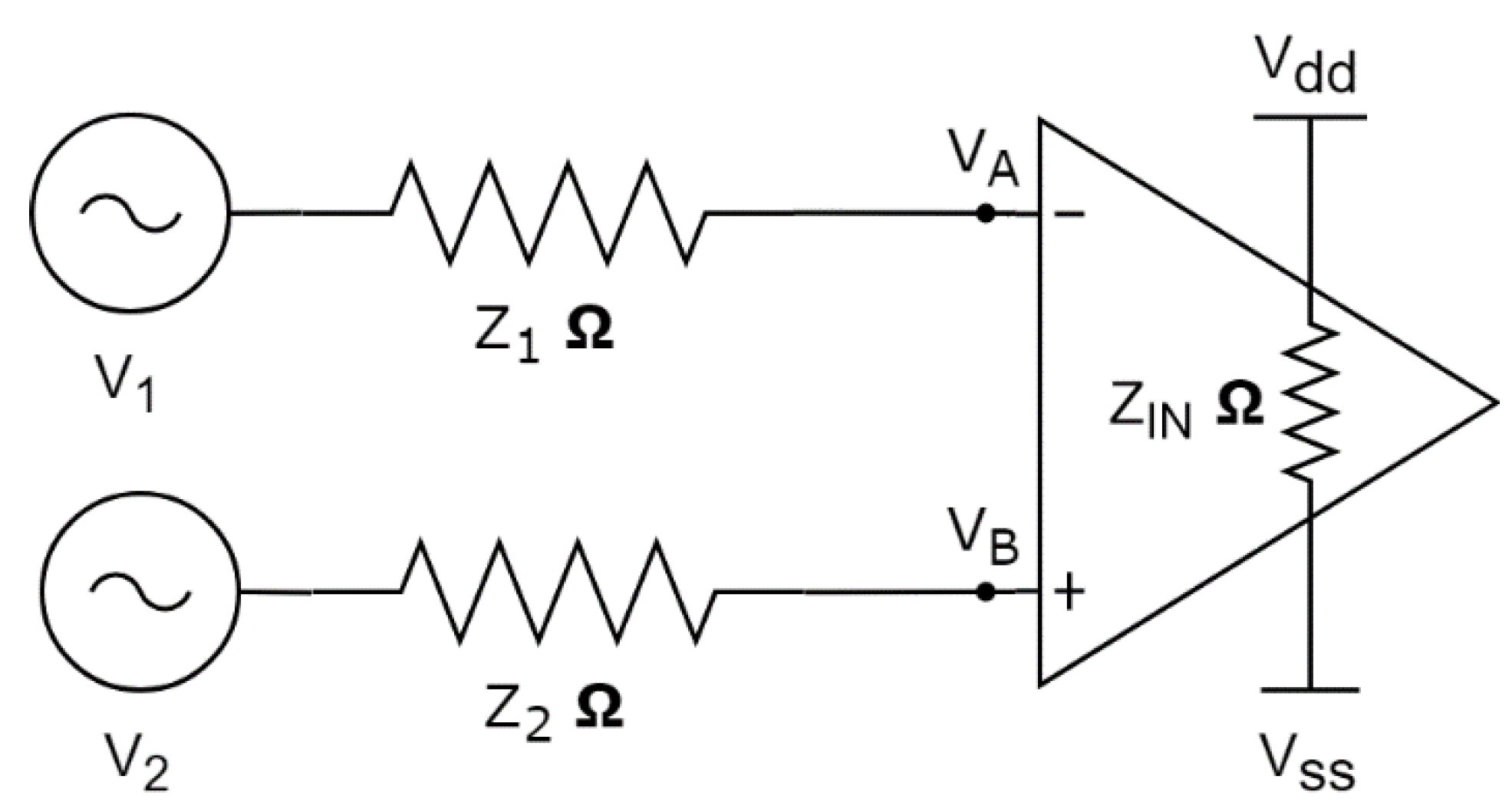
2.2.1. Acquisition System
2.2.2. EEG Simulation Framework
2.2.3. In Vivo EEG
3. Results
4. Discussion
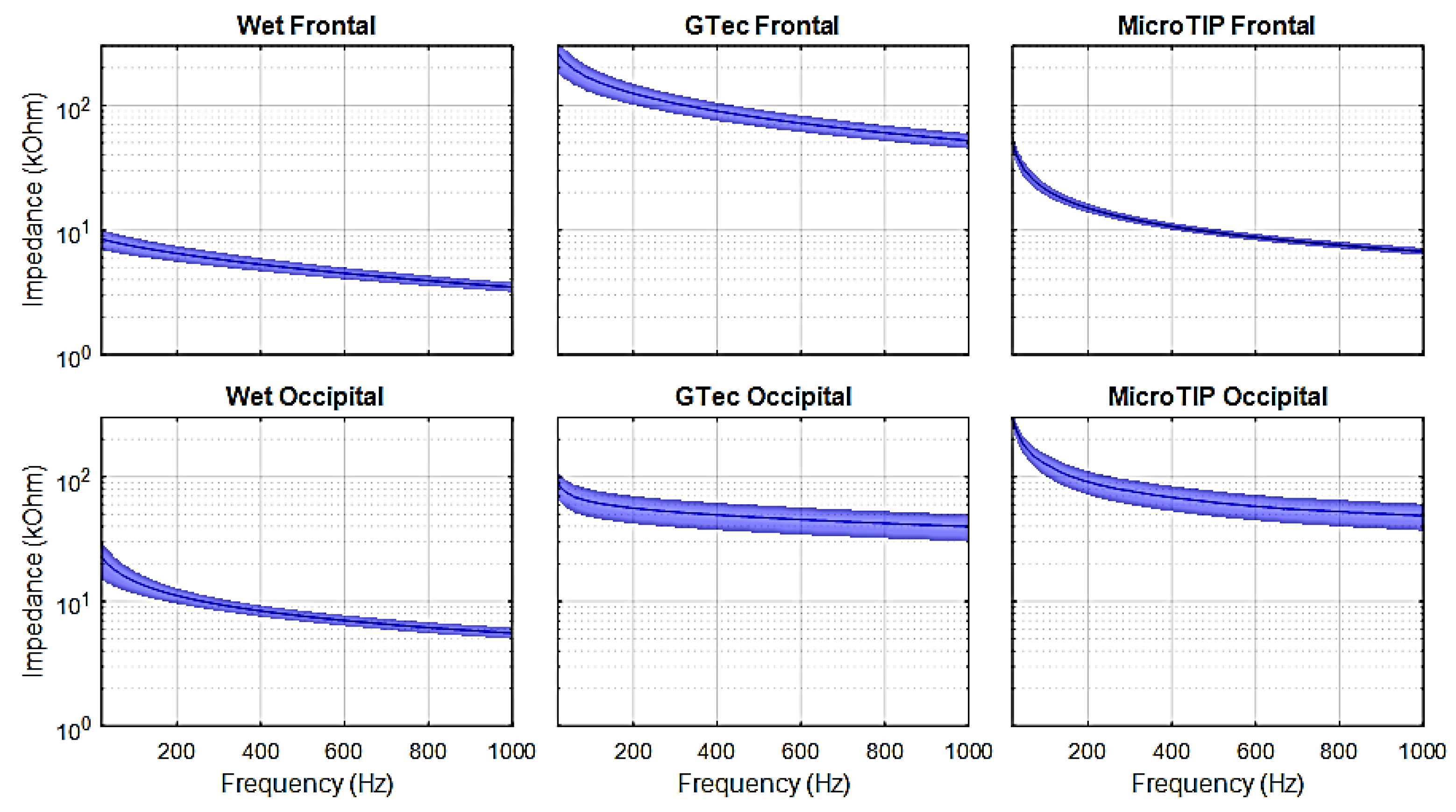
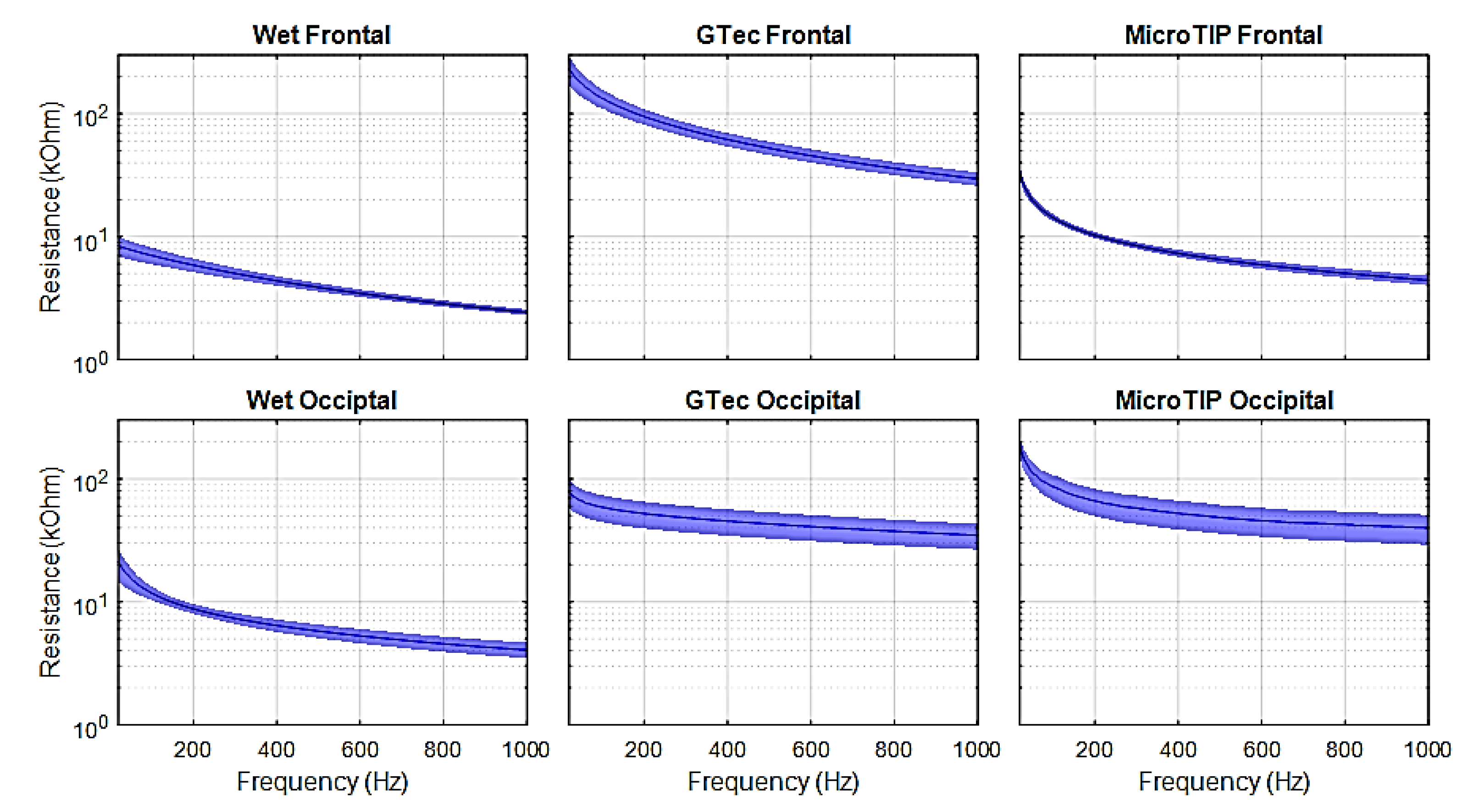
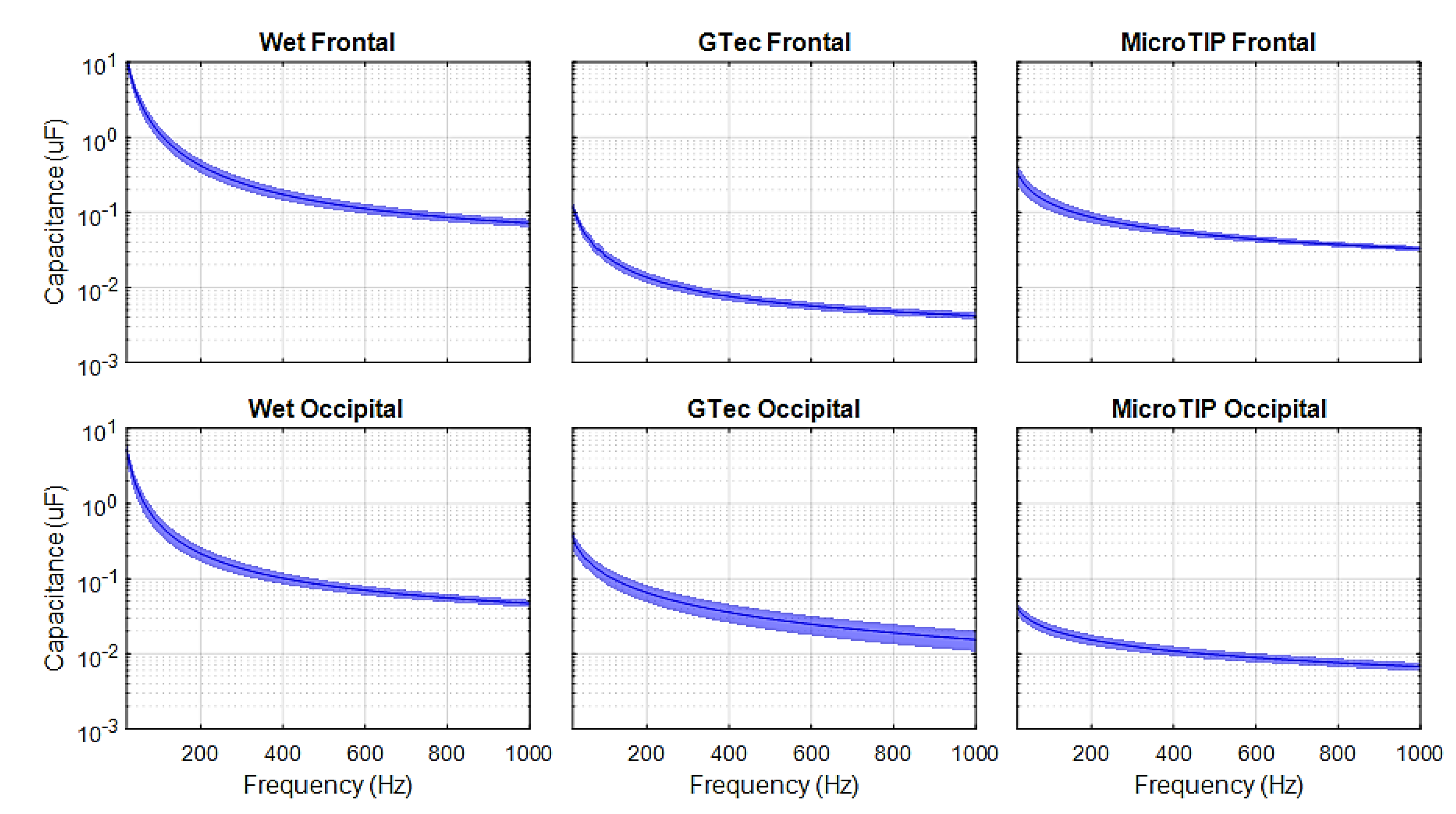
4.1. Skin-Electrode Impedance
4.2. EEG Simulation Framework
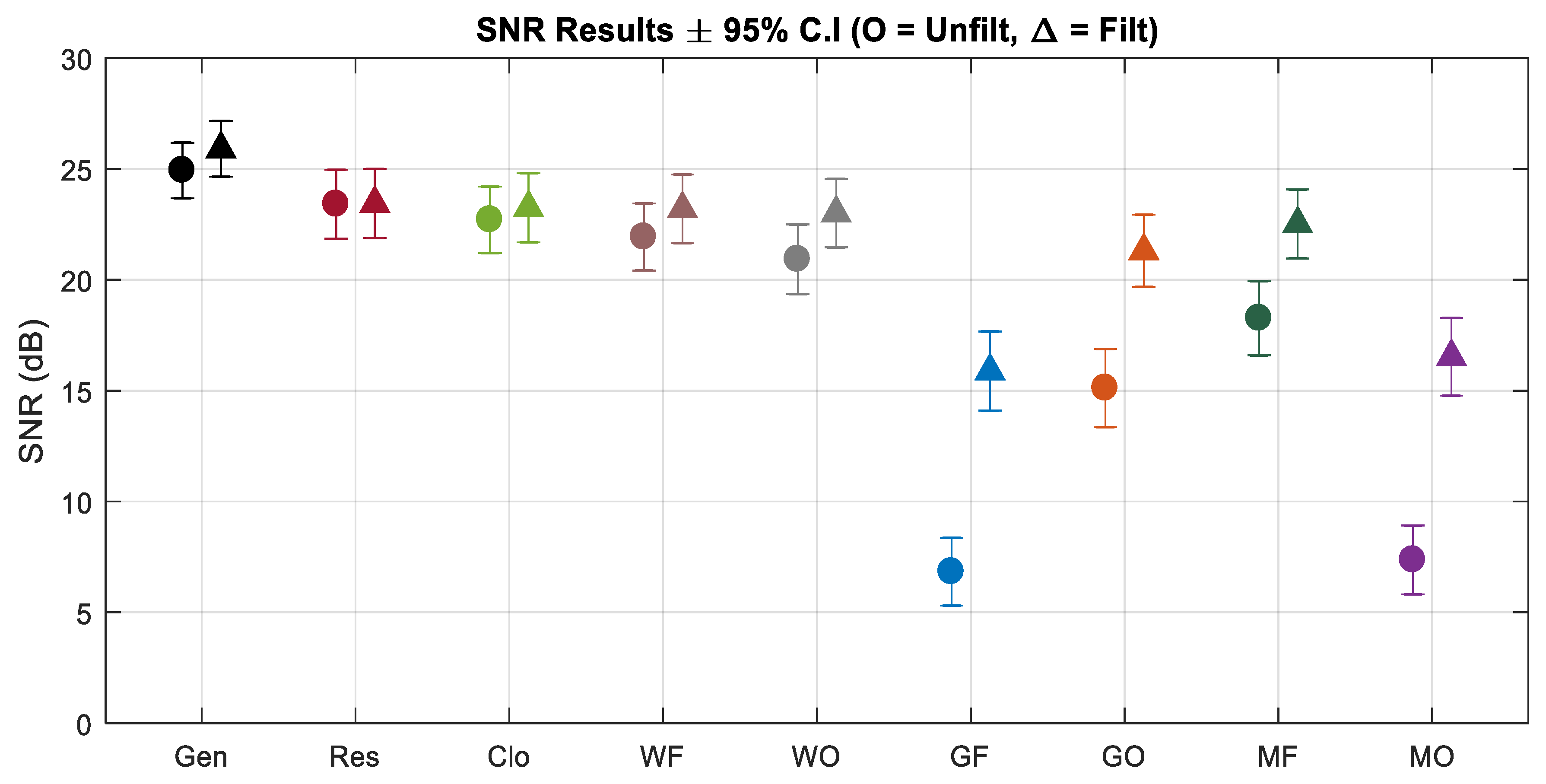
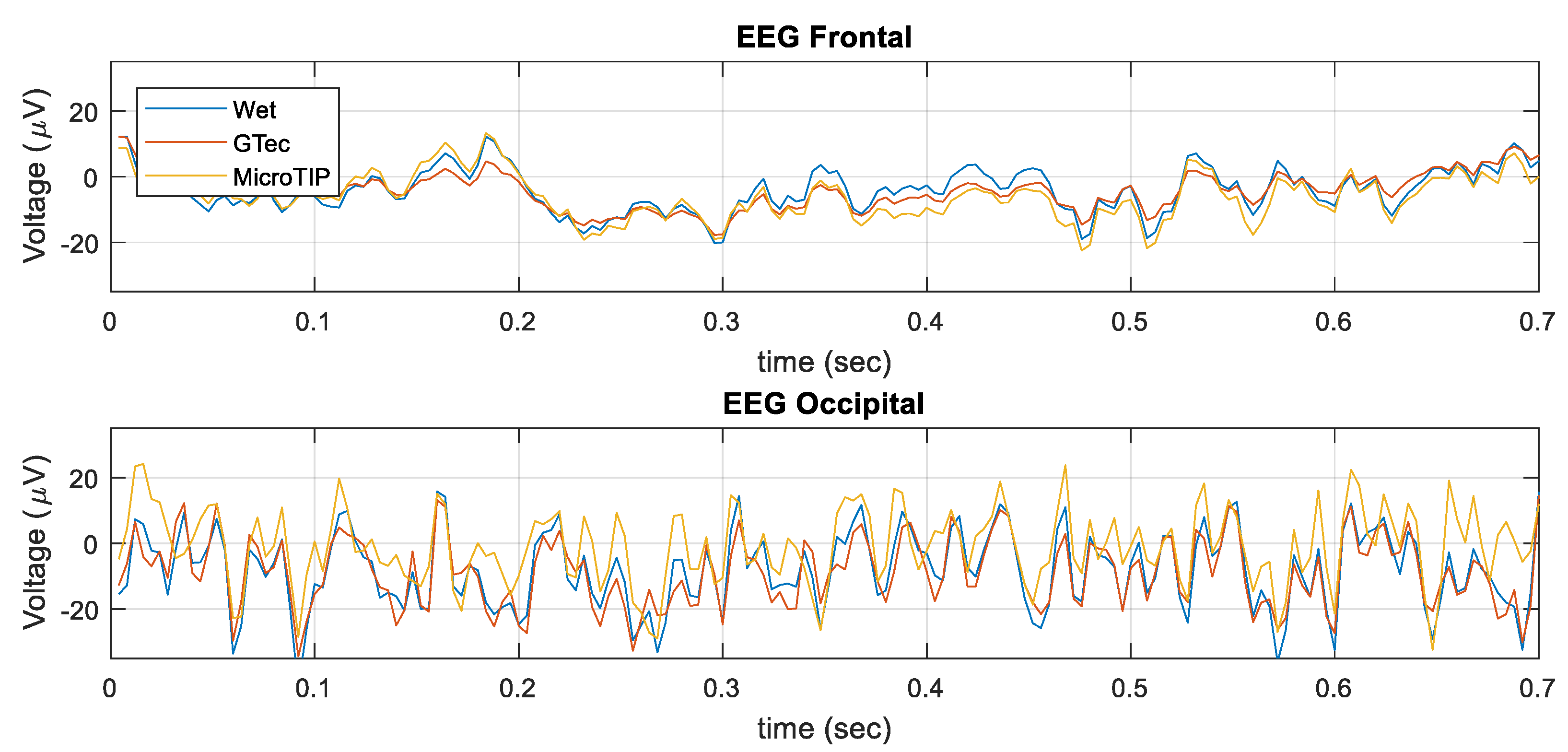
4.3. In-Vivo EEG
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murray, D.M.; Boylan, G.B.; Ali, I.; Ryan, C.A.; Murphy, B.P.; Connolly, S. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, 187–191. [Google Scholar] [CrossRef]
- Stevenson, N.J.; Clancy, R.R.; Vanhatalo, S.; Rosén, I.; Rennie, J.M.; Boylan, G.B. Interobserver agreement for neonatal seizure detection using multichannel EEG. Ann. Clin. Transl. Neurol. 2015, 2, 1002–1011. [Google Scholar] [CrossRef]
- Williams, R.P.; Banwell, B.; Berg, R.A.; Dlugos, D.J.; Donnelly, M.; Ichord, R.; Kessler, S.K.; Lavelle, J.; Massey, S.L.; Hewlett, J.; et al. Impact of an ICU EEG Monitoring Pathway on Timeliness of Therapeutic Intervention and Electrographic Seizure Termination. Epilepsia 2016, 57, 786–795. [Google Scholar] [CrossRef]
