Fingerprinting of Nitroaromatic Explosives Realized by Aphen-functionalized Titanium Dioxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Reagents and Equipment
2.2. Preparation of Aphen-functionalized Titanium Dioxide Nanosphere
2.2.1. Preparation of 5-amino-1,10-phenanthroline (Aphen)
2.2.2. Preparation of TiO2 Nanocrastals
2.2.3. Preparation of Aphen-functionalized TiO2 Nanocrystals (TiO2/Aphen)
2.3. Preparation of Sensor Chips
2.4. Gas Sensing Properties Testing
3. Results and Discussion
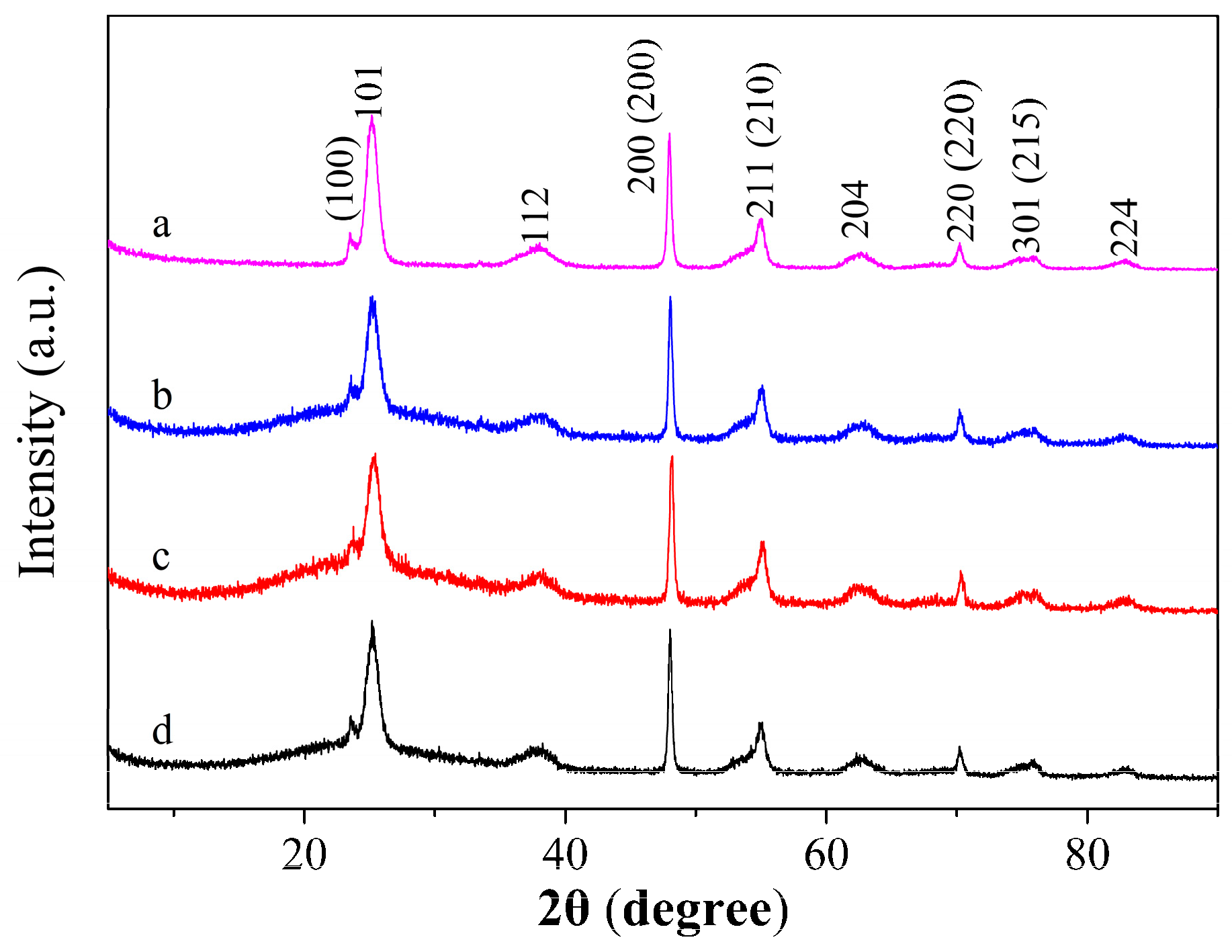
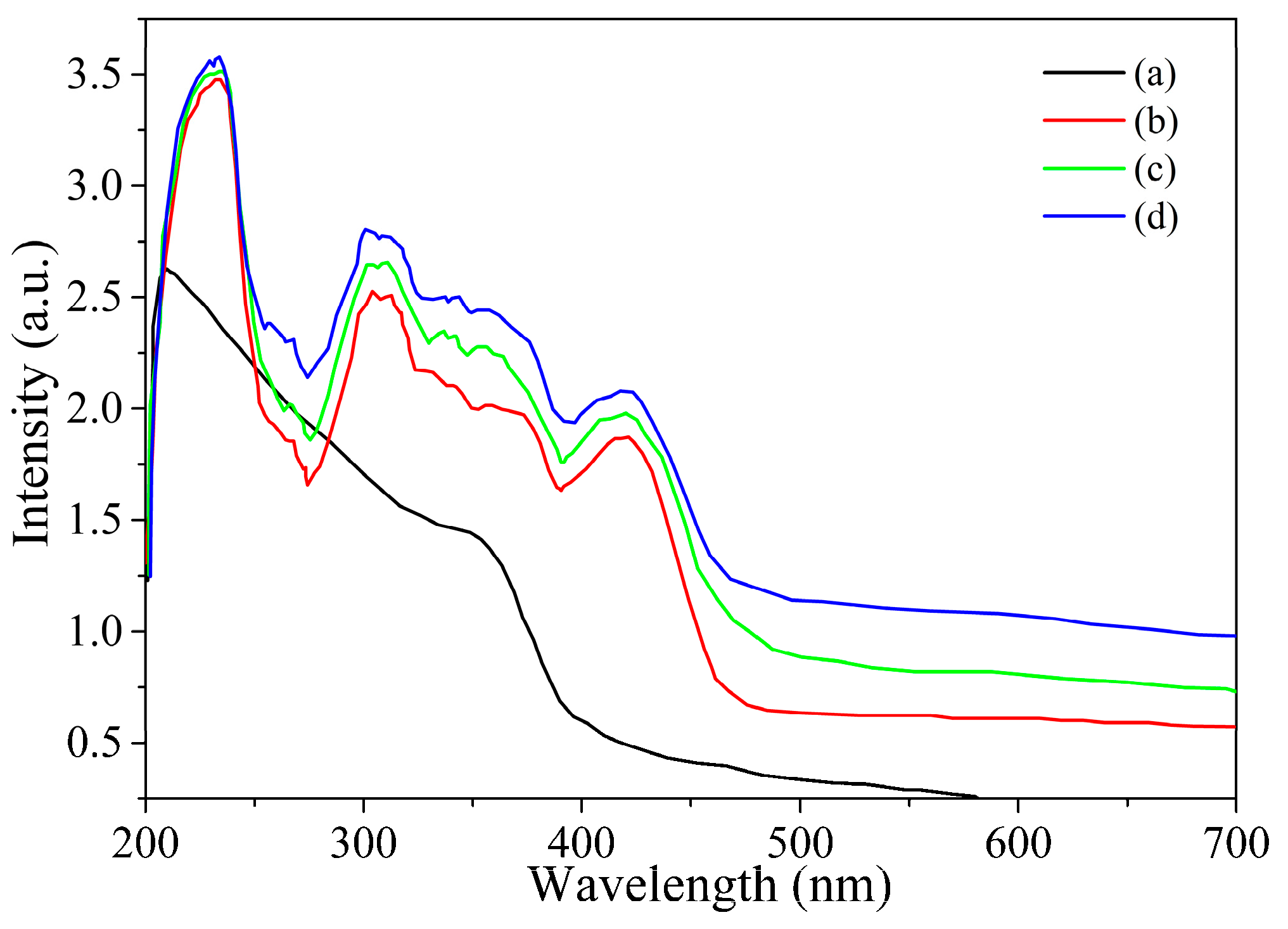
3.1. TiO2@Aphen Morphological and Structural Characterization
3.2. Gas Sensitivity Performance of TiO2@Aphen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brown, K.E.; Greenfield, M.T.; McGrane, S.D.; Moore, D.S. Advances in explosives analysis---part II: Photon and neutron methods. Anal. Bioanal. Chem. 2016, 408, 49–65. [Google Scholar] [CrossRef]
- Whetstone, Z.D.; Kearfott, K.J. A review of conventional explosives detection using active neutron interrogation. J. Radioanal. Nucl. Chem. 2014, 301, 629–639. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: from mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef]
- Zhang, S.-R.; Du, D.-Y.; Qin, J.-S.; Bao, S.-J.; Li, S.-L.; He, W.-W.; Lan, Y.-Q.; Shen, P.; Su, Z.-M. A Fluorescent Sensor for Highly Selective Detection of Nitroaromatic Explosives Based on a 2D, Extremely Stable, Metal–Organic Framework. Chem. A Eur. J. 2014, 20, 3589–3594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, Y.; Zhu, J.; Zhai, Y.; Dong, S. Simple and Sensitive Fluorescent and Electrochemical Trinitrotoluene Sensors Based on Aqueous Carbon Dots. Anal. Chem. 2015, 87, 2033–2036. [Google Scholar] [CrossRef] [PubMed]
- Sisco, E.; Forbes, T.P. Rapid detection of sugar alcohol precursors and corresponding nitrate ester explosives using direct analysis in real time mass spectrometry. Analyst 2015, 140, 2785–2796. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, M.; Nousiainen, M.; Sillanpää, M. Ion spectrometric detection technologies for ultra-traces of explosives: A review. Mass Spectrom. Rev. 2011, 30, 940–973. [Google Scholar] [CrossRef]
