Application of Electric Cell-Substrate Impedance Sensing to Investigate the Cytotoxic Effects of Andrographolide on U-87 MG Glioblastoma Cell Migration and Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Drug Treatment
2.2. Impedance Measurement
2.3. ECIS Wound Healing Assay
2.4. Alamar Blue Cell Viability Test
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
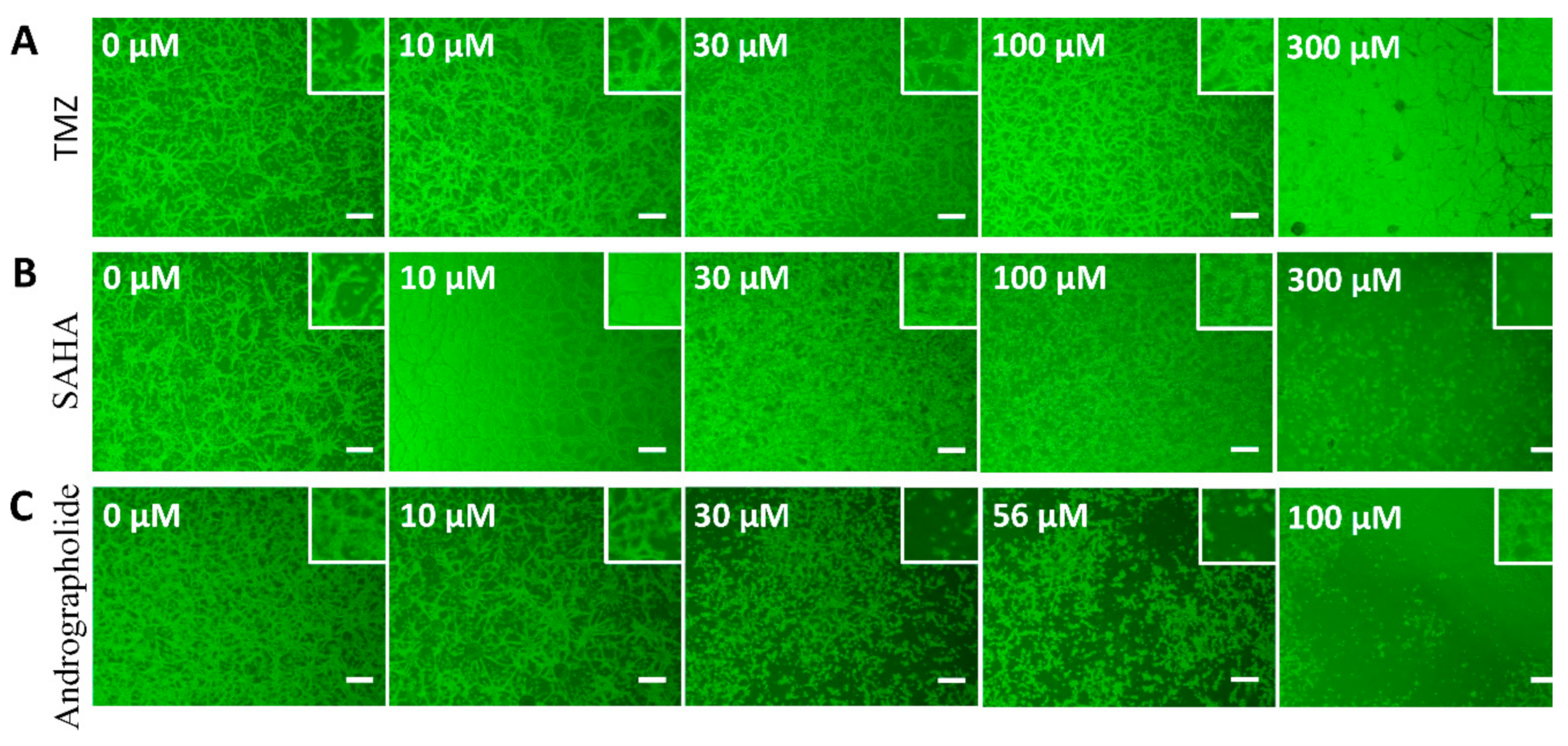
3.1. Cell Morphology
3.2. Cell Viability
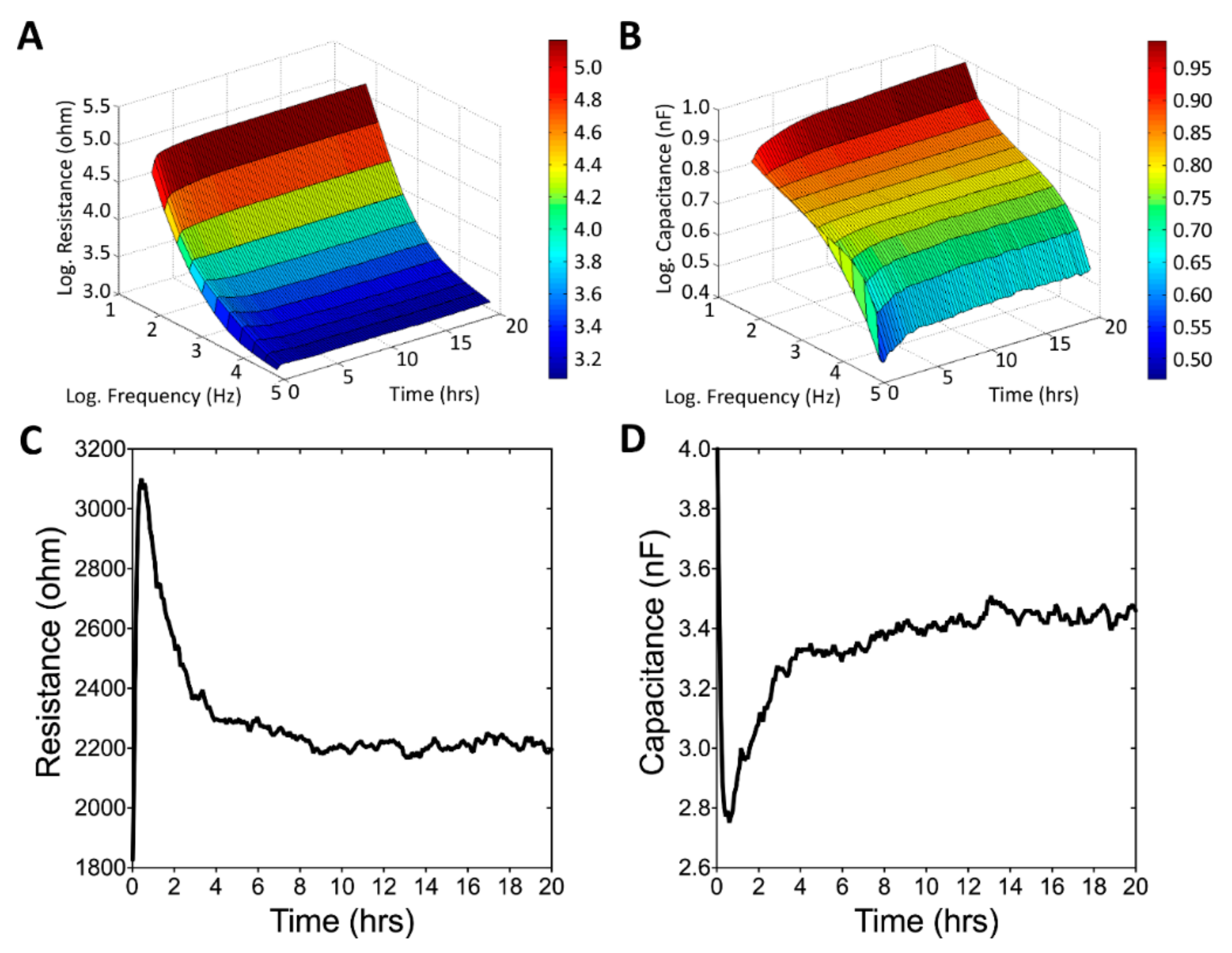
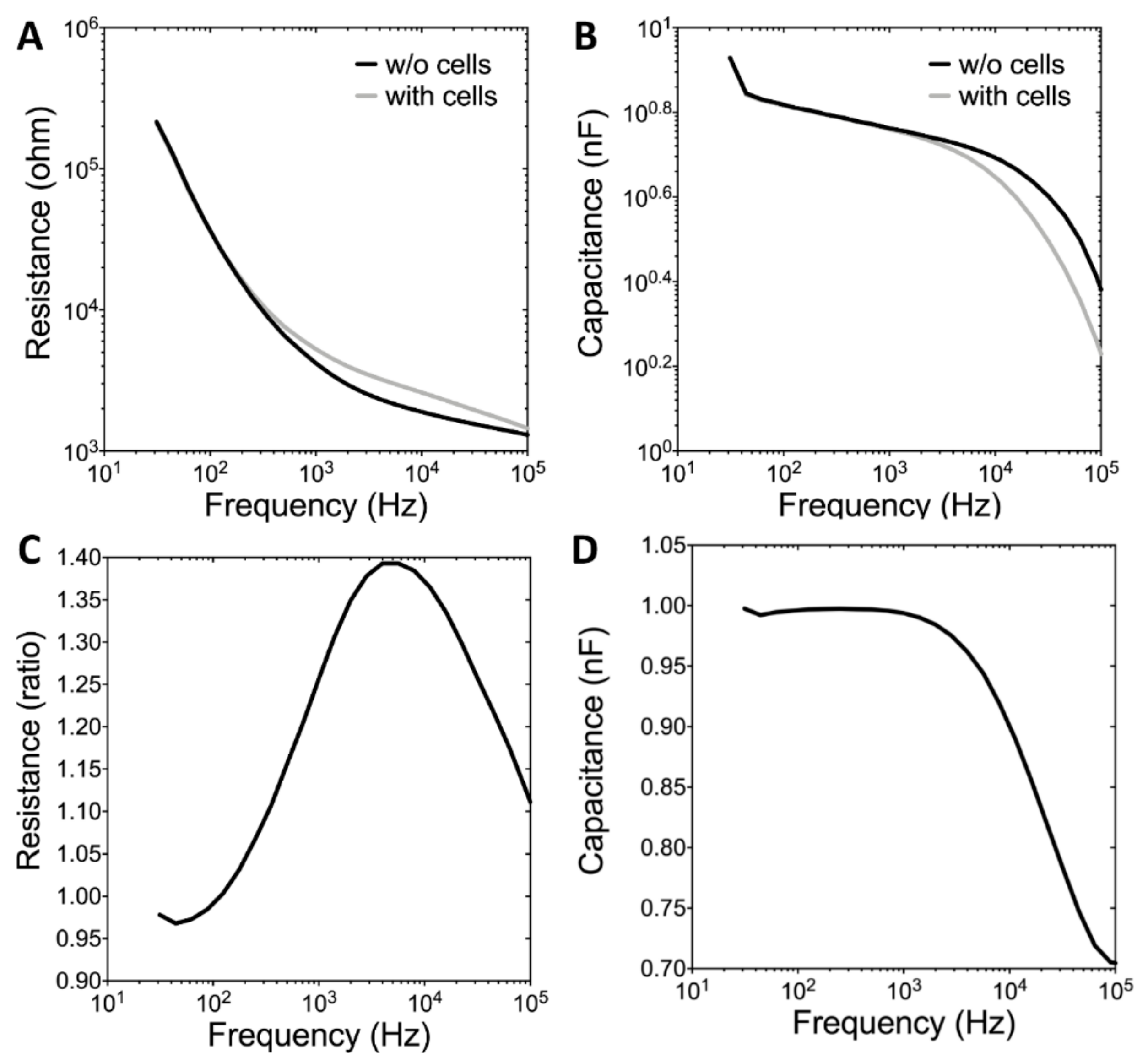
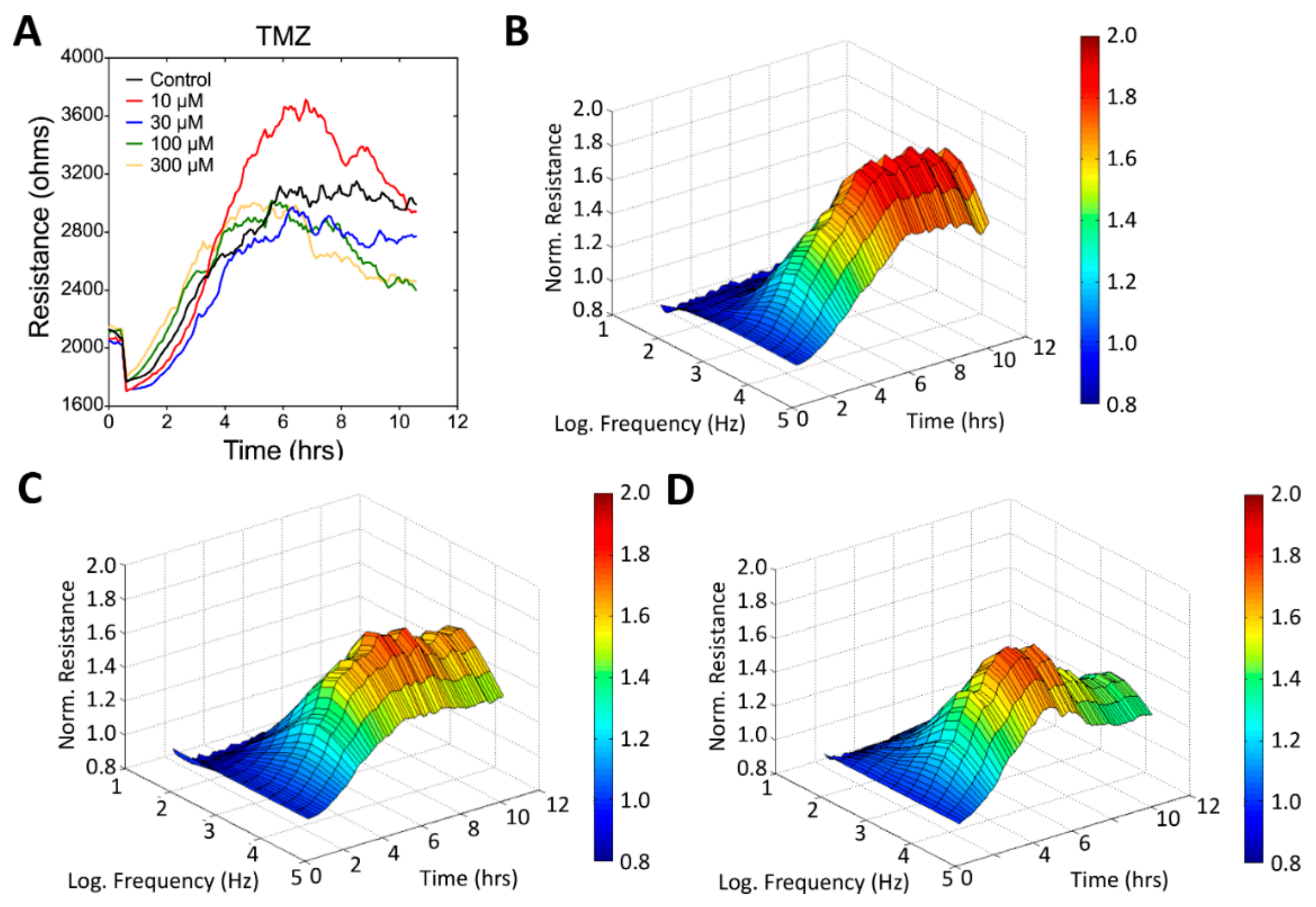
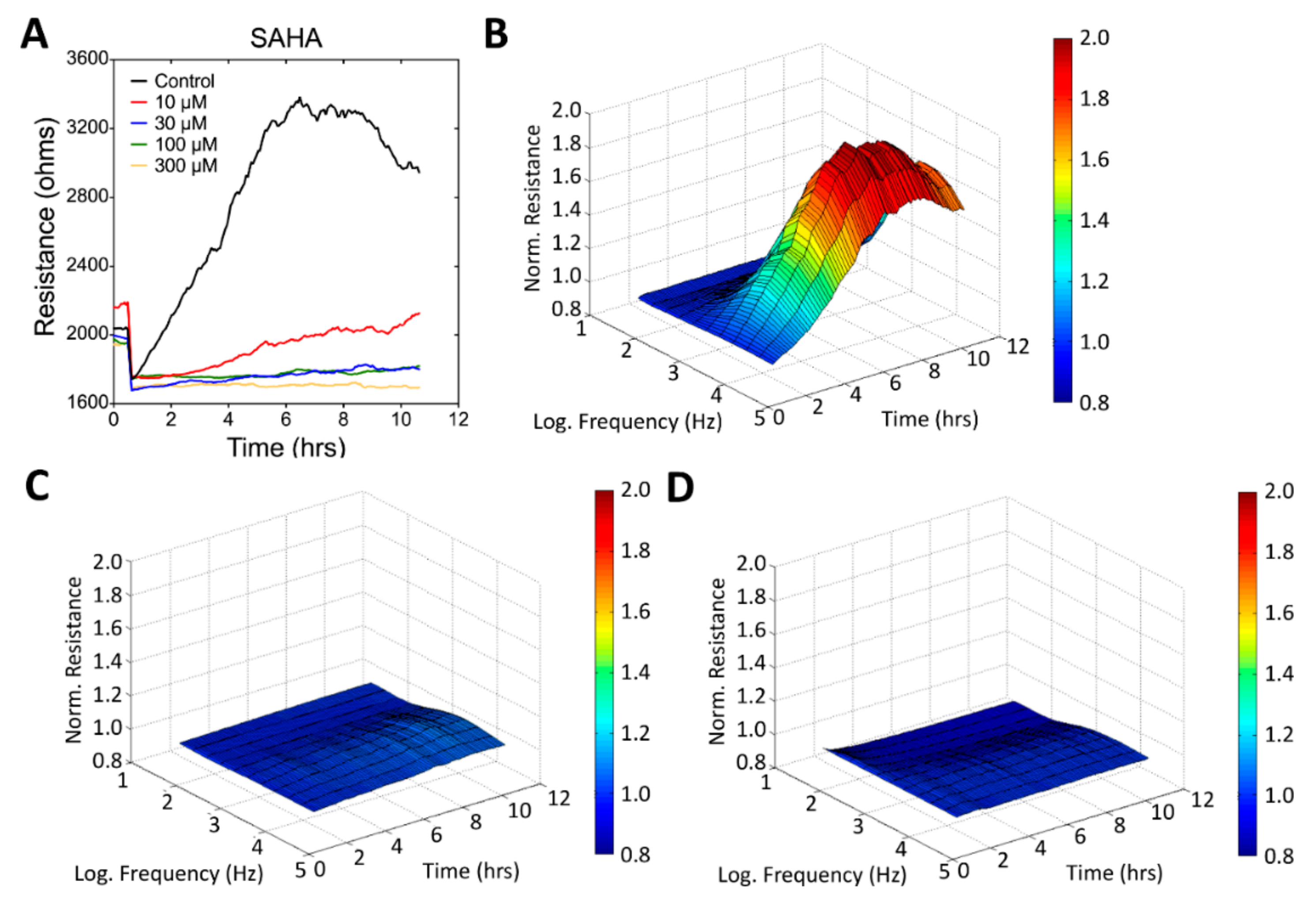
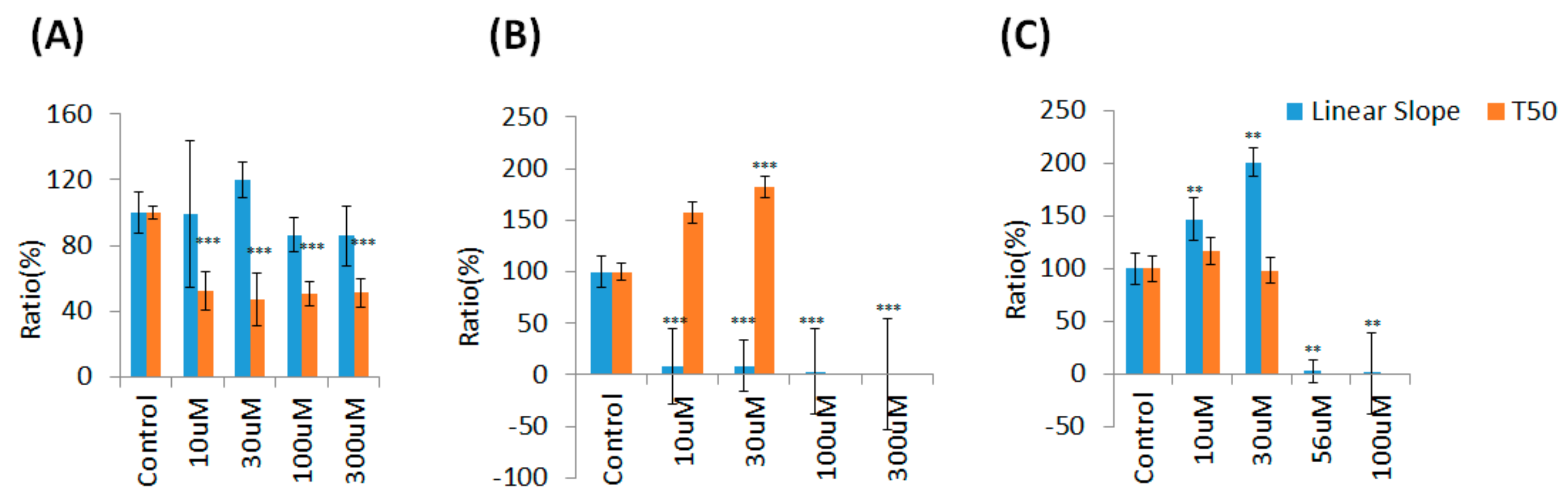
3.3. Real-Time Monitoring of U-87 MG Cell Attachment and Spreading
3.4. Wound Healing and Migration of U-87 MG Cells under Drug Treatment
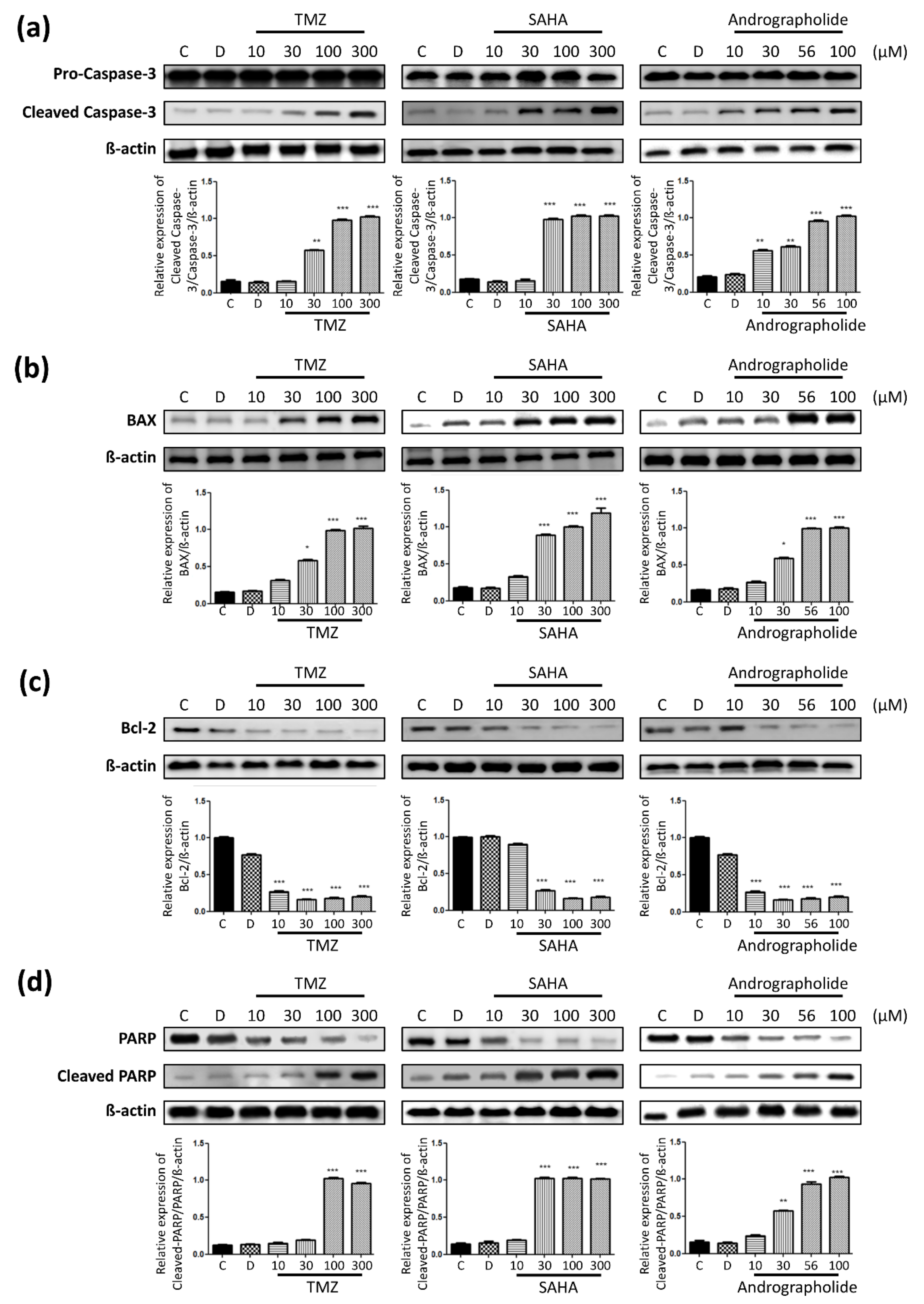
3.5. Apoptosis-Related Protein Expression in Drug-Treated U-87 MG Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rich, J.N.; Bigner, D.D. Development of novel targeted therapies in the treatment of malignant glioma. Nat. Rev. Drug Discov. 2004, 3, 430. [Google Scholar] [CrossRef]
- Holland, E.C. Glioblastoma multiforme: The terminator. Proc. Natl. Acad. Sci. USA 2000, 97, 6242–6244. [Google Scholar] [CrossRef]
