Validity of the Walked Distance Estimated by Wearable Devices in Stroke Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants Selection
2.2. Assessment of the Hemiplegia
2.3. Instrumentation
2.3.1. Actigraph GT3x
2.3.2. Sensewear Armband
2.3.3. Pedometer (ONStep 400, Geonaute)
2.4. Walked Distance
2.5. Test Protocol
- Measurement of the average step length over three trials of 20 steps.
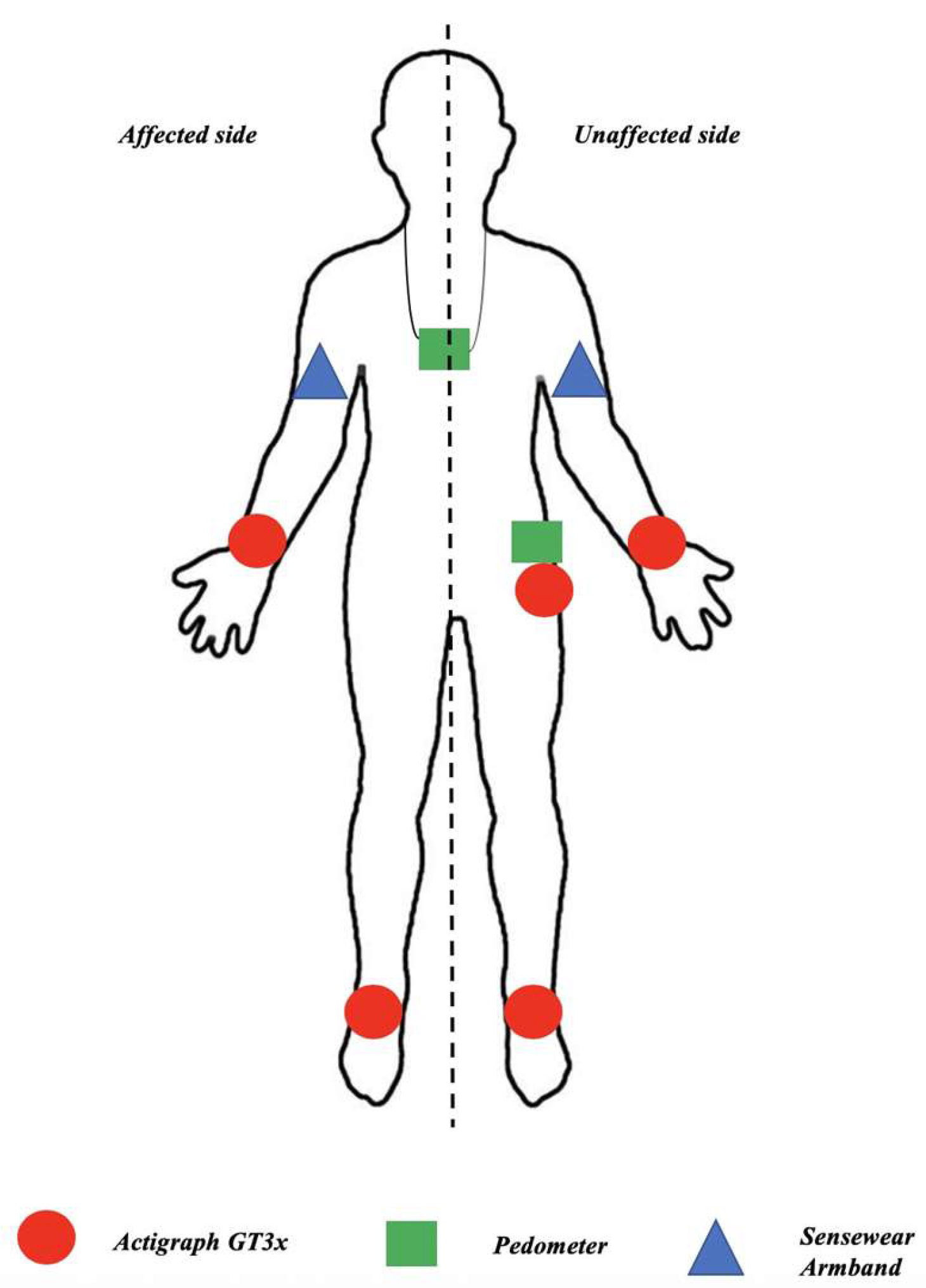
- Installation of the sensors. Actigraph GT3x devices were placed on the wrists and ankles on both the affected and unaffected sides, as well as at the unaffected hip. Sensewear Armbands were placed on both the affected and unaffected arms. Pedometers were placed at the unaffected hip and around the neck. The device placements are illustrated in Figure 1.
