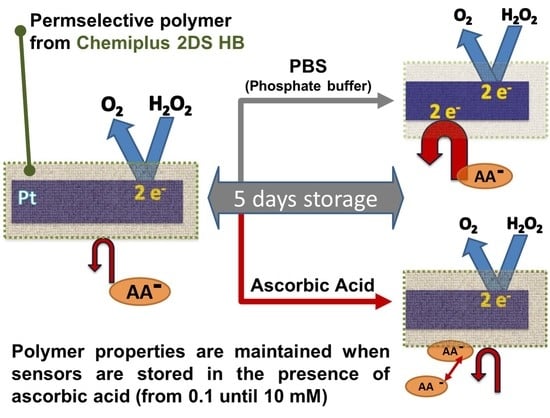
A Storage-Dependent Platinum Functionalization with a Commercial Pre-Polymer Useful for Hydrogen Peroxide and Ascorbic Acid Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Electrochemical Setup and Sensors Construction
2.3. Statistical Analysis
3. Results and Discussion
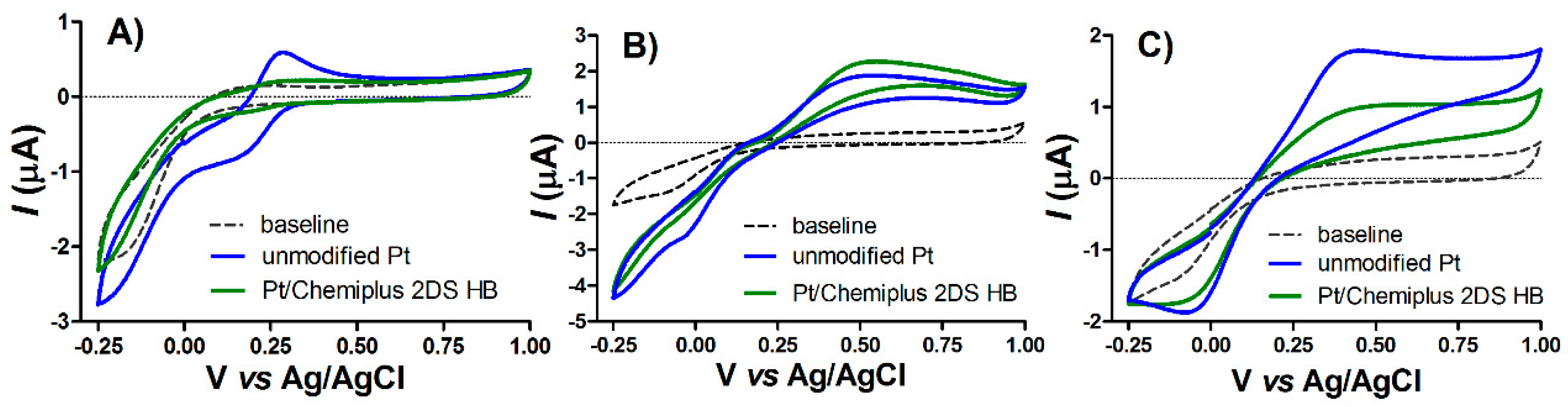
3.1. Voltammetric Characterization
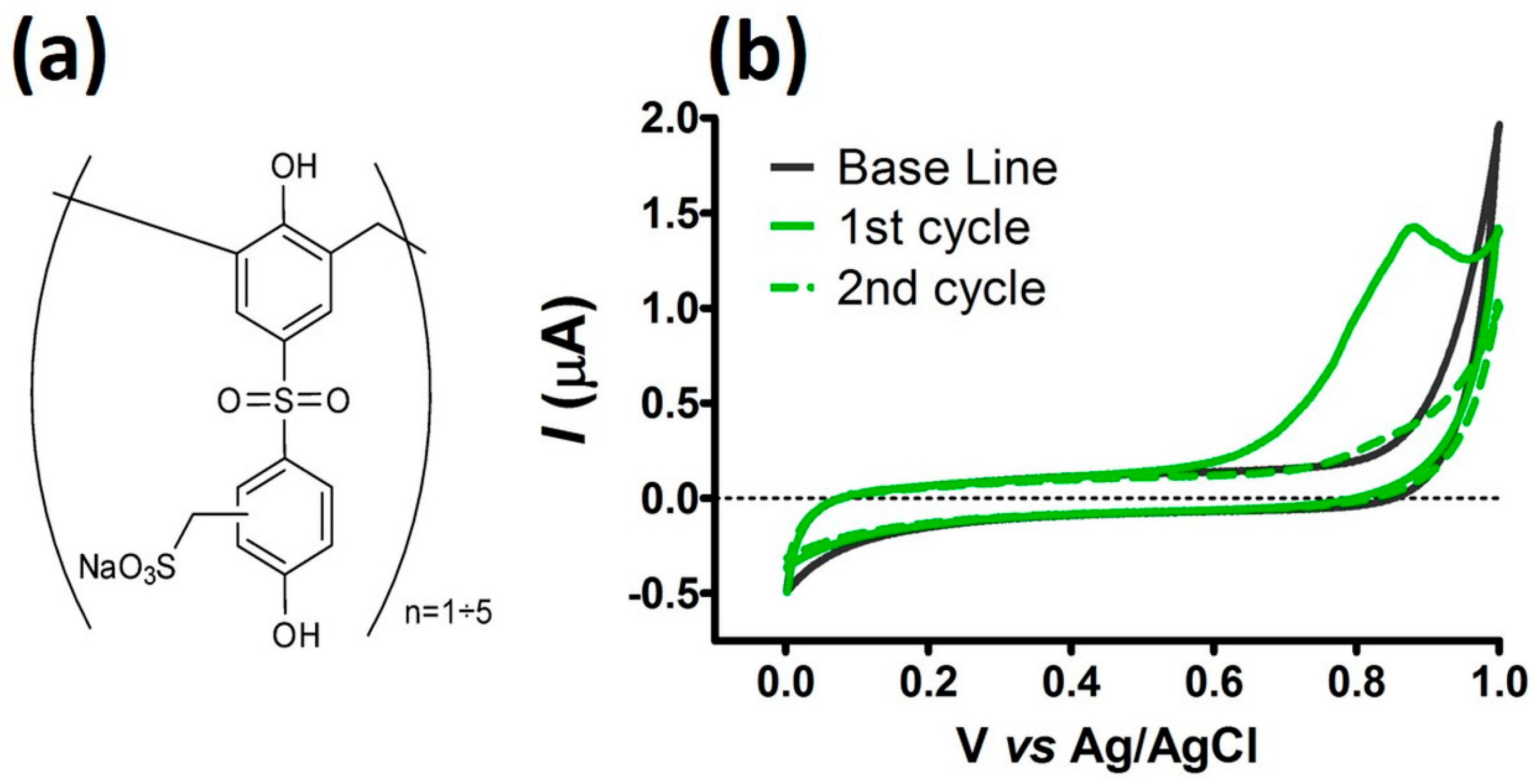
3.2. Surface Modification after CPA Treatment with Chemiplus 2DS HB
3.3. Storage Effect Characterization
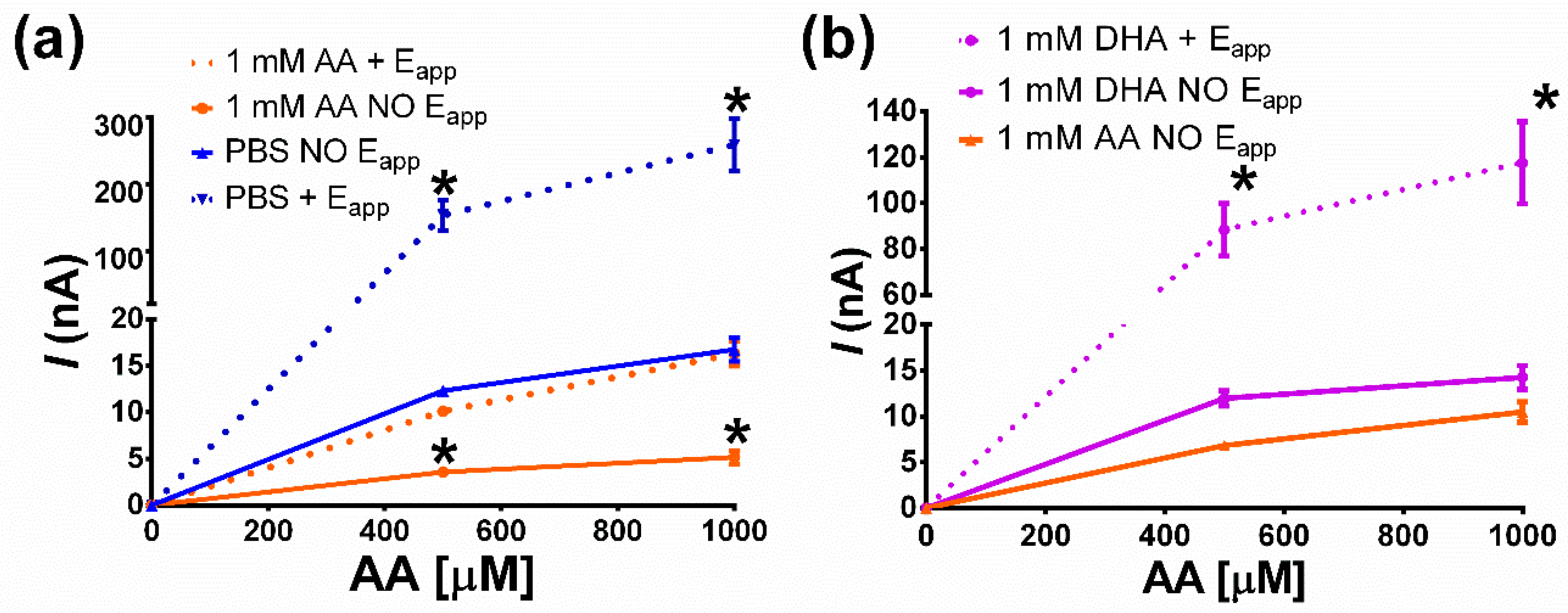
3.3.1. Storage Effect vs. AA Rejection: Under Constant Potential Applied
3.3.2. Storage Effect vs. AA Rejection: Constant Potential, AA, and DHA Experiments
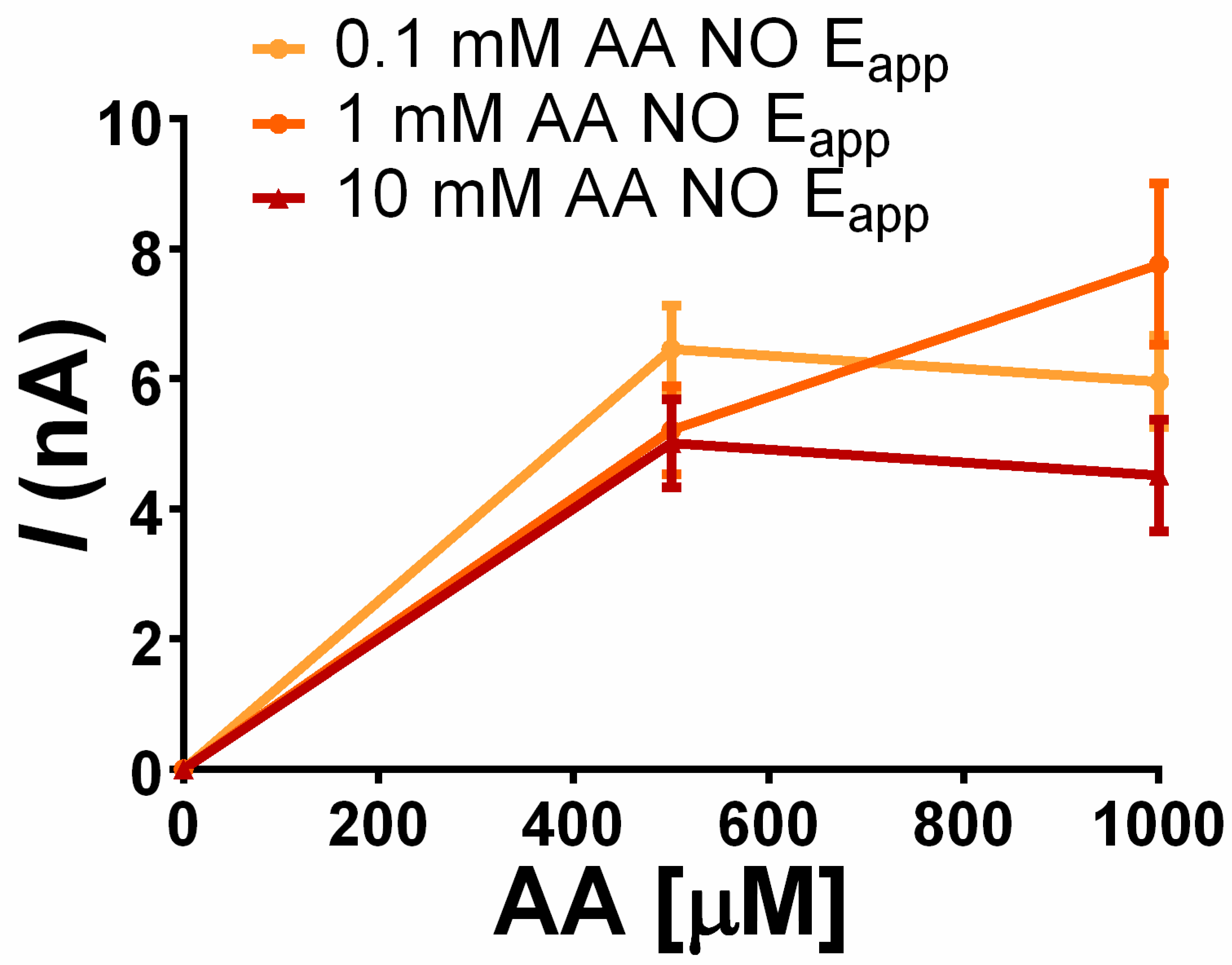
3.3.3. Storage Effect vs. AA Rejection: AA Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radical Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Davey, M.W.; Van Montagu, M.; Inze, D.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Review Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 860, 825–860. [Google Scholar] [CrossRef]
- Varvara, M.; Bozzo, G.; Disanto, C.; Pagliarone, C.N.; Celano, G.V. The use of the ascorbic acid as food additive and technical-legal issues. Ital. J. Food Saf. 2016, 5, 4313. [Google Scholar] [CrossRef]