- Thoresen, M.; Hellström-Westas, L.; Liu, X.; De Vries, L.S. Effect of Hypothermia on Amplitude-Integrated Electroencephalogram in Infants with Asphyxia. Pediatrics 2010, 126, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.; Goulding, R.; Filan, P.; Boylan, G.; Boylan, G. Overcoming the practical challenges of electroencephalography for very preterm infants in the neonatal intensive care unit. Acta Paediatr. 2015, 104, 152–157. [Google Scholar] [CrossRef]
- Fridman, I.; Cordeiro, M.; Rais-Bahrami, K.; McDonald, N.J.; Reese, J.J.; Massaro, A.N.; Conry, J.A.; Chang, T.; Soussou, W.; Tsuchida, T.N. Evaluation of Dry Sensors for Neonatal EEG recordings. J. Clin. Neurophysiol. 2016, 33, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Walls-Esquivel, E.; Vecchierini, M.; Héberlé, C.; Wallois, F. Electroencephalography (EEG) recording techniques and artefact detection in early premature babies. Neurophysiol. Clin. Neurophysiol. 2007, 37, 299–309. [Google Scholar] [CrossRef]
- Jasper, H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–375. [Google Scholar] [CrossRef]
- Shellhaas, R.A.; Chang, T.; Tsuchida, T.; Scher, M.S.; Riviello, J.J.; Abend, N.S.; Nguyen, S.; Wusthoff, C.J.; Clancy, R.R. The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. J. Clin. Neurophysiol. 2011, 28, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Prutchi, D.; Norris, M. Biopotential Amplifiers. In Design and Development of Medical Electronic Instrumentation; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 1–40. ISBN 978-0-471-67623-2. [Google Scholar]
- Nuwer, M.R.; Comi, G.; Emerson, R.; Fuglsang-Frederiksen, A.; Guérit, J.-M.; Hinrichs, H.; Ikeda, A.; Luccas, F.J.C.; Rappelsburger, P. IFCN standards for digital recording of clinical EEG. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 259–261. [Google Scholar] [CrossRef]
- Abend, N.S.; Topjian, A.A.; Williams, S. How much does it cost to identify a critically ill child experiencing electrographic seizures? J. Clin. Neurophysiol. 2015, 32, 257–264. [Google Scholar] [CrossRef]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Removal of Movement Artifact from High-Density EEG Recorded During Walking and Running. J. Neurophysiol. 2010, 103, 3526–3534. [Google Scholar] [CrossRef]