- The participants performed a six-minute walk test at a comfortable walking speed. During this walking period, the distance walked was measured by the examiner with the graduations marked on the floor of the corridor.
- Download of the data from all devices.
2.6. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Validity of the Analysis
3.3. Validity Parameters
4. Discussion
4.1. Strengths of the Study
4.2. Limitations
5. Conclusions
- The sensor type and its location on the body strongly impact the estimation of the walked distance in individuals with stroke sequelae.
- The pedometer (piezoelectric device) placed on the hip and the Actigraph activity monitor (triaxial accelerometer) worn on the hip on the non-affected side provided the closest estimations of the walked distance.
- Placing an Actigraph on the upper limbs caused a significant underestimation of the walked distance in individuals with stroke sequelae.
- The Sensewear Armband strongly underestimated the walking distance regardless of its placement on the affected or unaffected upper limb of the stroke individuals.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statements
References
- Barclay, R.E.; Stevenson, T.J.; Poluha, W.; Ripat, J.; Nett, C.; Srikesavan, C.S. Interventions for improving community ambulation in individuals with stroke. Cochrane Database Syst Rev. 2015, 13, CD010200. [Google Scholar] [CrossRef]
- Lord, S.E.; McPherson, K.; McNaughton, H.K.; Rochester, L.; Weatherall, M. Community ambulation after stroke: How important and obtainable is it and what measures appear predictive? Arch. Phys. Med. Rehabil. 2004, 85, 234–239. [Google Scholar] [CrossRef]
- Mayo, N.E.; Wood-Dauphinee, S.; Côté, R.; Durcan, L.; Carlton, J. Activity, participation, and quality of life 6 months poststroke. Arch. Phys. Med. Rehabil. 2002, 83, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Salbach, N.M.; O’Brien, K.; Brooks, D.; Irvin, E.; Martino, R.; Takhar, P.; Chan, S.; Howe, J.-A. Speed and Distance Requirements for Community Ambulation: A Systematic Review. Arch. Phys. Med. Rehabil. 2014, 95, 117–128. [Google Scholar] [CrossRef]
- Perry, J.; Garrett, M.; Gronley, J.K.; Mulroy, S.J. Classification of Walking Handicap in the Stroke Population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef]
- Patla, A.E.; Shumway-Cook, A. Dimensions of Mobility: Defining the Complexity and Difficulty Associated with Community Mobility. J. Aging Phys. Act. 1999, 7, 7–19. [Google Scholar] [CrossRef]
- Corrigan, R.; McBurney, H. Community ambulation: Environmental impacts and assessment inadequacies. Disabil. Rehabil. 2008, 30, 1411–1419. [Google Scholar] [CrossRef]
- Andrews, A.W.; Chinworth, S.A.; Bourassa, M.; Garvin, M.; Benton, D.; Tanner, S. Update on distance and velocity requirements for community ambulation. J. Geriatr. Phys. Ther. 2010, 33, 128–134. [Google Scholar]
- Billinger, S.A.; Arena, R.; Bernhardt, J.; Eng, J.J.; Franklin, B.A.; Johnson, C.M.; MacKay-Lyons, M.; Macko, R.F.; Mead, G.E.; Roth, E.J.; et al. Physical Activity and Exercise Recommendations for Stroke Survivors: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2014, 45, 2532–2553. [Google Scholar] [CrossRef] [PubMed]