- Schramm, S.; Léonço, D.; Hubert, C.; Tabet, J.-C.; Bridoux, M. Development and validation of an isotope dilution ultra-high performance liquid chromatography tandem mass spectrometry method for the reliable quantification of 1,3,5-Triamino-2,4,6-trinitrobenzene (TATB) and 14 other explosives and their degradation products in environmental water samples. Talanta 2015, 143, 271–278. [Google Scholar]
- Schnorr, J.M.; van der Zwaag, D.; Walish, J.J.; Weizmann, Y.; Swager, T.M. Sensory Arrays of Covalently Functionalized Single-Walled Carbon Nanotubes for Explosive Detection. Adv. Funct. Mater. 2013, 23, 5285–5291. [Google Scholar] [CrossRef]
- Zheng, Y.; Xincun, D.; Shengli, Z.; Linjuan, G.; Baiyi, Z.; Zhaofeng, W.; Haibo, Z. A High-Performance Nitro-Explosives Schottky Sensor Boosted by Interface Modulation. Adv. Funct. Mater. 2015, 25, 4039–4048. [Google Scholar]
- Qu, J.; Ge, Y.; Zu, B.; Li, Y.; Dou, X. Transition-Metal-Doped p-Type ZnO Nanoparticle-Based Sensory Array for Instant Discrimination of Explosive Vapors. Small 2016, 12, 1369–1377. [Google Scholar] [CrossRef]
- Zhao, J.; Li, N.; Yu, H.; Wei, Z.; Liao, M.; Chen, P.; Wang, S.; Shi, D.; Sun, Q.; Zhang, G. Highly Sensitive MoS2 Humidity Sensors Array for Noncontact Sensation. Adv. Mater. 2017, 29, 1702076. [Google Scholar] [CrossRef]
- Lü, X.; Hao, P.; Xie, G.; Duan, J.; Gao, L.; Liu, B. A Sensor Array Realized by a Single Flexible TiO2/POMs Film to Contactless Detection of Triacetone Triperoxide. Sensors 2019, 19, 915. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Yu, L.; Lou, X.W. (David) Rational Design of Three-Layered TiO2@Carbon@MoS2 Hierarchical Nanotubes for Enhanced Lithium Storage. Adv. Mater. 2017, 29, 1702724. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Correction: Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xu, Y.; Tian, B.; Lei, J.; Zhang, J.; Wang, L. Operando SERS self-monitoring photocatalytic oxidation of aminophenol on TiO2 semiconductor. Appl. Catal. B Environ. 2018, 224, 305–309. [Google Scholar] [CrossRef]
- Yoshihara, T.; Murayama, S.; Masuda, T.; Kikuchi, T.; Yoshida, K.; Hosaka, M.; Tobita, S. Mitochondria-targeted oxygen probes based on cationic iridium complexes with a 5-amino-1, 10-phenanthroline ligand. J. Photochem. Photobiol. A Chem. 2015, 299, 172–182. [Google Scholar] [CrossRef]