- Odabaee, M.; Freeman, W.J.; Colditz, P.B.; Ramon, C.; Vanhatalo, S. Spatial patterning of the neonatal EEG suggests a need for a high number of electrodes. NeuroImage 2013, 68, 229–235. [Google Scholar] [CrossRef]
- Lepola, P.; Myllymaa, S.; Töyräs, J.; Muraja-Murro, A.; Mervaala, E.; Lappalainen, R.; Myllymaa, K. Screen-printed EEG electrode set for emergency use. Sens. Actuators A Phys. 2014, 213, 19–26. [Google Scholar] [CrossRef]
- Ladino, L.D.; Voll, A.; Dash, D.; Sutherland, W.; Hernández-Ronquillo, L.; Téllez-Zenteno, J.F.; Moien-Afshari, F. StatNet Electroencephalogram: A Fast and Reliable Option to Diagnose Nonconvulsive Status Epilepticus in Emergency Setting. Can. J. Neurol. Sci. 2016, 43, 254–260. [Google Scholar] [CrossRef]
- Luttge, R.; Bystrova, S.N.; van Putten, M.J.A.M. Microneedle array electrode for human EEG recording. In Proceedings of the 4th European Conference of the International Federation for Medical and Biological Engineering, Antwerp, Belgium, 23–27 November 2008; Springer: Berlin/Heidelberg, Germany, 2009; Volume 22, pp. 1246–1249. [Google Scholar]