- English, C.; Manns, P.J.; Tucak, C.; Bernhardt, J. Physical Activity and Sedentary Behaviors in People With Stroke Living in the Community: A Systematic Review. Phys. Ther. 2014, 94, 185–196. [Google Scholar] [CrossRef]
- Balasubramanian, C.K.; Neptune, R.R.; Kautz, S.A. Variability in spatiotemporal step characteristics and its relationship to walking performance post-stroke. Gait Posture 2009, 29, 408–414. [Google Scholar] [CrossRef]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef]
- Lee, K.B.; Lim, S.H.; Ko, E.H.; Kim, Y.S.; Lee, K.S.; Hwang, B.Y. Factors related to community ambulation in patients with chronic stroke. Top. Stroke Rehabil. 2015, 22, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, N.; Kuys, S.S.; Downie, E.; Ng, P.; Brauer, S.G. Are Accelerometers and GPS Devices Valid, Reliable and Feasible Tools for Measurement of Community Ambulation After Stroke? Brain Impair. 2016, 17, 151–161. [Google Scholar] [CrossRef]
- Crouter, S.E.; Schneider, P.L.; Karabulut, M.; Bassett, D.R. Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med. Sci. Sports Exerc. 2003, 35, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.L.; Crouter, S.E.; Lukajic, O.; Bassett, D.R. Accuracy and Reliability of 10 Pedometers for Measuring Steps over a 400-m Walk. Med. Sci. Sports Exerc. 2003, 35, 1779–1784. [Google Scholar] [CrossRef]
- Chisholm, A.E.; Makepeace, S.; Inness, E.L.; Perry, S.D.; McIlroy, W.E.; Mansfield, A. Spatial-temporal gait variability poststroke: Variations in measurement and implications for measuring change. Arch. Phys. Med. Rehabil. 2014, 95, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Reisman, D.S.; Rudolph, K.S.; Farquhar, W.B. Influence of Speed on Walking Economy Poststroke. Neurorehabil. Neural Repair 2009, 23, 529–534. [Google Scholar] [CrossRef]
- Carroll, S.L.; Greig, C.A.; Lewis, S.J.; McMurdo, M.E.; Sniehotta, F.F.; Johnston, M.; Johnston, D.W.; Scopes, J.; Mead, G.E. The Use of Pedometers in Stroke Survivors: Are They Feasible and How Well Do They Detect Steps? Arch. Phys. Med. Rehabil. 2012, 93, 466–470. [Google Scholar] [CrossRef]
- Fulk, G.D.; Combs, S.A.; Danks, K.A.; Nirider, C.D.; Raja, B.; Reisman, D.S. Accuracy of 2 activity monitors in detecting steps in people with stroke and traumatic brain injury. Phys. Ther. 2014, 94, 222–229. [Google Scholar] [CrossRef]
- Klassen, T.D.; Simpson, L.A.; Lim, S.B.; Louie, D.R.; Parappilly, B.; Sakakibara, B.M.; Zbogar, D.; Eng, J.J. “Stepping Up” activity poststroke: Ankle-positioned accelerometer can accurately record steps during slow walking. Phys. Ther. 2016, 96, 355–360. [Google Scholar] [CrossRef]
- Demeurisse, G.; Demol, O.; Robaye, E. Motor evaluation in vascular hemiplegia. Eur. Neurol. 1980, 19, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Brun, V.; Mousbeh, Z.; Jouet-Pastre, B.; Benaim, C.; Kunnert, J.E.; Dhoms, G.; d’Angeli-Chevassut, M.; Torres, B.; Pélissier, J. Évaluation clinique de la marche de l’hémiplégique vasculaire: Proposition d’une modification de la functional ambulation classification. Ann. Phys. Rehabil. Med. 2000, 43, 14–20. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Bakken, L.N.; Kim, H.S.; Finset, A.; Lerdal, A. Stroke patients’ functions in personal activities of daily living in relation to sleep and socio-demographic and clinical variables in the acute phase after first-time stroke and at six months of follow-up. J. Clin. Nurs. 2012, 21, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- English, C.; Healy, G.N.; Coates, A.; Lewis, L.K.; Olds, T.; Bernhardt, J. Sitting time and physical activity after stroke: Physical ability is only part of the story. Top. Stroke Rehabil. 2016, 23, 36–42. [Google Scholar] [CrossRef]