- Jain, R.K.; Di Tomaso, E.; Duda, D.G.; Loeffler, J.S.; Sorensen, A.G.; Batchelor, T.T. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 2007, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Ellis, H.P.; Greenslade, M.; Powell, B.; Spiteri, I.; Sottoriva, A.; Kurian, K.M. Current challenges in glioblastoma: Intratumour heterogeneity, residual disease, and models to predict disease recurrence. Front. Oncol. 2015, 5, 251. [Google Scholar] [CrossRef] [PubMed]
- Barbarite, E.; Sick, J.T.; Berchmans, E.; Bregy, A.; Shah, A.H.; Elsayyad, N.; Komotar, R.J. The role of brachytherapy in the treatment of glioblastoma multiforme. Neurosurg. Rev. 2017, 40, 195–211. [Google Scholar] [CrossRef]
- Kim, J.T.; Kim, J.-S.; Ko, K.W.; Kong, D.-S.; Kang, C.-M.; Kim, M.H.; Son, M.J.; Song, H.S.; Shin, H.-J.; Lee, D.-S. Metronomic treatment of temozolomide inhibits tumor cell growth through reduction of angiogenesis and augmentation of apoptosis in orthotopic models of gliomas. Oncol. Rep. 2006, 16, 33–39. [Google Scholar] [CrossRef][Green Version]
- Chamberlain, M.C. Temozolomide: Therapeutic limitations in the treatment of adult high-grade gliomas. Expert Rev. Neurother. 2010, 10, 1537–1544. [Google Scholar] [CrossRef]
- Shi, L.; Chen, J.; Yang, J.; Pan, T.; Zhang, S.; Wang, Z. MiR-21 protected human glioblastoma U87MG cells from chemotherapeutic drug temozolomide induced apoptosis by decreasing Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 2010, 1352, 255–264. [Google Scholar] [CrossRef]
