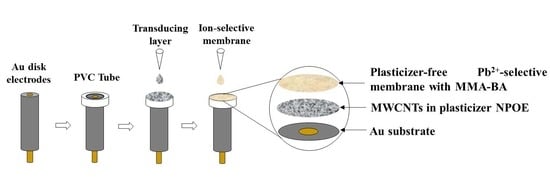
Disposable Multi-Walled Carbon Nanotubes-Based Plasticizer-Free Solid-Contact Pb2+-Selective Electrodes with a Sub-PPB Detection Limit †
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
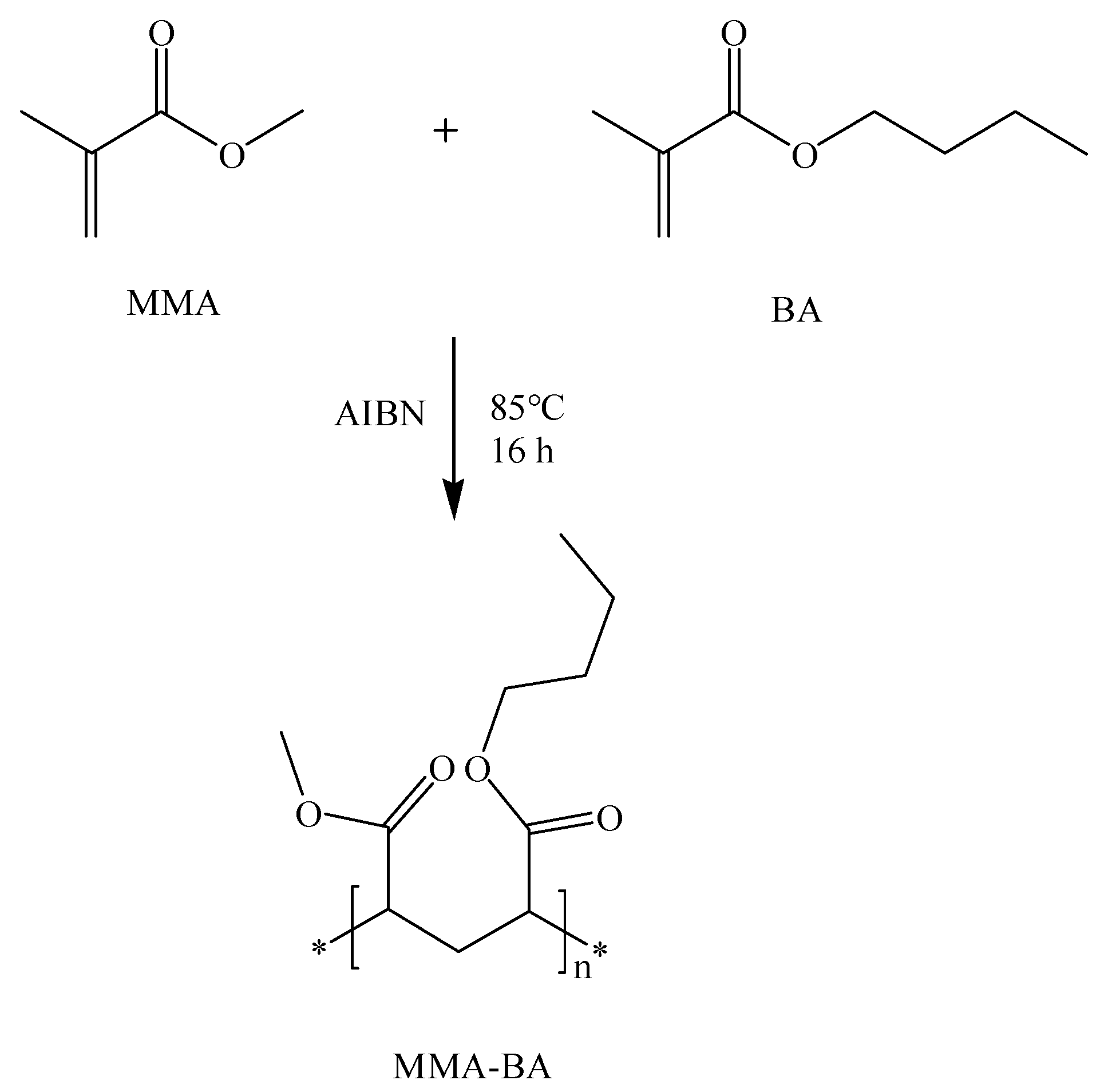
2.2. Polymer Preparation and Characterization
2.3. Electrode Fabrication
2.4. Apparatus and Measurements
3. Results and Discussion
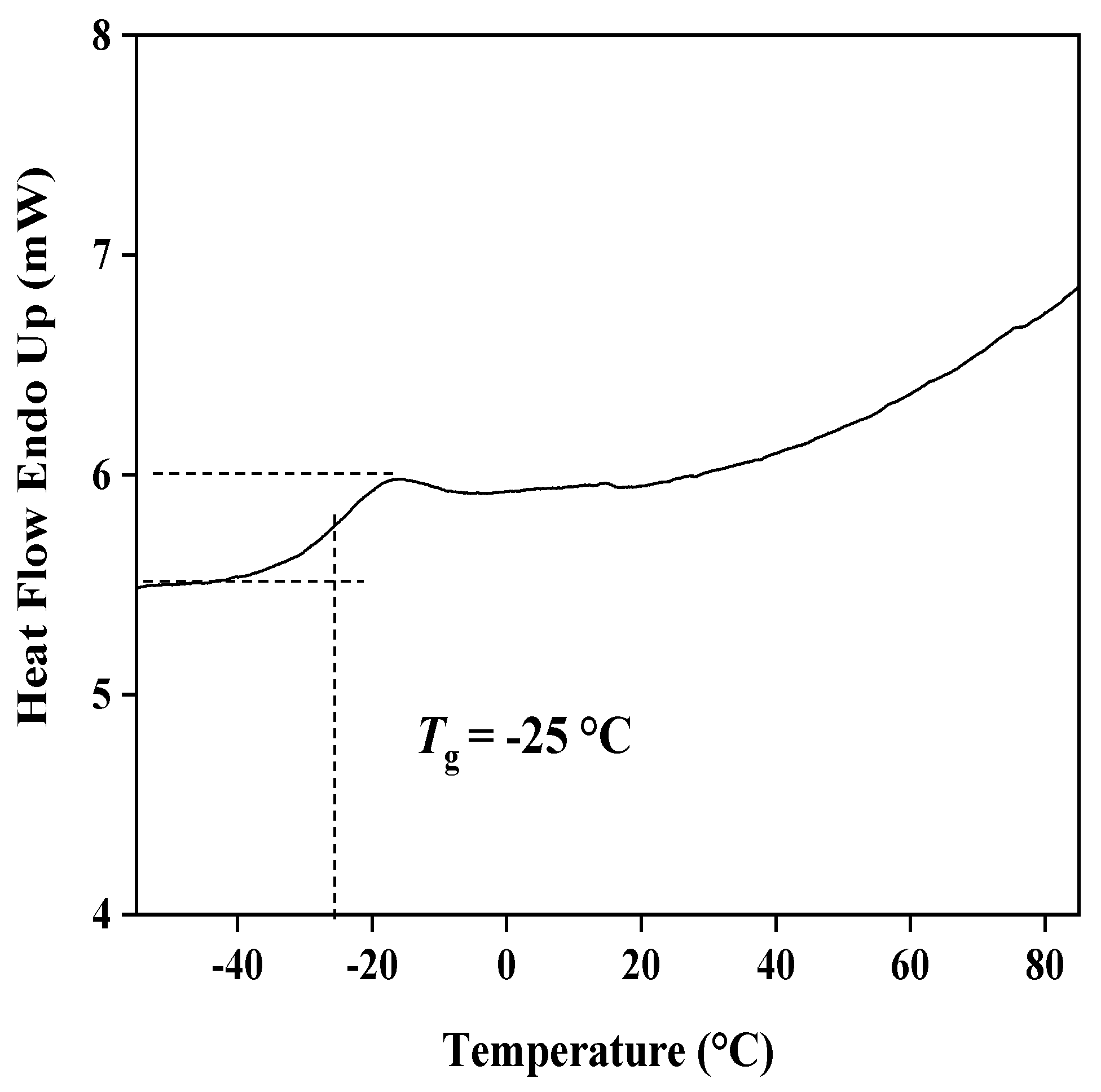
3.1. Characterization of the Copolymer
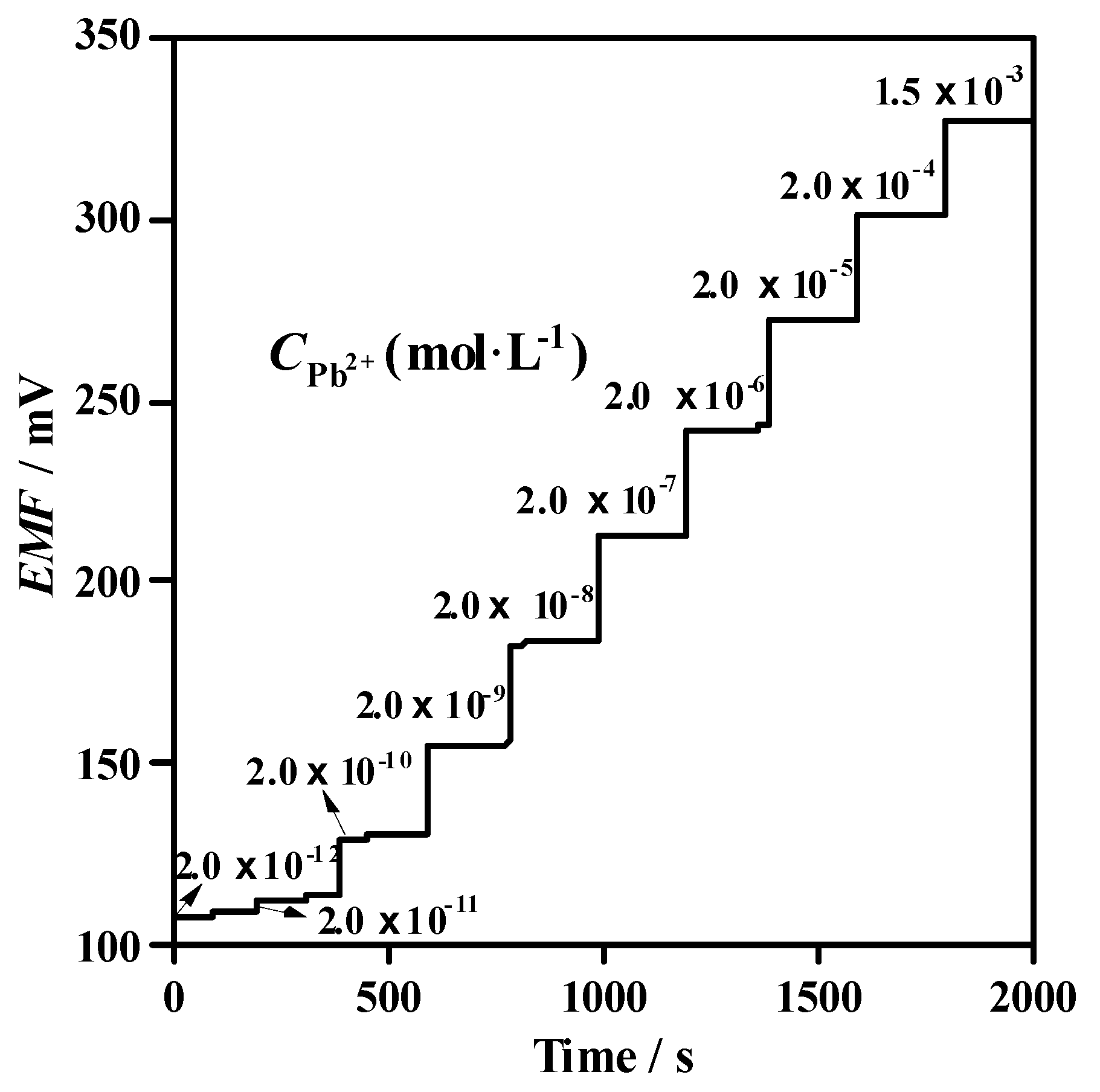
3.2. Potentiometric Behavior
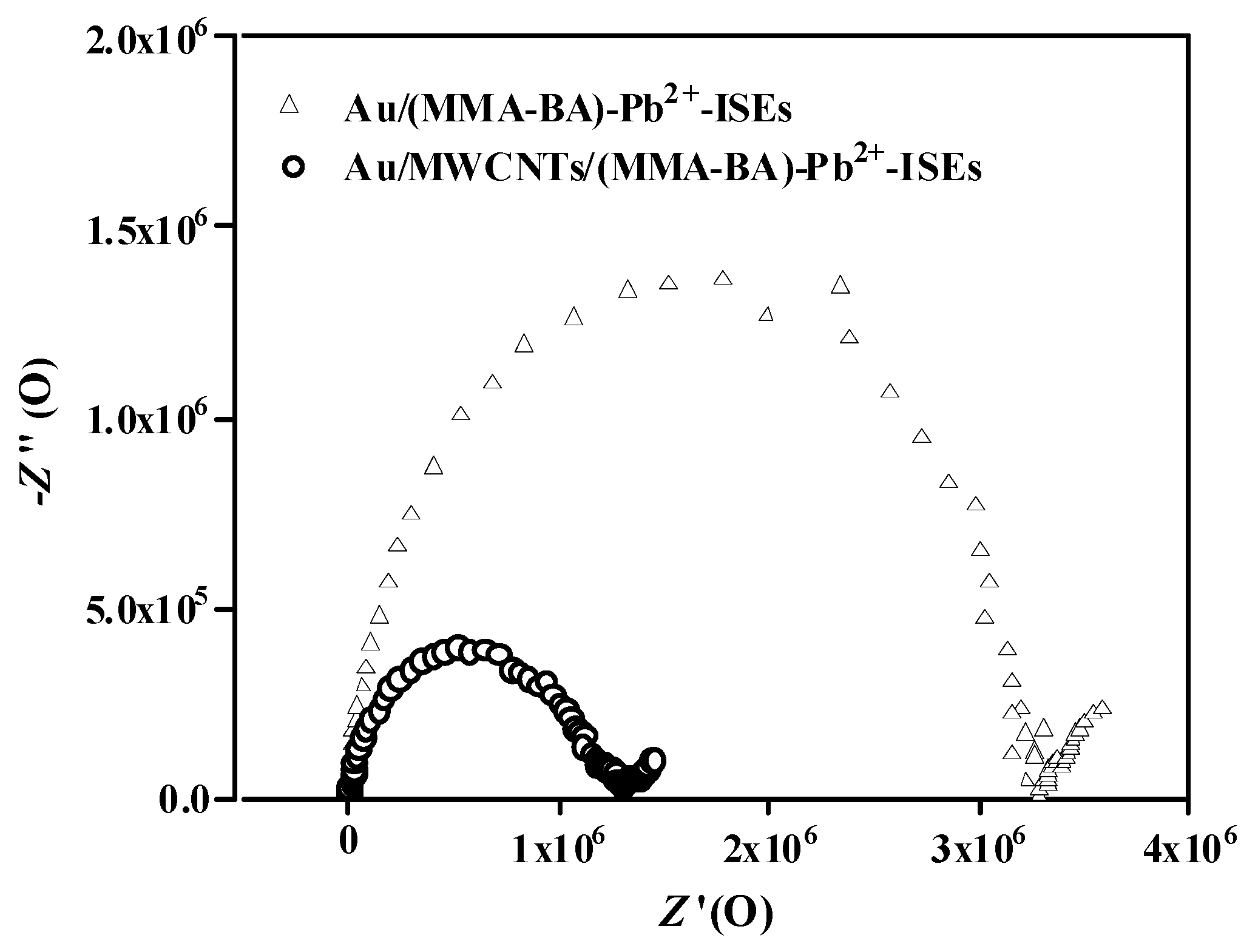
3.3. Impedance Measurements
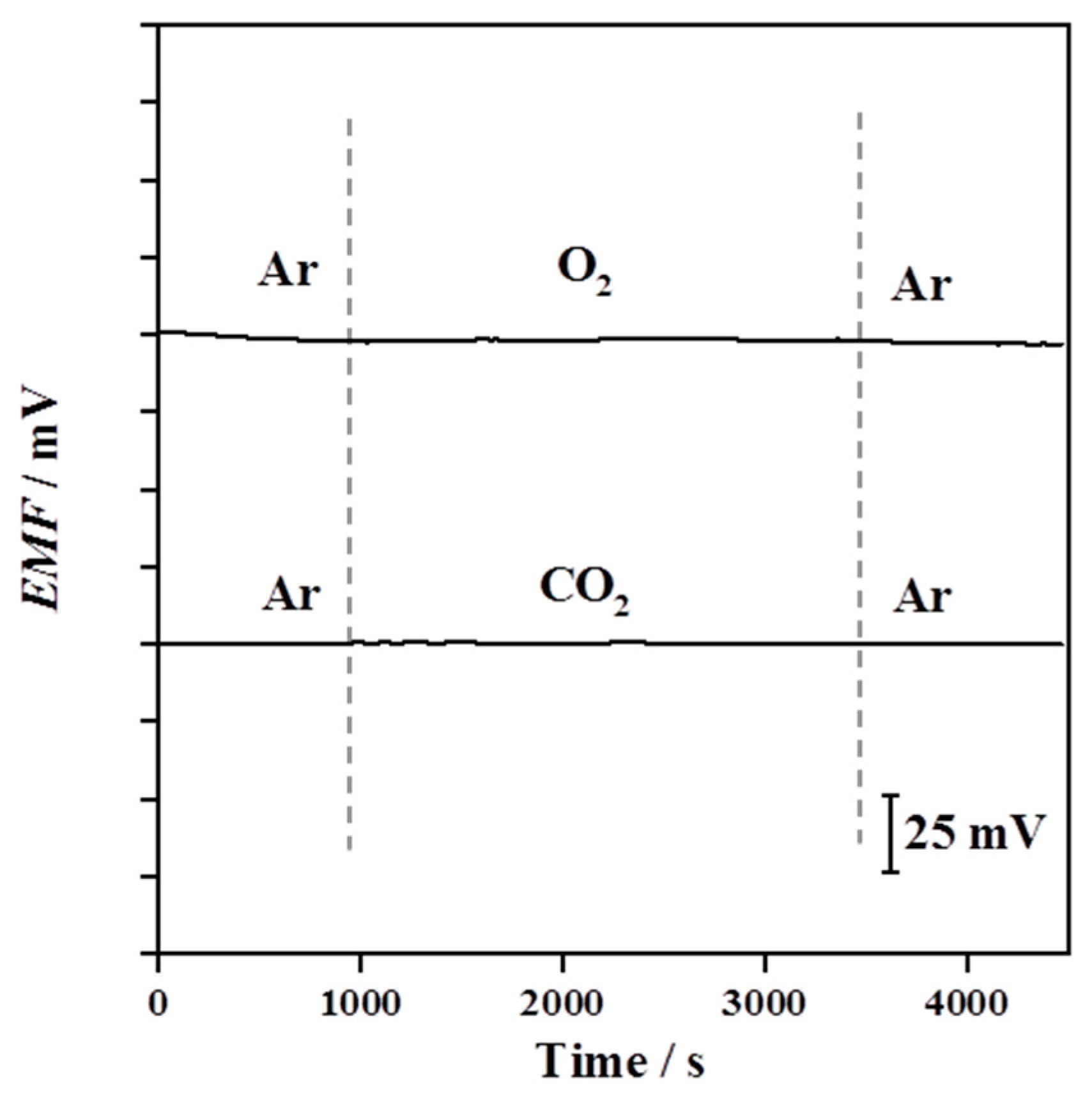
3.4. Influence of Oxygen and Carbon Dioxide
3.5. Potentiometric Water Layer Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Velde, L.; Angremont, E.; Olthuis, W. Solid contact potassium selective electrodes for biomedical applications—A review. Talanta 2016, 160, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Pretsch, E. Potentiometric sensors for trace-level analysis. Trends Anal. Chem. 2005, 24, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Choosang, J.; Numnuam, A.; Thavarungkul, P.; Kanatharana, P.; Radu, T.; Ullah, S.; Radu, A. Simultaneous detection of ammonium and nitrate in environmental samples using on ion-selective electrode and comparison with portable colorimetric assays. Sensors 2018, 18, 3555. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Feng, H.; Huang, M.R.; Gu, G.L.; Moloney, M.G. Ultrasensitive Pb (II) potentiometric sensor based on copolyaniline nanoparticles in a plasticizer-free membrane with a long lifetime. Anal. Chem. 2011, 84, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Golcs, Á.; Horváth, V.; Huszthy, P.; Tóth, T. Fast potentiometric analysis of lead in aqueous medium under competitive conditions using an acridono-crown ether neutral ionophore. Sensors 2018, 18, 1407. [Google Scholar] [CrossRef] [PubMed]