- Camarena, V.; Wang, G. The epigenetic role of vitamin C in health and disease. Cell. Mol. Life Sci. 2016, 73, 1645–1658. [Google Scholar] [CrossRef]
- Nakai, S.; Nakai, A.; Michida, T. Microencapsulation of Ascorbic Acid for Cosmetic by Utilizing Self-assembly of Phase Separated Polymer. Chem. Pharm. Bull. 2016, 64, 1514–1518. [Google Scholar] [CrossRef][Green Version]
- Osman, N.I.; Roman, S.; Bullock, A.J.; Chapple, C.R.; Macneil, S. The effect of ascorbic acid and fluid flow stimulation on the mechanical properties of a tissue engineered pelvic floor repair material. Proc. Inst. Mech. Eng. Part H. 2014, 228, 867–875. [Google Scholar] [CrossRef]
- Özcan, L.; Sahin, M.; Sahin, Y. Electrochemical preparation of a molecularly imprinted polypyrrole-modified pencil graphite electrode for determination of ascorbic acid. Sensors 2008, 8, 5792–5805. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Fafaneata, C. Electrochemical methods for ascorbic acid determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Barberis, A.; Spissu, Y.; Fadda, A.; Azara, E.; Bazzu, G.; Marceddu, S.; Angioni, A.; Sanna, D.; Schirra, M.; Serra, P.A. Simultaneous amperometric detection of ascorbic acid and antioxidant capacity in orange, blueberry and kiwi juice, by a telemetric system coupled with a fullerene- or nanotubes-modified ascorbate subtractive biosensor. Biosens. Bioelectron. 2015, 67, 214–223. [Google Scholar] [CrossRef]