- Venur, V.A.; Peereboom, D.M.; Ahluwalia, M.S. Current medical treatment of glioblastoma. In Current Understanding and Treatment of Gliomas; Springer: Cham, Switzerland, 2015; pp. 103–115. [Google Scholar]
- Zhai, Z.; Qu, X.; Li, H.; Ouyang, Z.; Yan, W.; Liu, G.; Liu, X.; Fan, Q.; Tang, T.; Dai, K. Inhibition of MDA-MB-231 breast cancer cell migration and invasion activity by andrographolide via suppression of nuclear factor-κB-dependent matrix metalloproteinase-9 expression. Mol. Med. Rep. 2015, 11, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.A.; Rifkind, R.A.; Richon, V.M.; Breslow, R.; Miller, T.; Kelly, W.K. Histone deacetylases and cancer: Causes and therapies. Nat. Rev. Cancer 2001, 1, 194. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Ryu, H.-W.; Won, H.-R.; Kwon, S.H. Advances in epigenetic glioblastoma therapy. Oncotarget 2017, 8, 18577. [Google Scholar] [CrossRef]
- Madhu, H.; Vasavada, A.; Johar, K. Andrographolide reduces proliferation and migration of lens epithelial cells by modulating PI3K/Akt pathway. Exp. Eye Res. 2014, 128, 23–26. [Google Scholar]
- Wen, L.; Xia, N.; Chen, X.; Li, Y.; Hong, Y.; Liu, Y.; Wang, Z. Activity of antibacterial, antiviral, anti-inflammatory in compounds andrographolide salt. Eur. J. Pharmacol. 2014, 740, 421–427. [Google Scholar] [CrossRef]