- Radu, A.; Peper, S.; Bakker, E.; Diamond, D. Guidelines for improving the lower detection limit of ion-selective electrodes: A systematic approach. Electroanalysis 2007, 19, 144–154. [Google Scholar] [CrossRef]
- Szigeti, Z.; Malon, A.; Vigassy, T.; Csokai, V.; Grün, A.; Wygladacz, K.; Ye, N.; Xu, C.; Chebny, V.J.; Rathore, R.; et al. Novel potentiometric and optical silver ion-selective sensors with subnanomolar detection limits. Anal. Chim. Acta 2006, 572, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Radu, A.; Peper, S.; Gonczy, C.; Runde, W.; Diamond, D. Trace-level determination of Cs+ using membrane-based ion-selective electrodes. Electroanalysis 2010, 18, 1379–1388. [Google Scholar] [CrossRef]
- Sokalski, T.; Ceresa, A.; Zwickl, T.; Pretsch, E. Large improvement of the lower detection limit of ion-selective polymer membrane electrodes. J. Am. Chem. Soc. 1997, 119, 11347–11348. [Google Scholar] [CrossRef]
- Michalska, A.; Maksymiuk, K. Conducting polymer membranes for low activity potentiometric ion sensing. Talanta 2004, 63, 109–117. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2019, 91, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric ion sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Bobacka, J. Conducting polymer-based solid-state ion-selective electrodes. Electroanalysis 2010, 18, 7–18. [Google Scholar] [CrossRef]
- Lange, U.; Roznyatovskaya, N.V.; Mirsky, V.M. Conducting polymers in chemical sensors and arrays. Anal. Chim. Acta 2008, 614, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.; Bobacka, J.; Ivaska, A.; Lewenstam, A. Influence of oxygen and carbon dioxide on the electrochemical stability of poly (3,4-ethylenedioxythiophene) used as ion-to-electron transducer in all-solid-state ion-selective electrodes. Sens. Actuators B Chem. 2002, 82, 7–13. [Google Scholar] [CrossRef]