- Civit, L.; Nassef, H.M.; Fragoso, A.; O’Sullivan, C.K. Amperometric determination of ascorbic acid in real samples using a disposable screen-printed electrode modified with electrografted o-aminophenol film. J. Agric. Food Chem. 2008, 56, 10452–10455. [Google Scholar] [CrossRef]
- Cosnier, S.; Holzinger, M. Electrosynthesized polymers for biosensing. Chem. Soc. Rev. 2011, 40, 2146–2156. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Jafari-Asl, M.; Rezaei, B. A novel enzyme-free amperometric sensor for hydrogen peroxide based on Nafion/exfoliated graphene oxide—Co3O4 nanocomposite. Talanta 2013, 103, 322–329. [Google Scholar] [CrossRef]
- Ciobanu, M.; Marin, L.; Cozan, V.; Bruma, M. Aromatic polysulfones used in sensor applications. Rev. Adv. Mater. Sci. 2009, 22, 89–96. [Google Scholar]
- Telipan, G.; Ignat, M.; Cozan, V. Use of the polysulfone polymer in NOX detection. J. Optoelectron. Adv. Mater. 2006, 8, 582–584. [Google Scholar]
- Giorno, L.; Drioli, E.; Carvoli, G.; Cassano, A.; Donato, L. Study of an enzyme membrane reactor with immobilized fumarase for production of L-malic acid. Biotechnol. Bioeng. 2001, 72, 77–84. [Google Scholar] [CrossRef]
- Prieto-Simón, B.; Fàbregas, E.; Hart, A. Evaluation of different strategies for the development of amperometric biosensors for L-lactate. Biosens. Bioelectron. 2007, 22, 2663–2668. [Google Scholar] [CrossRef]
