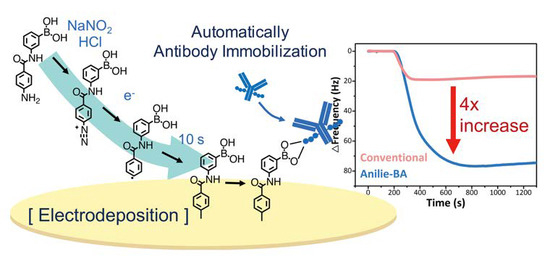
Effective Construction of a High-Capacity Boronic Acid Layer on a Quartz Crystal Microbalance Chip for High-Density Antibody Immobilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
2.3. Self-Assembly (SAM) Gold Electrode Modification
2.4. Electrochemical Cleaning Process
2.5. Electrodeposition
2.6. Cyclic Voltammetry (CV) and Electrochemical Impedance
2.7. QCM Measurement
3. Results and Discussion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bengtson, H.N.; Homolka, S.; Niemann, S.; Reis, A.J.; da Silva, P.E.; Gerasimova, Y.V.; Kolpashchikov, D.M.; Rohde, K.H. Multiplex detection of extensively drug resistant tuberculosis using binary deoxyribozyme sensors. Biosens. Bioelectron. 2017, 94, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Hoa, X.D.; Kirk, A.G.; Tabrizian, M. Towards integrated and sensitive surface plasmon resonance biosensors: A review of recent progress. Biosens. Bioelectron. 2007, 23, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Y.; Li, B.R.; Li, Y.K. Silicon nanowire field-effect-transistor based biosensors: From sensitive to ultra-sensitive. Biosens. Bioelectron. 2014, 60, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Li, B.R.; Hsieh, Y.J.; Chen, Y.X.; Chung, Y.T.; Pan, C.Y.; Chen, Y.T. An Ultrasensitive Nanowire-Transistor Biosensor for Detecting Dopamine Release from Living PC12 Cells under Hypoxic Stimulation. J. Am. Chem. Soc. 2013, 135, 16034–16037. [Google Scholar] [CrossRef] [PubMed]
- Li, B.R.; Chen, C.C.; Kumar, U.R.; Chen, Y.T. Advances in nanowire transistors for biological analysis and cellular investigation. Analyst 2014, 139, 1589–1608. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.T.; Lin, H.Y.G.; Han, Z.; Jin, T.; Millender, R.; Kimerling, L.C.; Agarwal, A. Label-Free Glucose Sensing Using Chip-Scale Mid-Infrared Integrated Photonics. Adv. Opt. Mater. 2016, 4, 1755–1759. [Google Scholar] [CrossRef]