- Lin, C.-T.; Liao, L.-D.; Liu, Y.-H.; Wang, I.-J.; Lin, B.-S.; Chang, J.-Y. Novel Dry Polymer Foam Electrodes for Long-Term EEG Measurement. IEEE Trans. Biomed. Eng. 2011, 58, 1200–1207. [Google Scholar] [CrossRef]
- Chen, Y.-H.; De Beeck, M.O.; Vanderheyden, L.; Carrette, E.; Mihajlovic, V.; Vanstreels, K.; Grundlehner, B.; Gadeyne, S.; Boon, P.; van Hoof, C. Soft, Comfortable Polymer Dry Electrodes for High Quality ECG and EEG Recording. Sensors 2014, 14, 23758–23780. [Google Scholar] [CrossRef]
- Liao, L.-D.; Wang, I.-J.; Chen, S.-F.; Chang, J.-Y.; Lin, C.-T.; Lin, C.-T. Design, Fabrication and Experimental Validation of a Novel Dry-Contact Sensor for Measuring Electroencephalography Signals without Skin Preparation. Sensors 2011, 11, 5819–5834. [Google Scholar] [CrossRef]
- Griss, P.; Tolvanen-Laakso, H.; Meriläinen, P.; Stemme, G. Characterization of micromachined spiked biopotential electrodes. IEEE Trans. Biomed. Eng. 2002, 49, 597–604. [Google Scholar] [CrossRef]
- Chiou, J.-C.; Ko, L.-W.; Lin, C.-T.; Hong, C.T.; Jung, T.-P.; Liang, S.-F.; Jeng, J.-L. Using novel MEMS EEG sensors in detecting drowsiness application. In Proceedings of the 2006 IEEE Biomedical Circuits and Systems Conference, London, UK, 29 November–1 December 2006; pp. 33–36. [Google Scholar] [CrossRef]
