Optical Fiber Gratings Immunoassays
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tilted Fiber Bragg Gratings Manufacturing
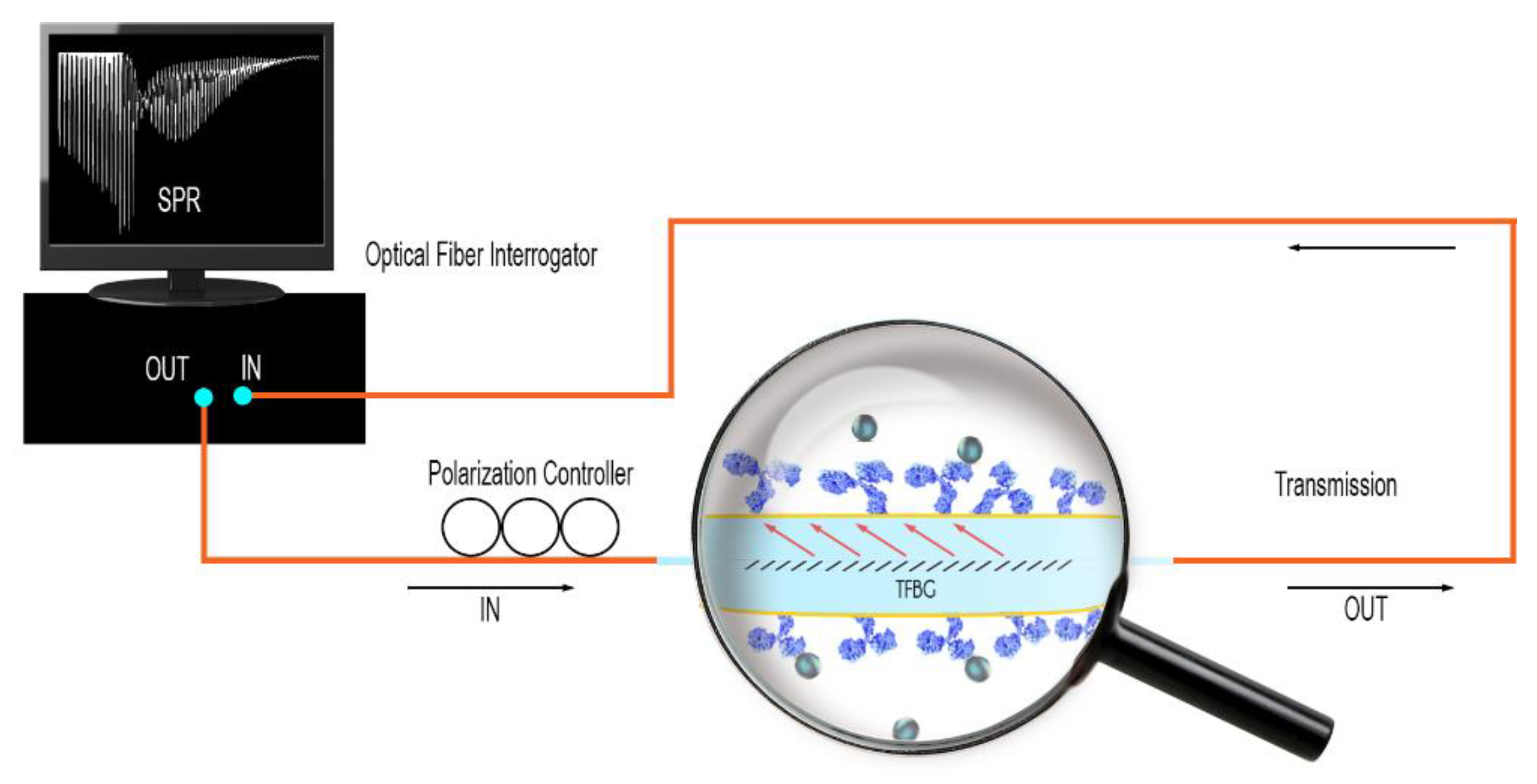
2.3. Optical Fiber Interrogation
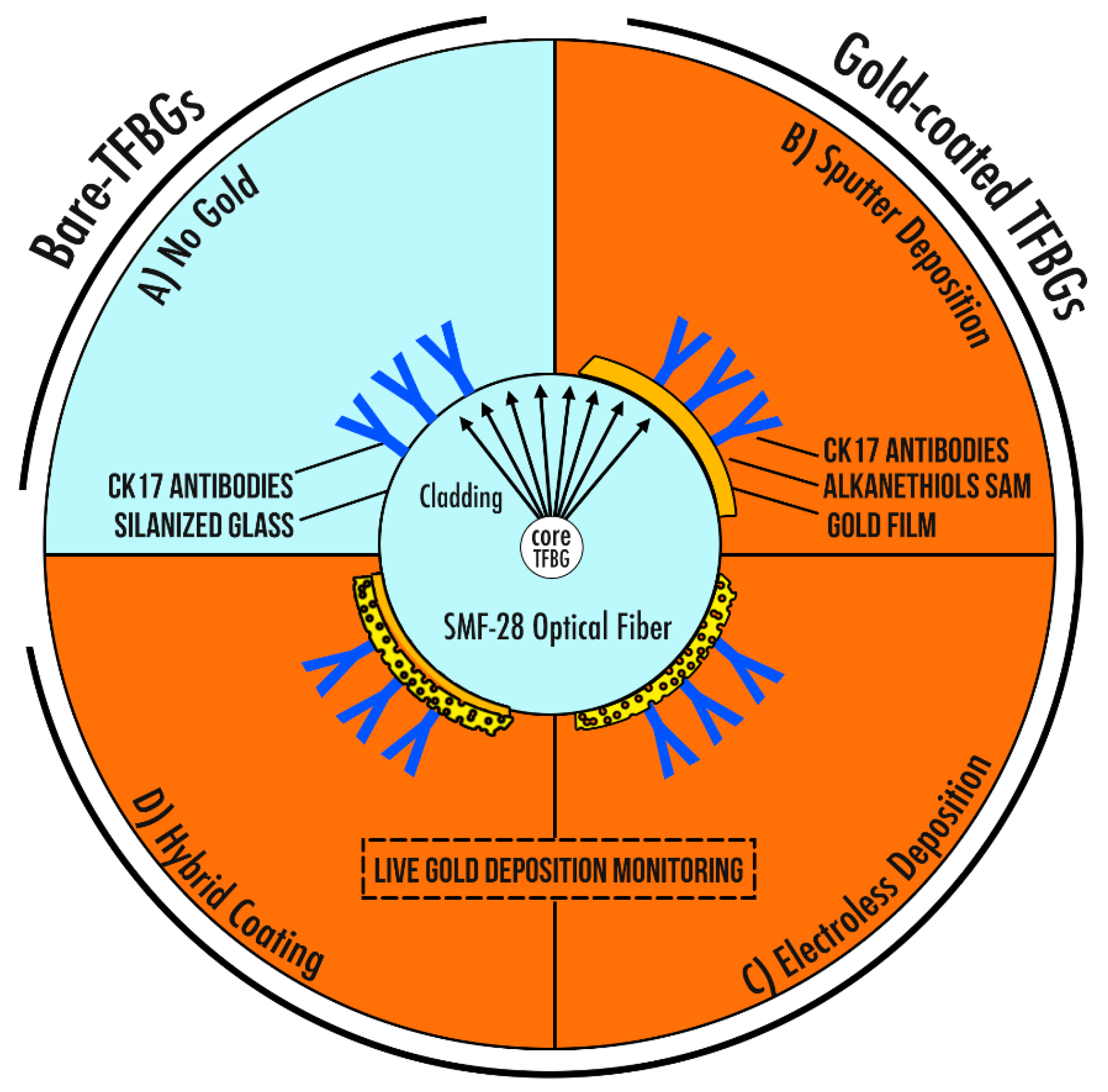
2.4. Description of the Four Different Sensing Configurations
2.4.1. Bare-TFBGs
2.4.2. Gold-Coated TFBGs: Sputter Deposition
2.4.3. Gold-Coated TFBGs: Electroless Deposition
2.4.4. Hybrid Gold-Coated TFBGs: Mix of Sputtering and ELP
2.5. Sensing Experiments
3. Results and Discussion
3.1. Spectral Sensitivity
3.2. Surface Influence on the Plasmonic Response
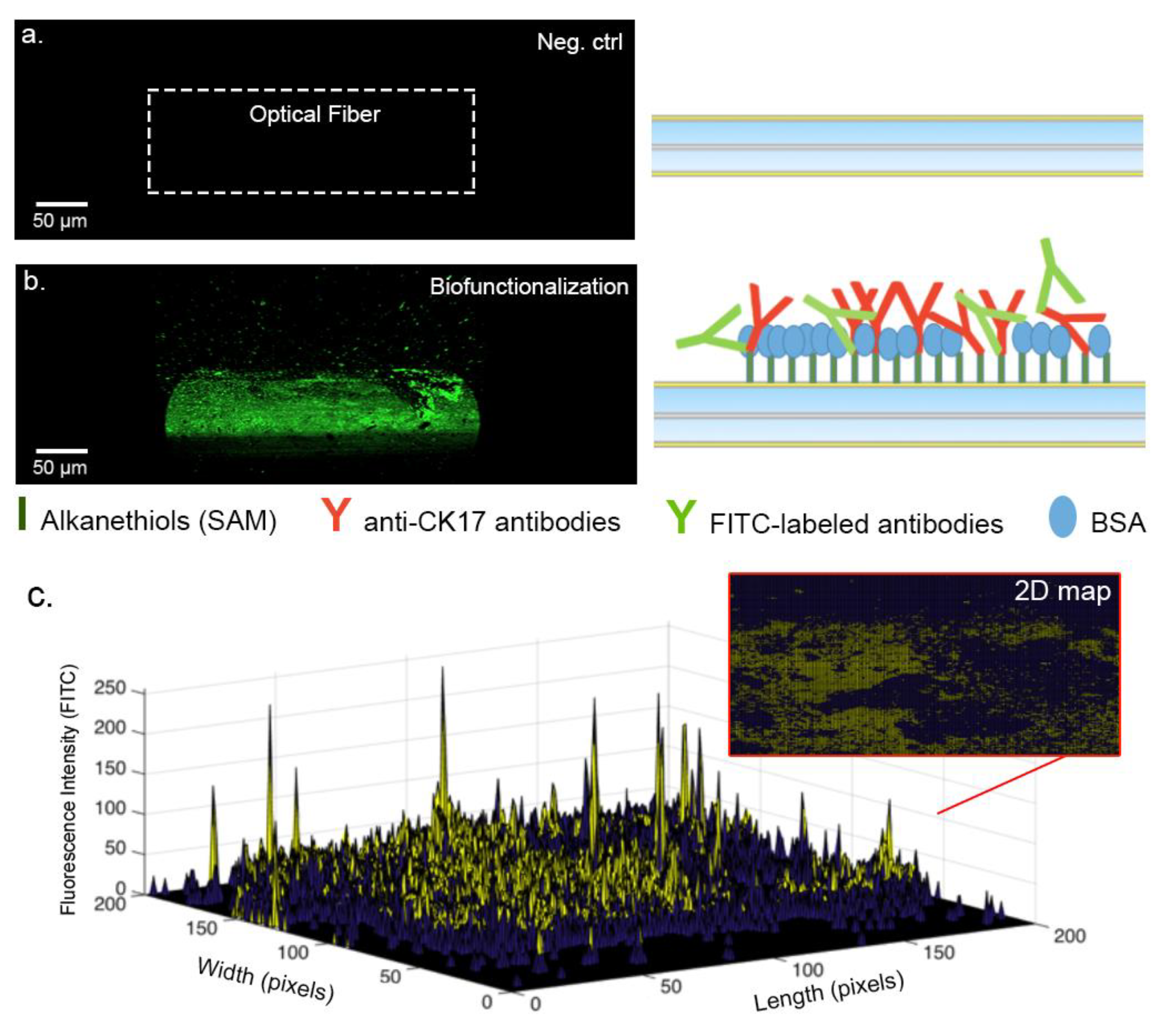
3.3. Analysis of the Biofunctionalized Surface
3.4. Biodetection of CK17 Proteins
3.5. Detection of CK17 Proteins in Resected Human Lung
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yin, M.; Gu, B.; An, Q.; Yang, C.; Guan, Y.L.; Yong, K. Recent development of fiber-optic chemical sensors and biosensors: Mechanisms, materials, micro/nano-fabrications and applications. Coord. Chem. Rev. 2018, 376, 348–392. [Google Scholar] [CrossRef]
- Gupta, B.D.; Kant, R. Recent advances in surface plasmon resonance based fiber optic chemical and biosensors utilizing bulk and nanostructures. Opt. Laser Technol. 2018, 101, 144–161. [Google Scholar] [CrossRef]
- Shan, D.; Gerhard, E.; Zhang, C.; Tierney, J.W.; Xie, D.; Liu, Z.; Yang, J. Polymeric biomaterials for biophotonic applications. Bioact. Mater. 2018, 3, 434–445. [Google Scholar] [CrossRef]
- Daems, D.; Pfeifer, W.; Rutten, I.; Sacca, B.; Spasic, D.; Lammertyn, J. 3D DNA origami as programmable anchoring points for bioreceptors in fiber optic surface plasmon resonance biosensing. ACS Appl. Mater. Interfaces 2018, 10, 23539–23547. [Google Scholar] [CrossRef]
- Pollet, J.; Delport, F.; Janssen, K.P.F.; Jans, K.; Maes, G.; Pfeiffer, H.; Wevers, M.; Lammertyn, J. Fiber optic SPR biosensing of DNA hybridization and DNA-protein interactions. Biosens. Bioelectron. 2009, 25, 864–869. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Zhou, X.; Zhang, Y. Review on the graphene based optical fiber chemical and biological sensors. Sens. Actuators B Chem. 2016, 231, 324–340. [Google Scholar] [CrossRef]