- Rajagopal, S.; Kumar, R.A.; Deevi, D.S.; Satyanarayana, C.; Rajagopalan, R. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J. Exp. Ther. Oncol. 2003, 3, 147–158. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Qiu, F.; Chen, L.; Miao, C.; Li, J.; Xiao, W.; Ma, E. Inactivation of PI3K/Akt signaling mediates proliferation inhibition and G2/M phase arrest induced by andrographolide in human glioblastoma cells. Life Sci. 2012, 90, 962–967. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Ding, Y.; Qi, C.-L.; Lan, T.; Li, J.-C.; He, X.-D.; Wang, L.-J. Inhibitory effect of andrographolide on cell proliferation of glioblastoma U87 cells and its mechanism. J. Jilin Univ. (Med. Ed.) 2013, 4, 008. [Google Scholar]
- Yang, S.-L.; Kuo, F.-H.; Chen, P.-N.; Hsieh, Y.-H.; Yu, N.-Y.; Yang, W.-E.; Hsieh, M.-J.; Yang, S.-F. Andrographolide suppresses the migratory ability of human glioblastoma multiforme cells by targeting ERK1/2-mediated matrix metalloproteinase-2 expression. Oncotarget 2017, 8, 105860. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Choon-Nam, O.; Shen, H.-M. Critical role of pro-apoptotic Bcl-2 family members in andrographolide-induced apoptosis in human cancer cells. Biochem. Pharmacol. 2006, 72, 132–144. [Google Scholar] [CrossRef]
- Zhao, F.; He, E.-Q.; Wang, L.; Liu, K. Anti-tumor activities of andrographolide, a diterpene from Andrographis paniculata, by inducing apoptosis and inhibiting VEGF level. J. Asian Nat. Prod. Res. 2008, 10, 467–473. [Google Scholar] [CrossRef]