- Mir, M.; Lugo, R.; Tahirbegi, I.B.; Samitier, J. Miniaturizable ion-selective arrays based on highly stable polymer membranes for biomedical applications. Sensors 2014, 14, 11844–11854. [Google Scholar] [CrossRef]
- Yin, T.; Pan, D.; Qin, W. A solid-contact Pb2+-selective polymeric membrane electrode with nafion-doped poly(pyrrole) as ion-to-electron transducer. J. Solid State Electrochem. 2012, 16, 499–504. [Google Scholar] [CrossRef]
- Sutter, J.; Radu, A.; Peper, S.; Bakker, E.; Pretsch, E. Solid-contact polymeric membrane electrodes with detection limits in the subnanomolar range. Anal. Chim. Acta 2004, 523, 53–59. [Google Scholar] [CrossRef]
- Sutter, J.; Lindner, E.; Gyurcsányi, R.E.; Pretsch, E. A polypyrrole-based solid-contact Pb2+-selective PVC-membrane electrode with a nanomolar detection limit. Anal. Bioanal. Chem. 2004, 380, 7–14. [Google Scholar] [CrossRef]
- Chumbimuni-Torres, K.Y.; Rubinova, N.; Radu, A.; Kubota, L.T.; Bakker, E. Solid contact potentiometric sensors for trace level measurements. Anal. Chem. 2006, 78, 1318–1322. [Google Scholar] [CrossRef]
- Lisak, G.; Grygolowicz-Pawlak, E.; Mazurkiewicz, M.; Malinowska, E.; Sokalski, T.; Bobacka, J.; Lewenstam, A. A New polyacrylate-based lead (II) ion-selective electrodes. Microchim. Acta 2009, 164, 293–297. [Google Scholar] [CrossRef]
- Heng, L.Y.; Hall, E.A. Producing “self-plasticizing” ion-selective membranes. Anal. Chem. 2000, 72, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Riu, J.; Bobacka, J.; Vallés, C.; Jiménez, P.; Benito, A.M.; Maser, W.K.; Rius, F.X. Reduced graphene oxide films as solid transducers in potentiometric all-solid-state ion-selective electrodes. J. Phys. Chem. C 2012, 116, 22570–22578. [Google Scholar] [CrossRef]