- Chiavaioli, F.; Biswas, P.; Trono, C.; Jana, S.; Bandyopadhyay, S.; Basumallick, N.; Giannetti, A.; Tombelli, S.; Bera, S.; Mallick, A.; et al. Sol-Gel-Based Titania-Silica Thin Film Overlay for Long Period Fiber Grating-Based Biosensors. Anal. Chem. 2015, 87, 12024–12031. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Biswas, P.; Chiavaioli, F.; Dey, T.K.; Basumallick, N.; Trono, C.; Giannetti, A.; Tombelli, S.; Baldini, F.; Bandyopadhyay, S. Long-period fiber grating: A specific design for biosensing applications. Appl. Opt. 2017, 56, 9846. [Google Scholar] [CrossRef]
- Chiavaioli, F.; Baldini, F.; Tombelli, S.; Trono, C.; Giannetti, A. Biosensing with optical fiber gratings. Nanophotonics 2017, 6, 663–679. [Google Scholar] [CrossRef]
- Caucheteur, C.; Voisin, V.; Albert, J. Near-infrared grating-assisted SPR optical fiber sensors: Design rules for ultimate refractometric sensitivity. Opt. Express 2015, 23, 2804–2809. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Y.; Xu, Y.; Wang, X.; Huang, Z.; Chen, J.; Li, Y. Ultrasensitive U-shaped fiber optic LSPR cytosensing for label-free and in situ evaluation of cell surface N-glycan expression. Sens. Actuators B Chem. 2019, 284, 582–588. [Google Scholar] [CrossRef]
- Christopher, C.; Subrahmanyam, A.; Sai, V.V.R. Gold Sputtered U-Bent Plastic Optical Fiber Probes as SPR- and LSPR-Based Compact Plasmonic Sensors. Plasmonics 2018, 13, 493–502. [Google Scholar] [CrossRef]
- Sai, V.V.R.; Kundu, T.; Mukherji, S. Novel U-bent fiber optic probe for localized surface plasmon resonance based biosensor. Biosens. Bioelectron. 2009, 24, 2804–2809. [Google Scholar] [CrossRef]
- Albert, J.; Lepinay, S.; Caucheteur, C.; Derosa, M.C. High resolution grating-assisted surface plasmon resonance fiber optic aptasensor. Methods 2013, 63, 239–254. [Google Scholar] [CrossRef]