- Yang, S.-H.; Wang, S.-M.; Syu, J.-P.; Chen, Y.; Wang, S.-D.; Peng, Y.-S.; Kuo, M.-F.; Kung, H.-N. Andrographolide Induces Apoptosis of C6 Glioma Cells via the ERK-p53-Caspase 7-PARP Pathway. BioMed Res. Int. 2014, 2014, 312847. [Google Scholar] [CrossRef]
- Bothiraja, C.; Pawar, A.P.; Shende, V.S.; Joshi, P.P. Acute and subacute toxicity study of andrographolide bioactive in rodents: Evidence for the medicinal use as an alternative medicine. Comp. Clin. Pathol. 2013, 22, 1123–1128. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. Micromotion of mammalian cells measured electrically. Proc. Natl. Acad. Sci. USA 1991, 88, 7896–7900. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. A morphological biosensor for mammalian cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Wang, Y.; Zhang, L.; Julien, R.; Tang, K.; Balasubramanian, N. Real-time monitoring approach: Assessment of effects of antibodies on the adhesion of NCI-H460 cancer cells to the extracellular matrix. Biosens. Bioelectron. 2008, 23, 1390–1396. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, J.; Xiao, L.; Tang, J.C.O.; Zhang, Y.; Wang, P.; Yang, M. Impedance studies of bio-behavior and chemosensitivity of cancer cells by micro-electrode arrays. Biosens. Bioelectron. 2009, 24, 1305–1310. [Google Scholar] [CrossRef]
- Lo, C.M.; Keese, C.R.; Giaever, I. Impedance analysis of MDCK cells measured by electric cell-substrate impedance sensing. Biophys. J. 1995, 69, 2800–2807. [Google Scholar] [CrossRef]