- Prieto-Simón, B.; Fàbregas, E. New redox mediator-modified polysulfone composite films for the development of dehydrogenase-based biosensors. Biosens. Bioelectron. 2006, 22, 131–137. [Google Scholar] [CrossRef]
- Rubinger, C.P.L.; Calado, H.D.R.; Rubinger, R.M.; Oliveira, H.; Donnici, C.L. Characterization of a sulfonated polycarbonate resistive humidity sensor. Sensors 2013, 13, 2023–2032. [Google Scholar] [CrossRef]
- Benmakrohaa, Y.; Christieb, I.; Desaib, M.; Vadgamab, P. Poly(vinyl chloride), polysulfone and sulfonated polyether-ether sulfone composite membranes for glucose and hydrogen peroxide perm-selectivity in amperometric biosensors. Analyst 1996, 121, 521–526. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Ghorbani, S.; Shockravi, A.; Mansourpanah, Y. The influence of sulfonated polyethersulfone (SPES) on surface nano-morphology and performance of polyethersulfone (PES) membrane. Appl. Surf. Sci. 2010, 256, 1825–1831. [Google Scholar] [CrossRef]
- Zheng, K.; Zhou, S.; Zhou, X. A low-cost and high-performance thin-film composite forward osmosis membrane based on an SPSU/PVC substrate. Sci. Rep. 2018, 8, 10022. [Google Scholar] [CrossRef]
- Kite, M.; Thomson, T. The chemist of tanning materials. In Conservation of Leather and Related Materials, 3rd ed.; Butterworth-Heinemann: Oxford, UK; Elsevier: Oxford, UK, 2006; pp. 22–35. [Google Scholar]