- Caucheteur, C.; Loyez, M.; González-Vila, Á.; Wattiez, R. Evaluation of gold layer configuration for plasmonic fiber grating biosensors. Opt. Express. 2018, 26, 24154–24163. [Google Scholar] [CrossRef]
- Ribaut, C.; Voisin, V.; Malachovská, V.; Dubois, V.; Mégret, P.; Wattiez, R.; Caucheteur, C. Small biomolecule immunosensing with plasmonic optical fiber grating sensor. Biosens. Bioelectron. 2016, 77, 315–322. [Google Scholar] [CrossRef]
- Gorodkiewicz, E.; Lukaszewski, Z. Recent Progress in Surface Plasmon Resonance. Biosensors 2018, 8, 132. [Google Scholar] [CrossRef]
- Ribaut, C.; Loyez, M.; Larrieu, J.C.; Chevineau, S.; Lambert, P.; Remmelink, M.; Wattiez, R.; Caucheteur, C. Cancer biomarker sensing using packaged plasmonic optical fiber gratings: Towards in vivo diagnosis. Biosens. Bioelectron. 2017, 92, 449–456. [Google Scholar] [CrossRef]
- Ferrigno, P.K. Non-antibody protein-based biosensors Affimer binders and peptide aptamers in biosensing. Essays Biochem. 2016, 19–25. [Google Scholar] [CrossRef]
- Hassan, E.M.; Mohamed, A.; DeRosa, M.C.; Willmore, W.G.; Hanaoka, Y.; Kiwa, T.; Ozaki, T. High-sensitivity detection of metastatic breast cancer cells via terahertz chemical microscopy using aptamers. Sens. Actuators B Chem. 2019, 287, 595–601. [Google Scholar] [CrossRef]
- Hassan, E.M.; Willmore, W.G.; Mckay, B.C.; Derosa, M.C. In vitro selections of mammaglobin A and mammaglobin B aptamers for the recognition of circulating breast tumor cells. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- Filipiak, M.S.; Rother, M.; Andoy, N.M.; Knudsen, A.C.; Grimm, S.; Bachran, C.; Swee, L.K.; Zaumseil, J.; Tarasov, A. Highly sensitive, selective and label-free protein detection in physiological solutions using carbon nanotube transistors with nanobody receptors. Sens. Actuators B Chem. 2018, 255, 1507–1516. [Google Scholar] [CrossRef]