- Chan, D.T.; Hsieh, S.Y.; Lau, C.K.; Kam, M.K.; Loong, H.H.; Tsang, W.K.; Poon, D.M.; Poon, W.S. Ten-year review of survival and management of malignant glioma in Hong Kong. Hong Kong Med. J. 2017, 23, 134–139. [Google Scholar] [CrossRef]
- Cheo, S.T.; Lim, G.H.; Lim, K.H. Glioblastoma multiforme outcomes of 107 patients treated in two Singapore institutions. Singap. Med. J. 2017, 58, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, M.S.; Langer, R.; Brem, H. Drug delivery to tumors of the central nervous system. Curr. Neurol. Neurosci. Rep. 2001, 1, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.; Begaud-Grimaud, G.; Bessette, B.; Verdier, M.; Battu, S.; Jauberteau, M.-O. Cancer stem cells from human glioma cell line are resistant to Fas-induced apoptosis. Int. J. Oncol. 2009, 34, 717–727. [Google Scholar] [PubMed]
- Ziegler, D.S.; Kung, A.L.; Kieran, M.W. Anti-apoptosis mechanisms in malignant gliomas. J. Clin. Oncol. 2008, 26, 493–500. [Google Scholar] [CrossRef]
- Chiao, M.-T.; Cheng, W.-Y.; Yang, Y.-C.; Shen, C.-C.; Ko, J.-L. Suberoylanilide hydroxamic acid (SAHA) causes tumor growth slowdown and triggers autophagy in glioblastoma stem cells. Autophagy 2013, 9, 1509–1526. [Google Scholar] [CrossRef]
- Woo, M.; Hakem, R.; Soengas, M.S.; Duncan, G.S.; Shahinian, A.; Kägi, D.; Hakem, A.; McCurrach, M.; Khoo, W.; Kaufman, S.A. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998, 12, 806–819. [Google Scholar] [CrossRef]