- Parra, E.J.; Crespo, G.A.; Riu, J.; Ruiz, A.; Rius, F.X. Ion-selective electrodes using multi-walled carbon nanotubes as ion-to-electron transducers for the detection of perchlorate. Analyst 2009, 134, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Heng, L.V.; Chern, L.H.; Ahmad, M. A hydrogen ion-selective sensor based on non-plasticised methacrylic-acrylic membranes. Sensors 2002, 2, 339–346. [Google Scholar] [CrossRef]
- Qin, Y.; Peper, S.; Bakker, E. Plasticizer-free polymer membraneion-selective electrodes containing a methacrylic copolymer matrix. Electroanalysis 2002, 14, 1375–1381. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, Y.; Tang, H.; Wang, M.; Qin, Y. Click-immobilized K+-selective ionophore for potentiometric and optical sensors. Sens. Actuators B Chem. 2012, 171, 556–562. [Google Scholar] [CrossRef]
- Püntener, M.; Vigassy, T.; Baier, E.; Ceresa, A.; Pretsch, E. Improving the lower detection limit of potentiometric sensors by covalently binding the ionophore to a polymer backbone. Anal. Chim. Acta 2004, 503, 187–194. [Google Scholar] [CrossRef]
- Michalska, A.J.; Appaih-Kusi, C.; Heng, L.Y.; Walkiewicz, S.; Hall, E.A. An experimental study of membrane materials and inner contacting layers for ion-selective K+ electrodes with a stable response and good dynamic range. Anal. Chem. 2004, 76, 2031–2039. [Google Scholar] [CrossRef]
- Michalska, A.; Wojciechowski, M.; Bulska, E.; Maksymiuk, K. Experimental study on stability of different solid contact arrangements of ion-selective electrodes. Talanta 2010, 82, 151–157. [Google Scholar] [CrossRef]
- Yu, S.; Yuan, Q.; Li, F.; Liu, Y. Improved potentiometric response of all-solid-state Pb2+-selective electrode. Talanta 2012, 101, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Anastasova, S.; Radu, A.; Matzeu, G.; Zuliani, C.; Mattinen, U.; Bobacka, J.; Diamond, D. Disposable solid-contact ion-selective electrodes for environmental monitoring of lead with ppb limit-of-detection. Electrochim. Acta 2012, 73, 93–97. [Google Scholar] [CrossRef]