- Guger, C.; Krausz, G.; Allison, B.Z.; Edlinger, G. Comparison of Dry and Gel Based Electrodes for P300 Brain–Computer Interfaces. Front. Neurosci. 2012, 6, 60. [Google Scholar] [CrossRef]
- Chi, Y.M.; Wang, Y.; Wang, Y.-T.; Jung, T.-P.; Kerth, T.; Cao, Y. A Practical Mobile Dry EEG System for Human Computer Interfaces. In Foundations of Augmented Cognition (AC 2013); Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 8027, pp. 649–655. [Google Scholar]
- Campbell, A.; Choudhury, T.; Hu, S.; Lu, H.; Mukerjee, M.K.; Rabbi, M.; Raizada, R.D. NeuroPhone: Brain-mobile phone interface using a wireless EEG headset. In Proceedings of the 2nd ACM SIGCOMM Workshop on Networking, Systems, and Applications on Mobile Handhelds, New Delhi, India, 30 August 2010; pp. 3–8. [Google Scholar] [CrossRef]
- Casson, A.; Yates, D.; Smith, S.; Duncan, J.; Rodriguez-Villegas, E. Wearable Electroencephalography. IEEE Eng. Med. Boil. Mag. 2010, 29, 44–56. [Google Scholar] [CrossRef] [PubMed]
- OpenEEG. Available online: openeeg.sourceforge.net/ (accessed on 3 April 2018).
- OpenBCI. Available online: openbci.com/ (accessed on 3 April 2018).