- van der Ploeg, H.P.; Streppel, K.R.M.; van der Beek, A.J.; van der Woude, L.H.V.; Vollenbroek-Hutten, M.; van Mechelen, W. The Physical Activity Scale for Individuals with Physical Disabilities: Test-retest reliability and comparison with an accelerometer. J. Phys. Act. Health 2007, 4, 96–100. [Google Scholar] [CrossRef]
- Gebruers, N.; Vanroy, C.; Truijen, S.; Engelborghs, S.; De Deyn, P.P. Monitoring of Physical Activity after Stroke: A Systematic Review of Accelerometry-Based Measures. Arch. Phys. Med. Rehabil. 2010, 91, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Manns, P.J.; Haennel, R.G. SenseWear Armband and Stroke: Validity of Energy Expenditure and Step Count Measurement during Walking. Stroke Res. Treat. 2012, 2012, 247165. [Google Scholar] [CrossRef]
- Mandigout, S.; Lacroix, J.; Ferry, B.; Vuillerme, N.; Compagnat, M.; Daviet, J.-C. Can energy expenditure be accurately assessed using accelerometry-based wearable motion detectors for physical activity monitoring in post-stroke patients in the subacute phase? Eur. J. Prev. Cardiol. 2017, 24, 2009–2016. [Google Scholar] [CrossRef]
- Bassett, D.R.; Troiano, R.P.; McClain, J.J.; Wolff, D.L. Accelerometer-based physical activity: Total volume per day and standardized measures. Med. Sci. Sports Exerc. 2015, 47, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Farrahi, V.; Niemelä, M.; Kangas, M.; Korpelainen, R.; Jämsä, T. Calibration and validation of accelerometer-based activity monitors: A systematic review of machine-learning approaches. Gait Posture 2019, 68, 285–299. [Google Scholar] [CrossRef]
- ActiGraph GT3X+. Available online: http://actigraphcorp.com/support/activity-monitors/gt3xplus/ (accessed on 6 March 2018).
- Rand, D.; Eng, J.J.; Tang, P.-F.; Jeng, J.-S.; Hung, C. How Active Are People With Stroke? Use of Accelerometers to Assess Physical Activity. Stroke 2009, 40, 163–168. [Google Scholar] [CrossRef]
- Podomètre ONstep 400—Geonaute. Available online: https://customercare.geonaute.com/hc/fr/sections/201652082-Podom%C3%A8tre-ONstep-400 (accessed on 12 June 2018).
- Lu, M.-J.; Zhong, W.-H.; Liu, Y.-X.; Miao, H.-Z.; Li, Y.-C.; Ji, M.-H. Sample Size for Assessing Agreement between Two Methods of Measurement by Bland-Altman Method. Int. J. Biostat. 2016, 12. [Google Scholar] [CrossRef]
- Korpan, S.M.; Schafer, J.L.; Wilson, K.C.; Webber, S.C. Effect of ActiGraph GT3X+ Position and Algorithm Choice on Step Count Accuracy in Older Adults. J. Aging Phys. Act. 2015, 23, 377–382. [Google Scholar] [CrossRef]
- Vanroy, C.; Vissers, D.; Cras, P.; Beyne, S.; Feys, H.; Vanlandewijck, Y.; Truijen, S. Physical activity monitoring in stroke: SenseWear Pro2 Activity accelerometer versus Yamax Digi-Walker SW-200 Pedometer. Disabil. Rehabil. 2013, 20, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Hallsworth, K.; Bluck, L.J.C.; Ford, G.A.; Rochester, L.; Trenell, M.I. Measuring Energy Expenditure After Stroke Validation of a Portable Device. Stroke 2012, 43, 1660–1662. [Google Scholar] [CrossRef] [PubMed]