- Li, B.R.; Shen, M.Y.; Yu, H.H.; Li, Y.K. Rapid construction of an effective antifouling layer on a Au surface via electrodeposition. Chem. Commun. 2014, 50, 6793–6796. [Google Scholar] [CrossRef] [PubMed]
- Okahata, Y.; Matsunobu, Y.; Ijiro, K.; Mukae, M.; Murakami, A.; Makino, K. Hybridization of nucleic acids immobilized on a quartz crystal microbalance. J. Am. Chem. Soc. 1992, 114, 8299–8300. [Google Scholar] [CrossRef]
- Yu, D.; Blankert, B.; Viré, J.C.; Kauffmann, J.M. Biosensors in drug discovery and drug analysis. Anal. Lett. 2005, 38, 1687–1701. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ando, T.; Takayama, M.; Egami, T.; Ohtsu, Y.; Sakurai, A.; Yoshida, T.; Taga, K.; Kamaya, H.; Ueda, I. Interaction between phospholipid monolayer and volatile anesthetics using quartz crystal oscillator methods. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 568–575. [Google Scholar] [CrossRef]
- Singh, R.; Mukherjee, M.D.; Sumana, G.; Gupta, R.K.; Sood, S.; Malhotra, B. Biosensors for pathogen detection: A smart approach towards clinical diagnosis. Sens. Actuators B Chem. 2014, 197, 385–404. [Google Scholar] [CrossRef]
- Yaqub, S.; Latif, U.; Dickert, F.L. Plastic antibodies as chemical sensor material for atrazine detection. Sens. Actuators B Chem. 2011, 160, 227–233. [Google Scholar] [CrossRef]
- Uludağ, Y.; Tothill, I.E. Development of a sensitive detection method of cancer biomarkers in human serum (75%) using a quartz crystal microbalance sensor and nanoparticles amplification system. Talanta 2010, 82, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, J. Oriented immobilization of proteins on solid supports for use in biosensors and biochips: A review. Microchim. Acta 2016, 183, 1–19. [Google Scholar] [CrossRef]