- Lépinay, S.; Ianoul, A.; Albert, J. Molecular imprinted polymer-coated optical fiber sensor for the identi fication of low molecular weight molecules. Talanta 2014, 128, 401–407. [Google Scholar] [CrossRef]
- Albert, J.; Shao, L.; Caucheteur, C. Tilted fiber Bragg grating sensors. Laser Photon. Rev. 2012, 26, 1–26. [Google Scholar] [CrossRef]
- Shevchenko, Y.Y.; Albert, J. Plasmon resonances in gold-coated tilted fiber Bragg gratings. Opt. Lett. 2007, 32, 211–213. [Google Scholar] [CrossRef]
- Caucheteur, C.; Guo, T.; Liu, F.; Guan, B.-O.; Albert, J. Ultrasensitive plasmonic sensing in air using optical fibre spectral combs. Nat. Commun. 2016, 7, 13371. [Google Scholar] [CrossRef]
- Loyez, M.; Albert, J.; Caucheteur, C.; Wattiez, R. Cytokeratins Biosensing Using Tilted Fiber Gratings. Biosensors 2018, 8, 74. [Google Scholar] [CrossRef]
- Guo, T.; González-Vila, Á.; Loyez, M.; Caucheteur, C. Plasmonic optical fiber-grating Immunosensing: A review. Sensors 2017, 17, 2732. [Google Scholar] [CrossRef]
- Loyez, M.; Ribaut, C.; Caucheteur, C.; Wattiez, R. Functionalized gold electroless-plated optical fiber gratings for reliable surface biosensing. Sens. Actuators B Chem. 2019, 280, 54–61. [Google Scholar] [CrossRef]
- Lahiri, A.; Kobayashi, S.-I. Electroless deposition of gold on silicon and its potential applications: Review. Surf. Eng. 2016, 32, 321–337. [Google Scholar] [CrossRef]
- Lepinay, S.; Staff, A.; Ianoul, A.; Albert, J. Improved detection limits of protein optical fiber biosensors coated with gold nanoparticles. Biosens. Bioelectron. 2014, 52, 337–344. [Google Scholar] [CrossRef]
- Arghir, I.; Delport, F.; Spasic, D.; Lammertyn, J. Smart design of fiber optic surfaces for improved plasmonic biosensing. New Biotechnol. 2015, 32, 473–484. [Google Scholar] [CrossRef]
- Lu, J.; Spasic, D.; Delport, F.; van Stappen, T.; Detrez, I.; Daems, D.; Vermeire, S.; Gils, A.; Lammertyn, J. A rapid immunoassay for detection of infliximab in whole blood using a fiber-optic SPR biosensor. Anal. Chem. 2017, 89, 3664–3671. [Google Scholar] [CrossRef]