| MEAN / MEDIAN | SD | MIN | MAX | |
|---|---|---|---|---|
| AGE (YEAR) | 64.60 | 14.80 | 34 | 88 |
| BMI (KG∙M−2) | 26.70 | 5.50 | 20 | 43 |
| TIME AFTER STROKE (DAYS) | 781 | 1492 | 9 | 5110 |
| DEMEURISSE UPPER LIMB SCORE (/100) | 68 | 1 | 100 | |
| DEMEURISSE LOWER LIMB SCORE (/100) | 77 | 43 | 100 | |
| MAS (/5) | 1 | 0 | 4 | |
| BARTHEL INDEX (/100) | 74 | 40 | 100 | |
| FACM (/8) | 5 | 4 | 8 | |
| SPEED (MS−1) | 0.56 | 0.30 | 0.06 | 1.22 |
| Pedometer Hip | Actigraph Ankle nH | Pedometer Chest | Actigraph Ankle H | Actigraph Wrist nH | Actigraph Hip | Actigraph Wrist H | Armband H | Armband nH | |
|---|---|---|---|---|---|---|---|---|---|
| Mean step count (step) | 514 | 410 | 406 | 387 | 237 | 221 | 212 | 195 | 170 |
| SD step count (step) | 251 | 188 | 295 | 216 | 166 | 235 | 161 | 249 | 196 |
| Mean Bias (m) | Percentage Difference (%) | 95% LoA Up (m) | 95% LoA Down (m) | Percentage 95%LoA (%) | r | p | RMSE (m) | Percentage RMSE (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Distance Actigraph Ankle nH | 22.58 | 10.70% | 87.45 | −42.29 | 30.80% | 0.95 | <0.001 | 30.79 | 14.60% |
| Distance Actigraph Ankle H | 32.50 | 15.40% | 111.39 | −46.38 | 37.40% | 0.93 | <0.001 | 40.20 | 19.00% |
| Distance Actigraph Hip | 101.78 | 48.30% | 222.37 | −18.81 | 57.20% | 0.86 | <0.001 | 62.28 | 29.50% |
| Distance Actigraph Wrist nH | 97.55 | 46.30% | 228.04 | −32.93 | 61.90% | 0.79 | <0.001 | 55.08 | 26.10% |
| Distance Actigraph Wrist H | 110.04 | 52.20% | 237.47 | −17.39 | 60.50% | 0.81 | <0.001 | 49.24 | 23.30% |
| Distance Armband nH | 127.26 | 60.40% | 286.80 | −32.28 | 75.70% | 0.68 | <0.001 | 65.92 | 31.30% |
| Distance Armband H | 120.62 | 57.20% | 288.25 | −47.01 | 79.60% | 0.72 | <0.001 | 83.01 | 39.40% |
| Distance Pedometer Chest | 27.20 | 12.90% | 156.42 | −102.02 | 61.30% | 0.91 | <0.001 | 61.67 | 29.20% |
| Distance Pedometer Hip | −20.51 | −9.70% | 28.68 | −69.70 | 23.30% | 0.98 | <0.001 | 23.12 | 10.90% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Compagnat, M.; Batcho, C.S.; David, R.; Vuillerme, N.; Salle, J.Y.; Daviet, J.C.; Mandigout, S. Validity of the Walked Distance Estimated by Wearable Devices in Stroke Individuals. Sensors 2019, 19, 2497. https://doi.org/10.3390/s19112497
Compagnat M, Batcho CS, David R, Vuillerme N, Salle JY, Daviet JC, Mandigout S. Validity of the Walked Distance Estimated by Wearable Devices in Stroke Individuals. Sensors. 2019; 19(11):2497. https://doi.org/10.3390/s19112497
Chicago/Turabian StyleCompagnat, Maxence, Charles Sebiyo Batcho, Romain David, Nicolas Vuillerme, Jean Yves Salle, Jean Christophe Daviet, and Stéphane Mandigout. 2019. "Validity of the Walked Distance Estimated by Wearable Devices in Stroke Individuals" Sensors 19, no. 11: 2497. https://doi.org/10.3390/s19112497
APA StyleCompagnat, M., Batcho, C. S., David, R., Vuillerme, N., Salle, J. Y., Daviet, J. C., & Mandigout, S. (2019). Validity of the Walked Distance Estimated by Wearable Devices in Stroke Individuals. Sensors, 19(11), 2497. https://doi.org/10.3390/s19112497