- Frey, J. Comparison of a consumer grade EEG amplifier with medical grade equipment in BCI applications. In Proceedings of the 6th International BCI Meeting, Pacific Grove, CA, USA, 30 May–3 June 2016. [Google Scholar]
- Liu, Y.; Jiang, X.; Cao, T.; Wan, F.; Mak, P.U.; Vai, M.I. Implementation of SSVEP based BCI with Emotiv EPOC. In Proceedings of the 2012 IEEE International Conference on Virtual Environments Human-Computer Interfaces and Measurement Systems (VECIMS), Tianjin, China, 2–4 July 2012; pp. 34–37. [Google Scholar]
- Lin, Y.-P.; Wang, Y.; Wei, C.-S.; Jung, T.-P. Assessing the quality of steady-state visual-evoked potentials for moving humans using a mobile electroencephalogram headset. Front. Hum. Neurosci. 2014, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Duvinage, M.; Castermans, T.; Petieau, M.; Hoellinger, T.; Cheron, G.; Dutoit, T. Performance of the Emotiv Epoc headset for P300-based applications. Biomed. Eng. Online 2013, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Hairston, W.D.; Whitaker, K.W.; Ries, A.J.; Vettel, J.M.; Bradford, J.C.; Kerick, S.E.; McDowell, K. Usability of four commercially-oriented EEG systems. J. Neural Eng. 2014, 11, 46018. [Google Scholar] [CrossRef] [PubMed]
- Bieszczad, J.; Collier, T.J.; Kynor, D.B.; Audette, W.E.; Kobylarz, E.J.; Diamond, S.G. Creation of a Human Head Phantom for Testing of Electroencephalography Equipment and Techniques. IEEE Trans. Biomed. Eng. 2012, 59, 2628–2634. [Google Scholar] [CrossRef]
- Wyckoff, S.N.; Sherlin, L.H.; Ford, N.L.; Dalke, D. Validation of a wireless dry electrode system for electroencephalography. J. Neuroeng. Rehabil. 2015, 12, 277. [Google Scholar] [CrossRef]
- Bakar, A.A.A.; Lim, Y.L.; Wilson, S.J.; Fuentes, M.; Bertling, K.; Bosch, T.; Rakic, A.D. Electrocardiographic signal detection using self-mixing interferometer technique with customized electro-optic phase modulator. In Proceedings of the 2012 IEEE 3rd International Conference on Photonics (ICP 2012), Penang, Malaysia, 1–3 October 2012. [Google Scholar]
- Gupta, R.; Bera, J.; Mitra, M. Development of an embedded system and MATLAB-based GUI for online acquisition and analysis of ECG signal. Measurement 2010, 43, 1119–1126. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Gomez, S.; O’Shea, A.; Salgado, E.; Huillca, K.; Mathieson, S.; Boylan, G.; Popovici, E.; Temko, A. Neonatal EEG Interpretation and Decision Support Framework for Mobile Platforms. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 4881–4884. [Google Scholar] [CrossRef]
- Poveda, J.; O’Sullivan, M.; Popovici, E.; Temko, A. Portable neonatal EEG monitoring and sonification on an Android device. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, Korea, 11–15 July 2017; pp. 2018–2021. [Google Scholar] [CrossRef]
- Neuman, M. Biopotential Electrodes. In The Biomedical Engineering Handbook; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-3540663515. [Google Scholar]
- Albulbul, A.; Wang, G.-J. Evaluating Major Electrode Types for Idle Biological Signal Measurements for Modern Medical Technology. Bioengineering 2016, 3, 20. [Google Scholar] [CrossRef]
- Ferree, T.C.; Luu, P.; Russell, G.S.; Tucker, D.M. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001, 112, 536–544. [Google Scholar] [CrossRef]
- O’Mahony, C.; Grygoryev, K.; Ciarlone, A.; Giannoni, G.; Kenthao, A.; Galvin, P. Design, fabrication and skin-electrode contact analysis of polymer microneedle-based ECG electrodes. J. Micromech. Microeng. 2016, 26, 84005. [Google Scholar] [CrossRef]
- IEC. IEC 60601-1. Medical Electrical Equipment—Part 1: General Requirements for Basic Safety and Essential Performance; IEC: Geneva, Switzerland, 2005. [Google Scholar]
- Keysight Technologies. E4980A Precision LCR Meter; Data Sheet; Keysight: Santa Rosa, CA, USA, 2018. [Google Scholar]
- Texas Instruments. ADS1299 Low-Noise, 8-Channel, 24-Bit, Analog-to-Digital Converter for EEG and Biopotential Measurements; Data Sheet; Texas Instruments: Dallas, TX, USA, 2017. [Google Scholar]
- IEC. IEC 60601-2-26. Medical Electrical Equipment—Part 2-26: Particular Requirements for the Basic Safety and Essential Performance of Electroencephalographs; IEC: Geneva, Switzerland, 2015. [Google Scholar]