- Lao, J.; Han, L.; Wu, Z.; Zhang, X.; Huang, Y.; Tang, Y. Gold Nanoparticle-Functionalized Surface Plasmon Resonance Optical Fiber Biosensor: In Situ Detection of Thrombin with 1 nM Detection Limit. J. Light. Technol. 2019, 37, 2748–2755. [Google Scholar] [CrossRef]
- Caucheteur, C.; Guo, T.; Albert, J. Review of plasmonic fiber optic biochemical sensors: Improving the limit of detection. Anal. Bioanal. Chem. 2015, 407, 3883–3897. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, S.; Zheng, X.; Wang, Y.; Xu, W. Optical Fiber LSPR Biosensor Prepared by Gold Nanoparticle Assembly on Polyelectrolyte Multilayer. Sensors 2010, 10, 3585–3596. [Google Scholar] [CrossRef]
- Chau, L.; Lin, Y.; Cheng, S.; Lin, T. Fiber-optic chemical and biochemical probes based on localized surface plasmon resonance. Sensors Actuators B Chem. 2006, 113, 100–105. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Cao, L.; Wen, Q. Keratin 17 Promotes Lung Adenocarcinoma Progression by Enhancing Cell Proliferation and Invasion. Med. Sci. Monit. 2018, 4782–4790. [Google Scholar] [CrossRef]
- Shoshan-barmatz, V.; Bishitz, Y.; Paul, A.; Krelin, Y.; Nakdimon, I.; Peled, N.; Lavon, A.; Rudoy-Zilberman, E.; Rafaely, Y. A molecular signature of lung cancer: Potential biomarkers for adenocarcinoma and squamous cell carcinoma. Oncotarget 2017, 8, 105492–105509. [Google Scholar] [CrossRef]
- Loyez, M.; Larrieu, J.-C.; Chevineau, S.; Remmelink, M.; Leduc, D.; Bondue, B.; Lambert, P.; Devière, J.; Wattiez, R.; Caucheteur, R. In situ cancer diagnosis through online plasmonics. Biosens. Bioelectron. 2019, 131, 104–112. [Google Scholar] [CrossRef]
- Sterzynska, K.; Budna, J.; Frydrych-tomczak, E.; Hreczycho, G.; Malinska, A.; Maciejewski, H.; Zabel, M. Silane-modified surfaces in specific antibody-mediated cell recognition. Folia Histochem. Cytobiol. 2014, 52, 250–255. [Google Scholar] [CrossRef]
- Bremer, M.G.E.G.; Duval, J.; Norde, W.; Lyklema, J. Electrostatic interactions between immunoglobulin (IgG) molecules and a charged sorbent. Colloids Surf. A 2004, 250, 29–42. [Google Scholar] [CrossRef]