- Liang, R.; Yin, T.; Qin, W. A simple approach for fabricating solid-contact ion-selective electrodes using nanomaterials as transducers. Anal. Chim. Acta 2015, 853, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, T.; Qin, W. An all-solid-state polymeric membrane Pb2+-selective electrode with bimodal pore C60 as solid contact. Anal. Chim. Acta 2015, 876, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, E.; Lewandowski, W.; Mieczkowski, J.; Maksymiuk, K.; Michalska, A. A Simple and disposable potentiometric sensors based on graphene or multi-walled carbon nanotubes–carbon–plastic potentiometric sensors. Analyst 2013, 138, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Gao, Y.; Wang, P. A general approach to one-step fabrication of single-piece nanocomposite membrane based Pb2+-selective electrodes. Sens. Actuators B Chem. 2019, 281, 705–712. [Google Scholar] [CrossRef]
- Didina, S.E.; Mitnik, L.L.; Koshmina, N.V.; Grekovich, A.L.; Mikhelson, K.N. Lead-selective electrodes based on liquid ion-exchangers. Sens. Actuators B Chem. 1994, 19, 396–399. [Google Scholar] [CrossRef]
- Koryta, J.; Dvořák, J. Principles of Electrochemistry; Willey: New York, NY, USA, 1987; p. 41. [Google Scholar]
- Qin, Y.; Peper, S.; Radu, A.; Ceresa, A.; Bakker, E. Plasticizer-free polymer containing a covalently immobilized Ca2+-selective ionophore for potentiometric and optical sensors. Anal. Chem. 2003, 75, 3038–3045. [Google Scholar] [CrossRef]
- Heng, L.Y.; Hall, E.A. Methacrylic–acrylic polymers in ion-selective membranes: Achieving the right polymer recipe. Anal. Chim. Acta 2000, 403, 77–89. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.; Zhao, Y.; Jiang, W.; Zhang, Z.; Yu, L. A solid-contact Pb2+-selective electrode based on electrospun polyaniline microfibers film as ion-to-electron transducer. Electrochim. Acta 2017, 231, 53–60. [Google Scholar] [CrossRef]