- Kirsch, D.G.; Doseff, A.; Chau, B.N.; Lim, D.-S.; de Souza-Pinto, N.C.; Hansford, R.; Kastan, M.B.; Lazebnik, Y.A.; Hardwick, J.M. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J. Boil. Chem. 1999, 274, 21155–21161. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.H.-Y.; Kirsch, D.G.; Clem, R.J.; Ravi, R.; Kastan, M.B.; Bedi, A.; Ueno, K.; Hardwick, J.M. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 1997, 278, 1966–1968. [Google Scholar] [CrossRef] [PubMed]
- Grandgirard, D.; Studer, E.; Monney, L.; Belser, T.; Fellay, I.; Borner, C.; Michel, M.R. Alphaviruses induce apoptosis in Bcl-2-overexpressing cells: Evidence for a caspase-mediated, proteolytic inactivation of Bcl-2. EMBO J. 1998, 17, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Clem, R.J.; Cheng, E.H.-Y.; Karp, C.L.; Kirsch, D.G.; Ueno, K.; Takahashi, A.; Kastan, M.B.; Griffin, D.E.; Earnshaw, W.C.; Veliuona, M.A. Modulation of cell death by Bcl-XL through caspase interaction. Proc. Natl. Acad. Sci. USA 1998, 95, 554–559. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Burgess, D.H.; Vanags, D.M.; Orrenius, S. Involvement of cellular proteolytic machinery in apoptosis. Biochem. Biophys. Res. Commun. 1997, 230, 481–488. [Google Scholar] [CrossRef]
- Durkacz, B.W.; Omidiji, O.; Gray, D.A.; Shall, S. (ADP-ribose) n participates in DNA excision repair. Nature 1980, 283, 593. [Google Scholar] [CrossRef]