- Gubala, V.; Crean, C.; Nooney, R.; Hearty, S.; McDonnell, B.; Heydon, K.; O’Kennedy, R.; MacCraith, B.D.; Williams, D.E. Kinetics of immunoassays with particles as labels: Effect of antibody coupling using dendrimers as linkers. Analyst 2011, 136, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Laib, S.; MacCraith, B.D. Immobilization of biomolecules on cycloolefin polymer supports. Anal. Chem. 2007, 79, 6264–6270. [Google Scholar] [CrossRef] [PubMed]
- Nishiyabu, R.; Shimizu, A. Boronic acid as an efficient anchor group for surface modification of solid polyvinyl alcohol. Chem. Commun. 2016, 52, 9765–9768. [Google Scholar] [CrossRef]
- Tinazli, A.; Tang, J.; Valiokas, R.; Picuric, S.; Lata, S.; Piehler, J.; Liedberg, B.; Tampé, R. High-affinity chelator thiols for switchable and oriented immobilization of histidine-tagged proteins: A generic platform for protein chip technologies. Chem. A Eur. J. 2005, 11, 5249–5259. [Google Scholar] [CrossRef]
- Trilling, A.K.; Harmsen, M.M.; Ruigrok, V.J.; Zuilhof, H.; Beekwilder, J. The effect of uniform capture molecule orientation on biosensor sensitivity: Dependence on analyte properties. Biosens. Bioelectron. 2013, 40, 219–226. [Google Scholar] [CrossRef]
- Ho, J.-A.A.; Hsu, W.-L.; Liao, W.-C.; Chiu, J.-K.; Chen, M.-L.; Chang, H.-C.; Li, C.-C. Ultrasensitive electrochemical detection of biotin using electrically addressable site-oriented antibody immobilization approach via aminophenyl boronic acid. Biosens. Bioelectron. 2010, 26, 1021–1027. [Google Scholar] [CrossRef]
- Sun, X.; Zhai, W.; Fossey, J.S.; James, T.D. Boronic acids for fluorescence imaging of carbohydrates. Chem. Commun. 2016, 52, 3456–3469. [Google Scholar] [CrossRef] [PubMed]