- Sammarco, U. Concia con sostanze organiche sintetiche. In Tecnologia Conciaria, 1st ed.; Editma: Rescaldina, Italy, 2007; pp. 186–455. [Google Scholar]
- Covington, A.D. Modern tanning chemistry. Chem. Soc. Rev. 1997, 26, 111–126. [Google Scholar] [CrossRef]
- Russell, A.; Copenhaver, J.W. Tanning Material. U.S. Patent 2,171,806, 5 September 1939. [Google Scholar]
- Orem, H.P. Process for Preparing 4,4′-Dihydroxy-Diphenyl Sulfone. U.S. Patent 3,366,692, 30 January 1968. [Google Scholar]
- Hsu, K.C.; Lee, Y.F. Water-soluble sulfonated phenolic resins. I. Synthesis. J. Appl. Polym. Sci. 1995, 57, 1501–1509. [Google Scholar] [CrossRef]
- Rezaei, B.; Damiri, S. Voltammetric behavior of multi-walled carbon nanotubes modified electrode-hexacyanoferrate(II) electrocatalyst system as a sensor for determination of captopril. Sens. Actuators B Chem. 2008, 134, 324–331. [Google Scholar] [CrossRef]
- Monti, P.; Calia, G.; Marceddu, S.; Dettori, M.A.; Fabbri, D.; Jaoua, S.; O’Neill, R.D.; Migheli, Q.; Delogu, G.; Serra, P.A. Low electro-synthesis potentials improve permselectivity of polymerized natural phenols in biosensor applications. Talanta 2017, 162, 151–158. [Google Scholar] [CrossRef]
- ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Available online: https://www.gmp-compliance.org/guidelines/gmp-guideline/ich-q2r1-validation-of-analytical-procedures-text-and-methodology (accessed on 27 May 2019).