- Guziński, M.; Lisak, G.; Sokalski, T.; Bobacka, J.; Ivaska, A.; Bocheńska, M.; Lewenstam, A. Solid-contact ion-selective electrodes with highly selective thioamide derivatives of p-tert-butylcalix[4]arene for the determination of lead (II) in environmental samples. Anal. Chem. 2013, 85, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, F.; Yin, T.; Liu, Y.; Pan, D.; Qin, W. A solid-contact Pb2+-selective electrode using poly(2-methoxy-5-(2′-ethylhexyloxy)-p-phenylene vinylene) as ion-to-electron transducer. Anal. Chim. Acta 2011, 702, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Wojciechowski, M.; Bulska, E.; Mieczkowski, J.; Maksymiuk, K. Poly (n-butyl acrylate) based lead (II) selective electrodes. Talanta 2009, 79, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Skompska, M.; Mieczkowski, J.; Zagórska, M.; Maksymiuk, K. Tailoring solution cast poly (3,4-dioctyloxythiophene) transducers for potentiometric all-solid-state ion-selective electrodes. Electroanalysis 2006, 18, 763–771. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Huang, M. Lead(II) ion-selective electrode based on polyaminoanthraquinone particles with intrinsic conductivity. Talanta 2008, 78, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Song, K.; Seo, H.R.; Choi, Y.K.; Jeon, S. Lead(II)-selective electrodes based on tetrakis(2-hydroxy-1-naphthyl)porphyrins: The effect of atropisomers. Sens. Actuators B Chem. 2004, 99, 323–329. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Bühlmann, P. Selectivity of potentiometric ion sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zou, X.U.; Stein, A.; Bühlmann, P. Ion-selective electrodes with colloid-imprinted mesoporous carbon as solid contact. Anal. Chem. 2014, 86, 7111–7118. [Google Scholar] [CrossRef] [PubMed]