- O’Sullivan, M.; Popovici, E.; Bocchino, A.; O’Mahony, C.; Boylan, G.; Temko, A. System Level Framework for Assessing the Accuracy of Neonatal EEG Acquisition. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 4339–4342. [Google Scholar]
- Shieldex. Shieldex Technik-tex P180+B; Statex: Bremen, Germany, 2018. [Google Scholar]
- Seeck, M.; Koessler, L.; Bast, T.; Leijten, F.; Michel, C.; Baumgartner, C.; He, B.; Beniczky, S. The standardized EEG electrode array of the IFCN. Clin. Neurophysiol. 2017, 128, 2070–2077. [Google Scholar] [CrossRef]
- Kohli, S.; Casson, A.J. Towards signal processing assisted hardware for continuous in-band electrode impedance monitoring (Invited paper). In Proceedings of the 2017 IEEE International Symposium on Circuits and Systems (ISCAS), Baltimore, MD, USA, 28–31 May 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Tulachan, B.; Singh, S.K.; Philip, D.; Das, M. Harvesting electricity from human hair. J. Cosmet. Sci. 2016, 67, 21–36. [Google Scholar]
- O’Sullivan, M.; Pena, J.P.; Bocchino, A.; O’Mahony, C.; Costello, D.; Popovici, E.; Temko, A. Comparison of electrode technologies for dry and portable EEG acquisition. In Proceedings of the 2017 7th IEEE International Workshop on Advances in Sensors and Interfaces (IWASI), Vieste, Italy, 15–16 June 2017; pp. 15–20. [Google Scholar] [CrossRef]
- Uktveris, T.; Jusas, V. Development of a Modular Board for EEG Signal Acquisition. Sensors 2018, 18, 2140. [Google Scholar] [CrossRef]
- Cherian, P.J.; Swarte, R.M.; Visser, G.H. Technical standards for recording and interpretation of neonatal electroencephalogram in clinical practice. Ann. Indian Acad. Neurol. 2009, 12, 58–70. [Google Scholar] [CrossRef]
| Sample Rate (Hz) | Resolution (bits) | (MO) | CMRR (dB) | Cross-Talk (dB) | |
|---|---|---|---|---|---|
| IFCN | 200 | 12 | 100 | 110 | 40 |
| OpenBCI | 250 | 24 | 1000 | 120 | 110 |
| Wet F | Wet O | g.tec F | g.tec O | Micro F | Micro O | |
|---|---|---|---|---|---|---|
| Impedance | 8.2 ± 1.5 | 19.9 ± 6.0 | 226.5 ± 62.4 | 77.9 ± 19.6 | 36.7 ± 5.7 | 214.9 ± 33.4 |
| Resistance | 8.1 ± 1.4 | 17.6 ± 4.4 | 198.2 ± 48.2 | 70.7 ± 17.5 | 24.0 ± 2.3 | 135.8 ± 26.1 |
| Correlation (±95% Conf. Int.) | SNR (±95% Conf. Int.) | 50 Hz Noise (µV) | |||
|---|---|---|---|---|---|
| Unfiltered | Filtered | Unfiltered | Filtered | ||
| Generator | 0.998 ± 0.005 | 0.998 ± 0.004 | 24.93 ± 1.3 | 25.9 ± 1.3 | 0.32 ± 0.11 |
| Resistor | 0.997 ± 0.015 | 0.997 ± 0.015 | 23.4 ± 1.6 | 23.4 ± 1.6 | 0.07 ± 0.03 |
| Cloth | 0.997 ± 0.015 | 0.997 ± 0.015 | 22.7 ± 1.5 | 23.2 ± 1.6 | 0.24 ± 0.11 |
| Wet Front. | 0.996 ± 0.016 | 0.997 ± 0.015 | 21.9 ± 1.5 | 23.2 ± 1.5 | 0.36 ± 0.15 |
| Wet Occip. | 0.995 ± 0.019 | 0.997 ± 0.015 | 20.9 ± 1.6 | 23.0 ± 1.5 | 0.51 ± 0.21 |
| g.tec Front. | 0.867 ± 0.555 | 0.982 ± 0.094 | 6.8 ± 1.5 | 15.9 ± 1.8 | 4.94 ± 2.11 |
| g.tec Occip. | 0.978 ± 0.107 | 0.995 ± 0.019 | 15.1 ± 1.8 | 21.3 ± 1.6 | 1.54 ± 0.65 |
| Micro Front. | 0.990 ± 0.041 | 0.996 ± 0.015 | 18.3 ± 1.7 | 22.5 ± 1.6 | 0.94 ± 0.40 |
| Micro Occip. | 0.881 ± 0.511 | 0.985 ± 0.076 | 7.4 ± 1.5 | 16.5 ± 1.8 | 4.59 ± 1.98 |
| g.tec Front. | g.tec Occip. | MicroTIP Front. | MicroTIP Occip | |
|---|---|---|---|---|
| Correlation | 0.827 ± 0.024 | 0.855 ± 0.009 | 0.915 ± 0.014 | 0.781 ± 0.008 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Sullivan, M.; Temko, A.; Bocchino, A.; O’Mahony, C.; Boylan, G.; Popovici, E. Analysis of a Low-Cost EEG Monitoring System and Dry Electrodes toward Clinical Use in the Neonatal ICU. Sensors 2019, 19, 2637. https://doi.org/10.3390/s19112637
O’Sullivan M, Temko A, Bocchino A, O’Mahony C, Boylan G, Popovici E. Analysis of a Low-Cost EEG Monitoring System and Dry Electrodes toward Clinical Use in the Neonatal ICU. Sensors. 2019; 19(11):2637. https://doi.org/10.3390/s19112637
Chicago/Turabian StyleO’Sullivan, Mark, Andriy Temko, Andrea Bocchino, Conor O’Mahony, Geraldine Boylan, and Emanuel Popovici. 2019. "Analysis of a Low-Cost EEG Monitoring System and Dry Electrodes toward Clinical Use in the Neonatal ICU" Sensors 19, no. 11: 2637. https://doi.org/10.3390/s19112637
APA StyleO’Sullivan, M., Temko, A., Bocchino, A., O’Mahony, C., Boylan, G., & Popovici, E. (2019). Analysis of a Low-Cost EEG Monitoring System and Dry Electrodes toward Clinical Use in the Neonatal ICU. Sensors, 19(11), 2637. https://doi.org/10.3390/s19112637