- Antohe, I.; Schouteden, K.; Goos, P.; Delport, F.; Spasic, D.; Lammertyn, J. Thermal annealing of gold coated fiber optic surfaces for improved plasmonic biosensing. Sens. Actuators B Chem. 2016, 229, 678–685. [Google Scholar] [CrossRef]
- Caucheteur, C.; Guo, T.; Albert, J. Polarization-Assisted Fiber Bragg Grating Sensors: Tutorial and Review. J. Light. Technol. 2017, 35, 3311–3322. [Google Scholar] [CrossRef]
- Saha, B.; Evers, T.H.; Prins, M.W.J. How Antibody Surface Coverage on Nanoparticles Determines the Activity and Kinetics of Antigen Capturing for Biosensing. Anal. Chem. 2014, 86, 8158–8166. [Google Scholar] [CrossRef]
- Brogan, K.L.; Wolfe, K.N.; Jones, P.A.; Schoenfisch, M.H. Direct oriented immobilization of F(ab’) antibody fragments on gold. Anal. Chim. Acta 2003, 496, 73–80. [Google Scholar] [CrossRef]
- De Michele, C.; de Los Rios, P.; Foffi, G.; Piazza, F. Simulation and Theory of Antibody Binding to Crowded Antigen-Covered Surfaces. PLoS Comput. Biol. 2016, 12, e1004752. [Google Scholar] [CrossRef]
- Malaspina, D.C.; Longo, G.; Szleifer, I. Behavior of ligand binding assays with crowded surfaces: Molecular model of antigen capture by antibody-conjugated nanoparticle. PLoS ONE 2017, 12, e0185518. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loyez, M.; Lobry, M.; Wattiez, R.; Caucheteur, C. Optical Fiber Gratings Immunoassays. Sensors 2019, 19, 2595. https://doi.org/10.3390/s19112595
Loyez M, Lobry M, Wattiez R, Caucheteur C. Optical Fiber Gratings Immunoassays. Sensors. 2019; 19(11):2595. https://doi.org/10.3390/s19112595
Chicago/Turabian StyleLoyez, Médéric, Maxime Lobry, Ruddy Wattiez, and Christophe Caucheteur. 2019. "Optical Fiber Gratings Immunoassays" Sensors 19, no. 11: 2595. https://doi.org/10.3390/s19112595
APA StyleLoyez, M., Lobry, M., Wattiez, R., & Caucheteur, C. (2019). Optical Fiber Gratings Immunoassays. Sensors, 19(11), 2595. https://doi.org/10.3390/s19112595