- Kuramitz, H.; Matsushita, M.; Tanaka, S. Electrochemical removal of bisphenol A based on the anodic polymerization using a column type carbon fiber electrode. Water Res. 2004, 38, 2331–2338. [Google Scholar] [CrossRef]
- Pailleret, A.; Magan-Oliva, N.; Ollivier, S.; Arrigan, D.W.M. Electrochemical oxidation of a hexasulfonated calix[6]arene. J. Electroanal. Chem. 2001, 508, 81–88. [Google Scholar] [CrossRef]
- Calia, G.; Monti, P.; Marceddu, S.; Dettori, M.A.; Fabbri, D.; Jaoua, S.; O’Neill, R.D.; Serra, P.A.; Delogu, G.; Migheli, Q. Electropolymerized phenol derivatives as permselective polymers for biosensor applications. Analyst 2015, 140, 3607–3615. [Google Scholar] [CrossRef]
- Ernst, H.; Knoll, M. Electrochemical characterisation of uric acid and ascorbic acid at a platinum electrode. Anal. Chim. Acta 2001, 449, 129–134. [Google Scholar] [CrossRef]
- La, D.D.; Anuradha, A.; Hundal, A.K.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. pH-Dependent self-assembly of water-soluble sulfonate-tetraphenylethylene with aggregation-induced emission. Supramol. Chem. 2018, 30, 1–8. [Google Scholar] [CrossRef]
- Březina, M.; Koryta, J.; Loučka, T.; Maršíková, D.; Pradáč, J. Adsorption and kinetics of oxidation of ascorbic acid at platinum electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1972, 40, 13–17. [Google Scholar] [CrossRef]
- Hess, S.C.; Grass, R.N.; Stark, W.J. MOF Channels within porous polymer film: Flexible, self-supporting ZIF-8 Poly(ether sulfone) composite membrane. Chem. Mater. 2016, 28, 7638–7644. [Google Scholar] [CrossRef]
- Mahmoudifard, M.; Soudi, S.; Soleimani, M.; Hosseinzadeh, S.; Esmaeili, E.; Vossoughi, M. Efficient protein immobilization on polyethersolfone electrospun nanofibrous membrane via covalent binding for biosensing applications. Mater. Sci. Eng. C 2016, 58, 586–594. [Google Scholar] [CrossRef]
- van den Hurk, R.; Evoy, S. A review of membrane-based biosensors for pathogen detection. Sensors 2015, 15, 14045–14078. [Google Scholar] [CrossRef]
- Ayyaru, S.; Dharmalingam, S. Enhanced response of microbial fuel cell using sulfonated poly ether ether ketone membrane as a biochemical oxygen demand sensor. Anal. Chim. Acta 2014, 818, 15–22. [Google Scholar] [CrossRef]
- Poźniak, G.; Krajewska, B.; Trochimczuk, W. Urease immobilized on modified polysulphone membrane: Preparation and properties. Biomaterials 1995, 16, 129–134. [Google Scholar] [CrossRef]
- Karabinas, P.; Jannakoudakis, D. Kinetic parameters and mechanism of the electrochemical oxidation of L-ascorbic acid on platinum electrodes in acid solutions. J. Electroanal. Chem. Interfacial Electrochem. 1984, 160, 159–167. [Google Scholar] [CrossRef]

| H2O2 Behavior | ||||||||||