- Duval, F.; van Beek, T.A.; Zuilhof, H. Key steps towards the oriented immobilization of antibodies using boronic acids. Analyst 2015, 140, 6467–6472. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Ahmed, M.U. A carbon nanofiber-based label free immunosensor for high sensitive detection of recombinant bovine somatotropin. Biosens. Bioelectron. 2015, 70, 48–53. [Google Scholar] [CrossRef] [PubMed]
- González-González, M.; Bartolome, R.; Jara-Acevedo, R.; Casado-Vela, J.; Dasilva, N.; Matarraz, S.; García, J.; Alcazar, J.; Sayagues, J.; Orfao, A. Evaluation of homo-and hetero-functionally activated glass surfaces for optimized antibody arrays. Anal. Biochem. 2014, 450, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.D.; Adak, A.K.; Yu, C.C.; Hsiao, W.C.; Lin, H.J.; Chen, M.L.; Lin, C.C. Fabrication of Highly Stable Glyco-Gold Nanoparticles and Development of a Glyco-Gold Nanoparticle-Based Oriented Immobilized Antibody Microarray for Lectin (GOAL) Assay. Chem. A Eur. J. 2015, 21, 3956–3967. [Google Scholar] [CrossRef]
- Humbert, C.; Buck, M.; Calderone, A.; Vigneron, J.P.; Meunier, V.; Champagne, B.; Zheng, W.Q.; Tadjeddine, A.; Thiry, P.; Peremans, A. In Situ Monitoring of the Self-Assembly of p-Nitroanilino Terminated Thiol on Gold: A Study by IR-vis Sum-Frequency Generation Spectroscopy. Phys. Status Solidi A 1999, 175, 129–136. [Google Scholar] [CrossRef]
- Liao, W.-C.; Ho, J.-A.A. Improved activity of immobilized antibody by paratope orientation controller: Probing paratope orientation by electrochemical strategy and surface plasmon resonance spectroscopy. Biosens. Bioelectron. 2014, 55, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Mévellec, V.; Roussel, S.; Tessier, L.; Chancolon, J.; Mayne-L’Hermite, M.; Deniau, G.; Viel, P.; Palacin, S. Grafting polymers on surfaces: A new powerful and versatile diazonium salt-based one-step process in aqueous media. Chem. Mater. 2007, 19, 6323–6330. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Jahne, M.; Rogers, S.; Suni, I.I. Detection of Listeria monocytogenes by electrochemical impedance spectroscopy. Electroanalysis 2013, 25, 2231–2237. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Pali, M.; Lee, H.J.; Lee, T.R.; Suni, I.I. Impedance biosensor incorporating a Carboxylate-Terminated Bidentate Thiol for antibody immobilization. J. Electrochem. Soc. 2016, 163, 125–130. [Google Scholar] [CrossRef]
- Matharu, Z.; Bandodkar, A.J.; Gupta, V.; Malhotra, B.D. Fundamentals and application of ordered molecular assemblies to affinity biosensing. Chem. Soc. Rev. 2012, 41, 1363–1402. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Adak, A.K.; Yeh, N.C.; Yang, W.B.; Chuang, Y.J.; Wong, C.H.; Hwang, K.C.; Hwu, J.R.R.; Hsieh, S.L.; Lin, C.C. Fabrication of an Oriented Fc-Fused Lectin Microarray through Boronate Formation. Angew. Chem. Int. Ed. 2008, 47, 8627–8630. [Google Scholar] [CrossRef] [PubMed]
- Brooks, W.L.; Sumerlin, B.S. Synthesis and applications of boronic acid-containing polymers: From materials to medicine. Chem. Rev. 2015, 116, 1375–1397. [Google Scholar] [CrossRef] [PubMed]
- Belanger, D.; Pinson, J. Electrografting: A powerful method for surface modification. Chem. Soc. Rev. 2011, 40, 3995–4048. [Google Scholar] [CrossRef] [PubMed]
- Le, X.T.; Viel, P.; Jégou, P.; Garcia, A.; Berthelot, T.; Bui, T.H.; Palacin, S. Diazonium-induced anchoring process: An application to improve the monovalent selectivity of cation exchange membranes. J. Mater. Chem. 2010, 20, 3750–3757. [Google Scholar] [CrossRef]

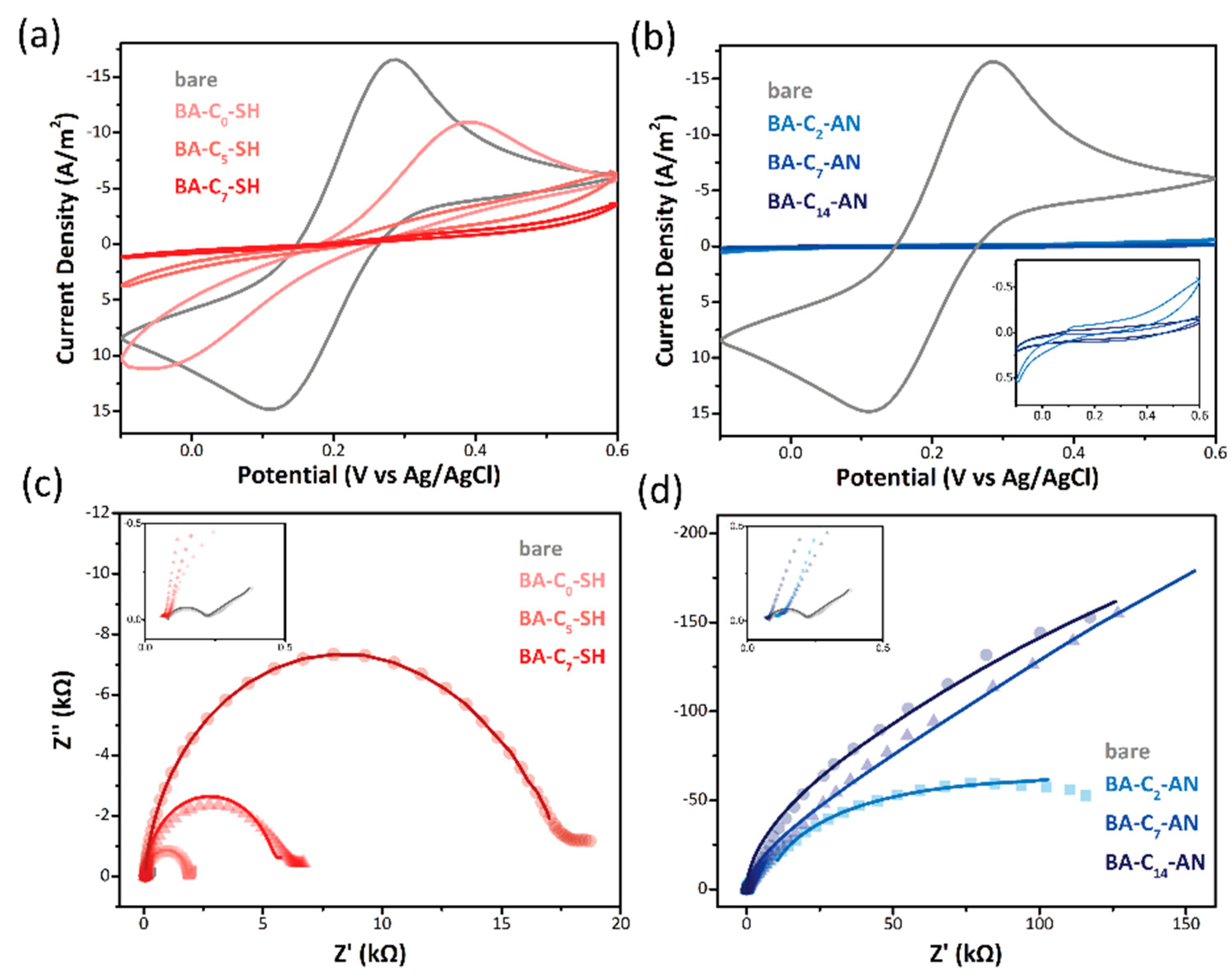
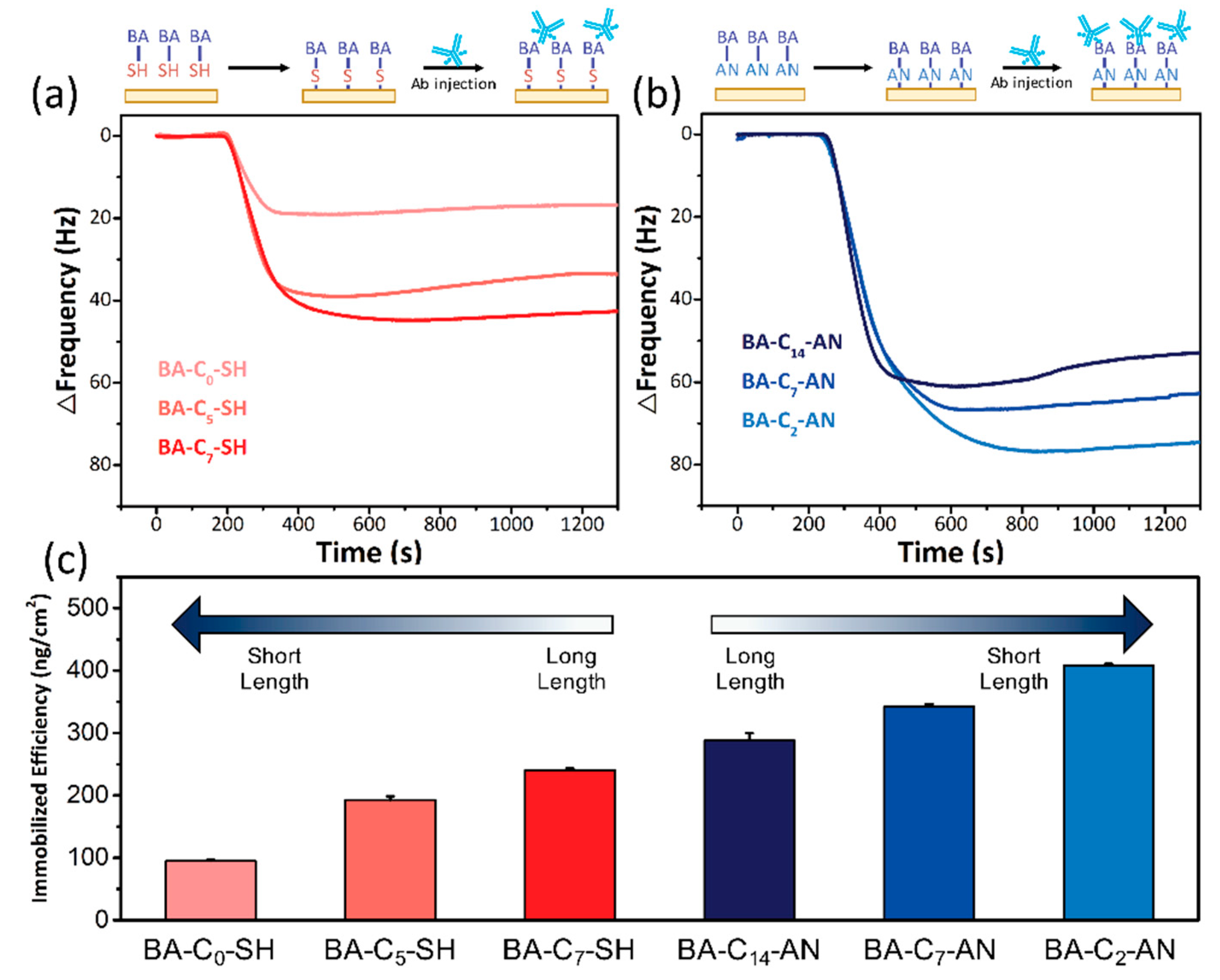
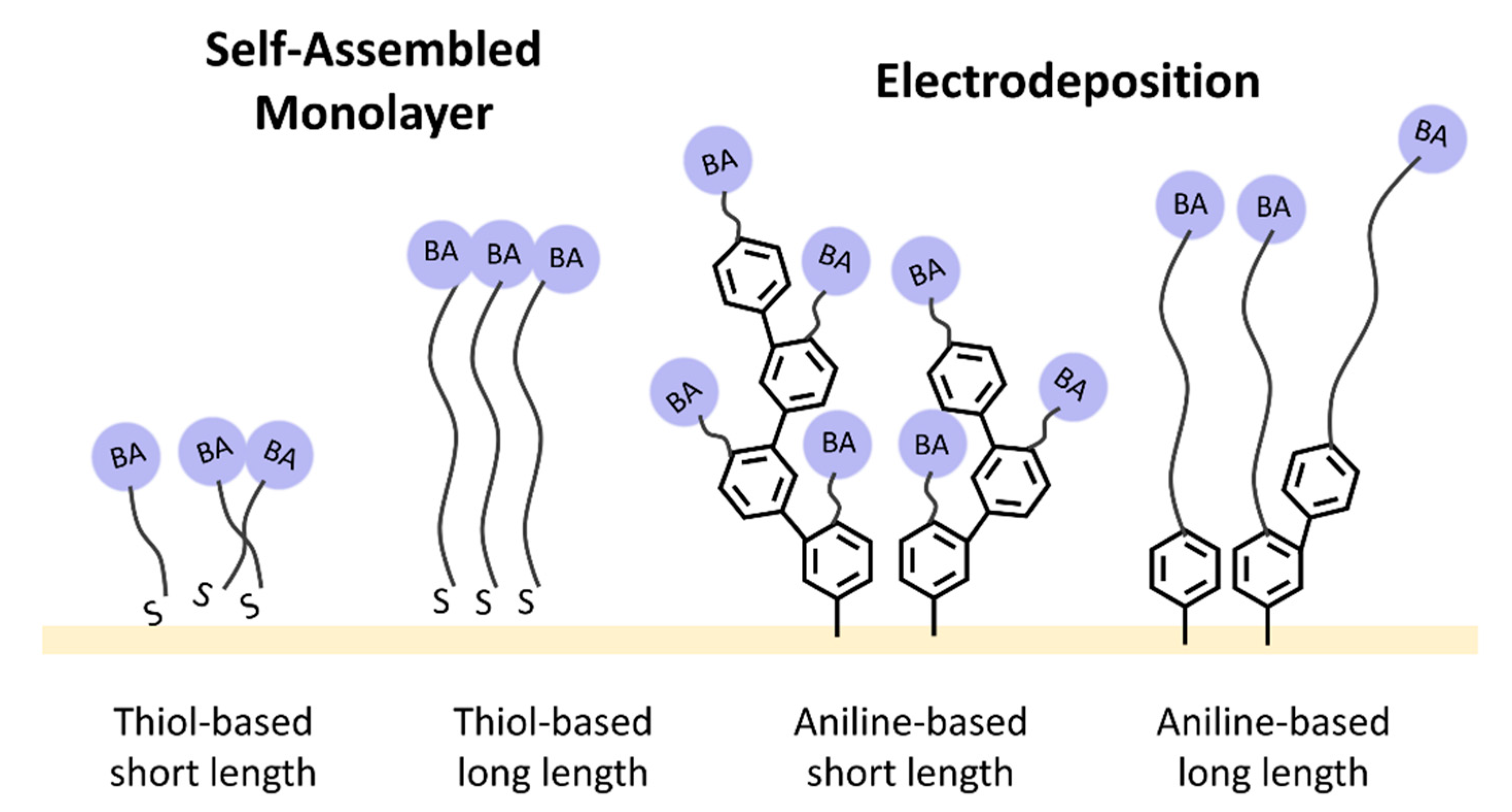
| Modification Method | Bare Electrode | Self-Assembled Monolayer Modified | Electrodeposition Modified | ||||
|---|---|---|---|---|---|---|---|