- Veder, J.; Marco, R.D.; Clarke, G.; Chester, R.; Nelson, A.; Prince, K.; Pretsch, E.; Bakker, E. Elimination of undesirable water layers in solid-contact polymeric ion-selective electrodes. Anal. Chem. 2008, 80, 6731–6740. [Google Scholar] [CrossRef]



| Composition Based on Feed (mol Fraction) | Average Molecular Weight (Daltons) | Polydispersity | |||||
|---|---|---|---|---|---|---|---|
| MMA/BA = 1:3 | 9837 | 15,487 | 12,441 | 31,759 | 1.50 | 2.05 | 1.57 |
| Low Detection Limit (mol·L−1) | Response Slope (mV·decade−1) | Electrode Substrate | Ion-to-Electron Conducting Layer and Deposition Method | Ion-Selective Membrane Composition | Reference |
|---|---|---|---|---|---|
| 1.0 × 10−10 | 29.1 ± 0.5 | Au disk electrodes | multi-walled carbon nanotubes (MWCNTs)/solution drop-casting | PVC, NPOE, lead ionophore IV, NaTFPB | this work |
| 6.3 × 10−10 | 29.1 ± 0.7 | glassy carbon disk electrodes | electrospun polyaniline microfibers film/solution drop−casting | PVC, NPOE, lead ionophore IV, NaTFPB | [41] |
| 5.0 × 10−10 | 28.8 ± 1.2 | glassy carbon disk electrodes | bimodal pore C60/electrodeposition | PVC, NPOE, lead ionophore IV, ETH 500, NaTFPB | [34] |
| 7.9 × 10−10 | 28.4 ± 0.4 | glassy carbon disk electrodes | poly(3,4-ethylenedioxythiophene) doped with polystyrene sulfonate anion (PEDOT-PSS)/electrodeposition | PVC, NPOE, lead ionophore IV, potassium tetrakis(p-chlorophenyl)-borate | [42] |
| 1.2 × 10−9 | 23.4 ± 0.0 | screen printer electrodes | poly(3-octylthiophene-2,5-diyl)/solution drop−casting | PVC, NPOE, lead ionophore IV, NaTFPB | [32] |
| 6.3 × 10−10 | 29.1 ± 0.8 | Au disk electrodes | poly(2-methoxy-5-(2′-ethylhexyloxy)-p-phenylene vinylene) (MEH-PPV)/solution drop-casting | PVC, NPOE, lead ionophore IV, NaTFPB | [43] |
| 1.0 × 10−9 | 26.2 ± 0.3 | glassy carbon disk electrodes | poly(octylthiophene) (POT)/solution drop-casting | hydroxyethyl methacrylate-butyl acrylate, lead ionophore IV, NaTFPB | [44] |