- Shervedani, R.K.; Foroushani, M.S.; Dehaghi, S.B. Functionalization of gold mercaptopropionic acid self-assembled monolayer with 5-amino-1,10-phenanthroline: Interaction with iron(II) and application for selective recognition of guanine. Electrochim. Acta 2015, 164, 344–352. [Google Scholar] [CrossRef]
- Feist, B.; Pilch, M.; Nycz, J. Graphene oxide chemically modified with 5-amino-1,10-phenanthroline as sorbent for separation and preconcentration of trace amount of lead(II). Microchim. Acta 2019, 186, 91. [Google Scholar] [CrossRef]
- Smith, G.F.; Cagle, F.W. The improved synthesis of 5-nitro-1, 10-phenanthroline. J. Org. Chem. 1947, 12, 781–784. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, J.; Xu, D.; Cheng, B.; Yu, J. Enhanced photocatalytic H2-production activity of anatase TiO2 nanosheet by selectively depositing dual-cocatalysts on {101} and {001} facets. Appl. Catal. B Environ. 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Liu, X.; Dong, G.; Li, S.; Lu, G.; Bi, Y. Direct Observation of Charge Separation on Anatase TiO2 Crystals with Selectively Etched {001} Facets. J. Am. Chem. Soc. 2016, 138, 2917–2920. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-J.; Ye, Z.-J.; Lu, H.-W.; Hu, B.; Li, Y.-H.; Chen, D.-Q.; Zhong, J.-S.; Yu, Z.-T.; Zou, Z.-G. Constructing Anatase TiO2 Nanosheets with Exposed (001) Facets/Layered MoS2 Two-Dimensional Nanojunctions for Enhanced Solar Hydrogen Generation. ACS Catal. 2016, 6, 532–541. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Opra, D.P.; Sinebryukhov, S.L.; Kuryavyi, V.G.; Ustinov, А.Y.; Sergienko, V.I. Structural and electrochemical investigation of nanostructured C: TiO2–TiOF2 composite synthesized in plasma by an original method of pulsed high-voltage discharge. J. Alloys Compd. 2015, 621, 364–370. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, G.; Liu, J.; Wang, Z.; An, C.; Zhang, J. Synthesis of heterostructured Pd@TiO2/TiOF2 nanohybrids with enhanced photocatalytic performance. Mater. Res. Bull. 2016, 80, 337–343. [Google Scholar] [CrossRef]