| Compound Name | BA-C0-SH | BA-C5-SH | BA-C7-SH | BA-C2-AN | BA-C7-AN | BA-C14-AN | |
| Rs (kΩ) | 0.085 | 0.081 | 0.087 | 0.087 | 0.126 | 0.144 | 0.115 |
| Rct (kΩ) | 0.123 | 1.735 | 4.939 | 12.651 | 27.927 | 43.577 | 63.454 |
| CPE (µF) | 2.8 | 0.641 | 0.784 | 0.590 | 1.359 | 1.19 | 1.83 |
| Modification Method | Self-Assembled Monolayer Modified | Electrodeposition Modified | ||||
|---|---|---|---|---|---|---|
| Compound Name | BA-C0-SH | BA-C5-SH | BA-C7-SH | BA-C14-AN | BA-C7-AN | BA-C2-AN |
| Δ Frequency (Hz) | 17.3 ± 0.4 | 35.1 ± 1.1 | 43.9 ± 0.5 | 52.6 ± 2.1 | 62.5 ± 0.8 | 74.5 ± 0.5 |
| Total Antibody (ng) | 12.8 | 26 | 32.5 | 38.9 | 46.2 | 55.1 |
| Immobilization efficiency (ng/cm2) | 94.8 | 192.6 | 240.7 | 288.1 | 342.2 | 408.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.-H.; Huang, S.-C.; Chen, K.-P.; Li, B.-R.; Li, Y.-K. Effective Construction of a High-Capacity Boronic Acid Layer on a Quartz Crystal Microbalance Chip for High-Density Antibody Immobilization. Sensors 2019, 19, 28. https://doi.org/10.3390/s19010028
Lin P-H, Huang S-C, Chen K-P, Li B-R, Li Y-K. Effective Construction of a High-Capacity Boronic Acid Layer on a Quartz Crystal Microbalance Chip for High-Density Antibody Immobilization. Sensors. 2019; 19(1):28. https://doi.org/10.3390/s19010028
Chicago/Turabian StyleLin, Pei-Heng, Sheng-Cih Huang, Kuang-Po Chen, Bor-Ran Li, and Yaw-Kuen Li. 2019. "Effective Construction of a High-Capacity Boronic Acid Layer on a Quartz Crystal Microbalance Chip for High-Density Antibody Immobilization" Sensors 19, no. 1: 28. https://doi.org/10.3390/s19010028
APA StyleLin, P.-H., Huang, S.-C., Chen, K.-P., Li, B.-R., & Li, Y.-K. (2019). Effective Construction of a High-Capacity Boronic Acid Layer on a Quartz Crystal Microbalance Chip for High-Density Antibody Immobilization. Sensors, 19(1), 28. https://doi.org/10.3390/s19010028