| Storage Condition Group: | Bare Pt (Day 0) | After Construction (Day 0) | After Storage (Day 7) | |||||||
| Slope (nA µM−1) | R2 | Slope (nA µM−1) | R2 | LOD (µM l−1) | LOQ (µM l−1) | Slope (nA µM−1) | R2 | LOD (µM l−1) | LOQ (µM l−1) | |
| Stored in 1 mM AA | 0.83 ± 0.03 | 0.99 | 0.52 ± 0.01 | 0.99 | 0.19 ± 0.03 | 0.65 ± 0.07 | 0.15 ± 0.02 | 0.99 | 0.55 ± 0.06 | 1.85 ± 0.31 |
| Stored in PBS | 0.76 ± 0.03 | 0.99 | 0.51 ± 0.02 | 0.99 | 0.17 ± 0.03 | 0.56 ± 0.06 | 0.94 ± 0.21 | 0.98 | 0.06 ± 0.01 | 0.20 ± 0.04 |
| AA Behaviour | ||||||||||
| Storage Condition Group: | Bare Pt (Day 0) | After Construction (Day 0) | After Storage (Day 7) | |||||||
| ΔI (nA) | R2 | ΔI (nA) | R2 | ΔI (nA) | R2 | |||||
| Stored in 1 mM AA | 185 ± 5 | 0.99 | 6.10 ± 2.93 | - | 8.55 ± 4.46 | - | ||||
| Stored in PBS | 182 ± 10 | 0.99 | 2.25 ± 0.53 | - | 99.3 ± 23.7 | - | ||||
| Sensors Set Name (n = 4) | Storage Solution | Constant Potential Applied (+700 mV vs. Ag/AgCl) |
|---|---|---|
| PBS + Eapp | Phosphate buffer (PBS) | YES |
| PBS NO Eapp | Phosphate buffer (PBS) | NO |
| 1 mM AA + Eapp | PBS + 1 mM Ascorbic Acid | YES |
| 10 mM AA NO Eapp | PBS + 10 mM Ascorbic Acid | NO |
| 1 mM AA NO Eapp | PBS + 1 mM Ascorbic Acid | NO |
| 0.1 mM AA NO Eapp | PBS + 0.1 mM Ascorbic Acid | NO |
| 1 mM DHA + Eapp | PBS + 1 mM Dehydroascorbic Acid | YES |
| 1 mM DHA NO Eapp | PBS + 1 mM Dehydroascorbic Acid | NO |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monti, P.; Migheli, Q.; Bartiromo, A.R.; Pauciulo, A.; Gliubizzi, R.; Marceddu, S.; Serra, P.A.; Delogu, G. A Storage-Dependent Platinum Functionalization with a Commercial Pre-Polymer Useful for Hydrogen Peroxide and Ascorbic Acid Detection. Sensors 2019, 19, 2435. https://doi.org/10.3390/s19112435
Monti P, Migheli Q, Bartiromo AR, Pauciulo A, Gliubizzi R, Marceddu S, Serra PA, Delogu G. A Storage-Dependent Platinum Functionalization with a Commercial Pre-Polymer Useful for Hydrogen Peroxide and Ascorbic Acid Detection. Sensors. 2019; 19(11):2435. https://doi.org/10.3390/s19112435
Chicago/Turabian StyleMonti, Patrizia, Quirico Migheli, Andrea R. Bartiromo, Antonio Pauciulo, Rocco Gliubizzi, Salvatore Marceddu, Pier A. Serra, and Giovanna Delogu. 2019. "A Storage-Dependent Platinum Functionalization with a Commercial Pre-Polymer Useful for Hydrogen Peroxide and Ascorbic Acid Detection" Sensors 19, no. 11: 2435. https://doi.org/10.3390/s19112435
APA StyleMonti, P., Migheli, Q., Bartiromo, A. R., Pauciulo, A., Gliubizzi, R., Marceddu, S., Serra, P. A., & Delogu, G. (2019). A Storage-Dependent Platinum Functionalization with a Commercial Pre-Polymer Useful for Hydrogen Peroxide and Ascorbic Acid Detection. Sensors, 19(11), 2435. https://doi.org/10.3390/s19112435