- Jung, M.-J.; Kim, Y.; Lee, Y.-S. Enhancement of the electrochemical capacitance of TiOF2 obtained via control of the crystal structure. J. Ind. Eng. Chem. 2017, 47, 187–193. [Google Scholar] [CrossRef]
- Gao, J.; Lu, C.; Lu, X. APhen-functionalized nanoparticles—Polymer fluorescent nanocomposites via ligand exchange and in situ bulk polymerization. J. Mater. Chem. 2007, 17, 4591–4597. [Google Scholar] [CrossRef]
- Du, Y.; Lu, C.; Su, Z.; Gao, J.; Fu, Y.; Lu, X. Synthesis and properties of transparent luminescent nanocomposites with surface functionalized semiconductor nanocrystals. J. Solid State Chem. 2008, 181, 2279–2284. [Google Scholar]
- Govindaiah, P.; Lee, J.M.; Jung, Y.J.; Lee, S.J.; Kim, J.H. Tunable fluorescent poly (styrene-co-methacrylic acid)/Au-Aphen hybrid nanoparticles via surface immobilization. J. Mater. Chem 2009, 19, 3529–3537. [Google Scholar] [CrossRef]
- Isaacs, M.; Sykes, A.G.; Ronco, S. Synthesis, characterization and photophysical properties of mixed ligand tris (polypyridyl) chromium (III) complexes, [Cr(phen)2L]3+. Inorganica Chim. Acta 2006, 359, 3847–3854. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, L.-C.; Gong, Y.; Niu, P.; Wang, J.-Q.; Gu, L.; Chen, X.; Liu, G.; Wang, L.; Cheng, H.-M. An Unusual Strong Visible-Light Absorption Band in Red Anatase TiO2 Photocatalyst Induced by Atomic Hydrogen-Occupied Oxygen Vacancies. Adv. Mater. 2018, 30, 1704479. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, G.; Liu, B. Fingerprinting of Nitroaromatic Explosives Realized by Aphen-functionalized Titanium Dioxide. Sensors 2019, 19, 2407. https://doi.org/10.3390/s19102407
Xie G, Liu B. Fingerprinting of Nitroaromatic Explosives Realized by Aphen-functionalized Titanium Dioxide. Sensors. 2019; 19(10):2407. https://doi.org/10.3390/s19102407
Chicago/Turabian StyleXie, Guanshun, and Bingxin Liu. 2019. "Fingerprinting of Nitroaromatic Explosives Realized by Aphen-functionalized Titanium Dioxide" Sensors 19, no. 10: 2407. https://doi.org/10.3390/s19102407
APA StyleXie, G., & Liu, B. (2019). Fingerprinting of Nitroaromatic Explosives Realized by Aphen-functionalized Titanium Dioxide. Sensors, 19(10), 2407. https://doi.org/10.3390/s19102407