| 1.2 × 10−8 | 27.9 ± 0.3 | glassy carbon disk electrodes | poly(3,4-dioctyloxythiophene) doped with lead ionophore IV/electrodeposition | PVC, DOS, lead ionophore IV, NaTFPB | [45] |
| 5.0 × 10−10 | Nernstian response | Au disk electrodes | poly(octylthiophene) (POT)/solution drop-casting | Methylmethacrylate-decylmethacrylate, ETH500, lead ionophore IV, NaTFPB | [18] |
| Ion J | ||||||||
|---|---|---|---|---|---|---|---|---|
| J | Na+ | K+ | Li+ | Ca2+ | Mg2+ | Cu2+ | Ag+ | |
| Electrode Type | ||||||||
| Au/MWCNTs/(MMA-BA)-Pb2+-ISEs | −8.49 ± 0.2 | −8.48 ± 0.1 | −7.93 ± 0.2 | −11.07 ± 0.3 | −11.07 ± 0.2 | −5.70 ± 0.3 | −0.47 ± 2.1 | |
| Au/PPy/(PVC-DOS)-Pb2+-ISEs [19] | −6.3 ± 0.1 | −6.6 ± 0.1 | Not given | −13.6 ± 0.2 | Not given | Not given | Not given | |
| Au/POT/(MMA-DMA)-Pb2+-ISEs [18] | −8.7 ± 0.2 | −8.7 ± 0.2 | Not given | −14.3 ± 0.2 | Not given | Not given | Not given | |
| Au/MEH-PPV/(PVC-NPOE)-Pb2+-ISEs [43] | −6.6 ± 0.1 | Not given | Not given | −16.5 ± 0.2 | Not given | −4.6 ± 0.1 | Not given | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Gao, Y.; Yan, R.; Huang, H.; Wang, P.
Disposable Multi-Walled Carbon Nanotubes-Based Plasticizer-Free Solid-Contact Pb2+-Selective Electrodes with a Sub-PPB Detection Limit
Liu Y, Gao Y, Yan R, Huang H, Wang P.
Disposable Multi-Walled Carbon Nanotubes-Based Plasticizer-Free Solid-Contact Pb2+-Selective Electrodes with a Sub-PPB Detection Limit
Liu, Yueling, Yingying Gao, Rui Yan, Haobo Huang, and Ping Wang.
2019. "Disposable Multi-Walled Carbon Nanotubes-Based Plasticizer-Free Solid-Contact Pb2+-Selective Electrodes with a Sub-PPB Detection Limit
Liu, Y., Gao, Y., Yan, R., Huang, H., & Wang, P.
(2019). Disposable Multi-Walled Carbon Nanotubes-Based Plasticizer-Free Solid-Contact Pb2+-Selective Electrodes with a Sub-PPB Detection Limit