- Friedman, H.S.; Kerby, T.; Calvert, H. Temozolomide and treatment of malignant glioma. Clin. Cancer Res. 2000, 6, 2585–2597. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, S.-P.; Batsaikhan, B.; Huang, H.-M.; Wang, J.-Y. Application of Electric Cell-Substrate Impedance Sensing to Investigate the Cytotoxic Effects of Andrographolide on U-87 MG Glioblastoma Cell Migration and Apoptosis. Sensors 2019, 19, 2275. https://doi.org/10.3390/s19102275
Chiu S-P, Batsaikhan B, Huang H-M, Wang J-Y. Application of Electric Cell-Substrate Impedance Sensing to Investigate the Cytotoxic Effects of Andrographolide on U-87 MG Glioblastoma Cell Migration and Apoptosis. Sensors. 2019; 19(10):2275. https://doi.org/10.3390/s19102275
Chicago/Turabian StyleChiu, Sheng-Po, Buyandelger Batsaikhan, Huei-Mei Huang, and Jia-Yi Wang. 2019. "Application of Electric Cell-Substrate Impedance Sensing to Investigate the Cytotoxic Effects of Andrographolide on U-87 MG Glioblastoma Cell Migration and Apoptosis" Sensors 19, no. 10: 2275. https://doi.org/10.3390/s19102275
APA StyleChiu, S.-P., Batsaikhan, B., Huang, H.-M., & Wang, J.-Y. (2019). Application of Electric Cell-Substrate Impedance Sensing to Investigate the Cytotoxic Effects of Andrographolide on U-87 MG Glioblastoma Cell Migration and Apoptosis. Sensors, 19(10), 2275. https://doi.org/10.3390/s19102275



