Cascaded-Microrings Biosensors Fabricated on a Polymer Platform
Abstract
:1. Introduction
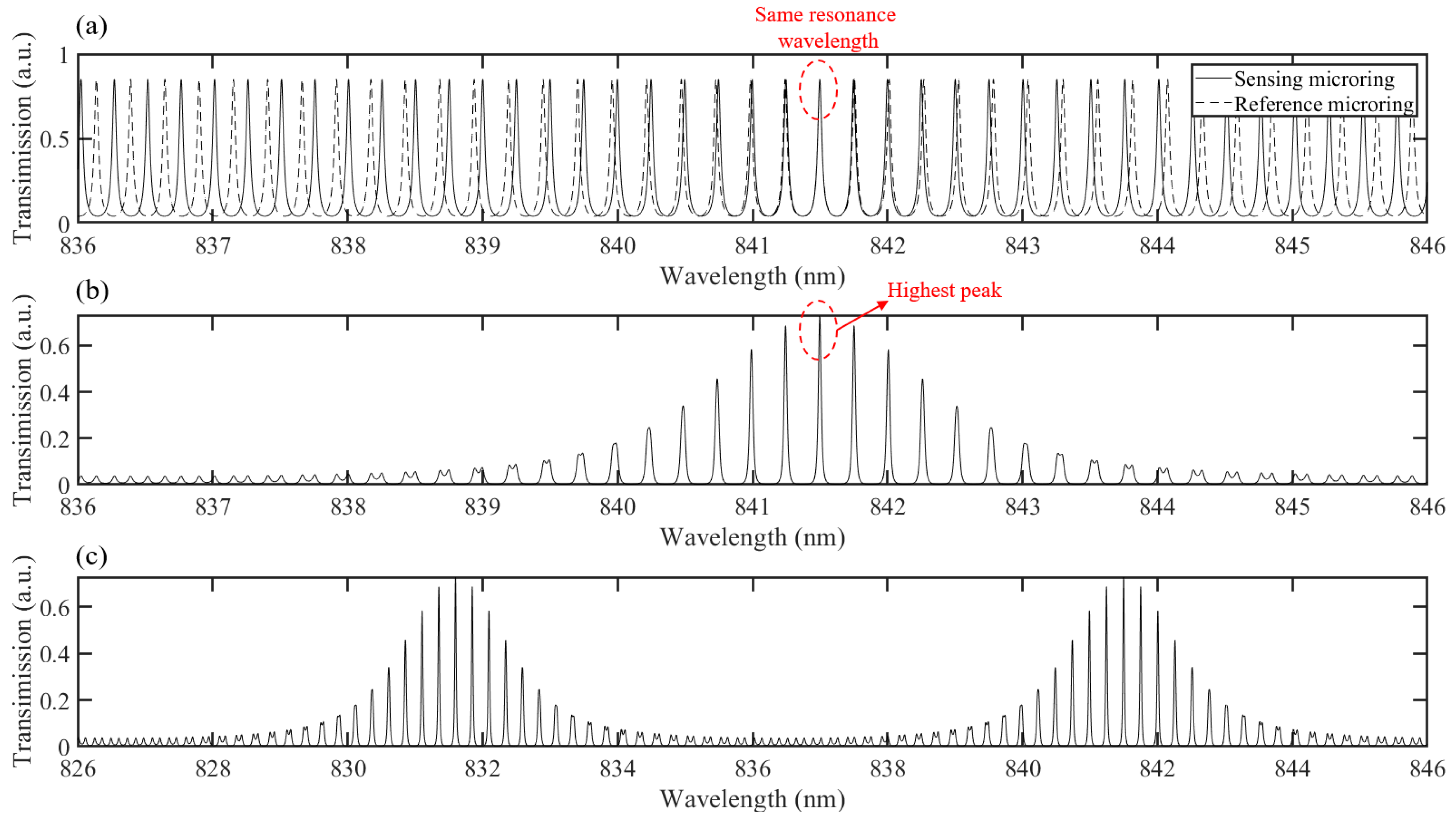
2. Theoretical Analysis and Design
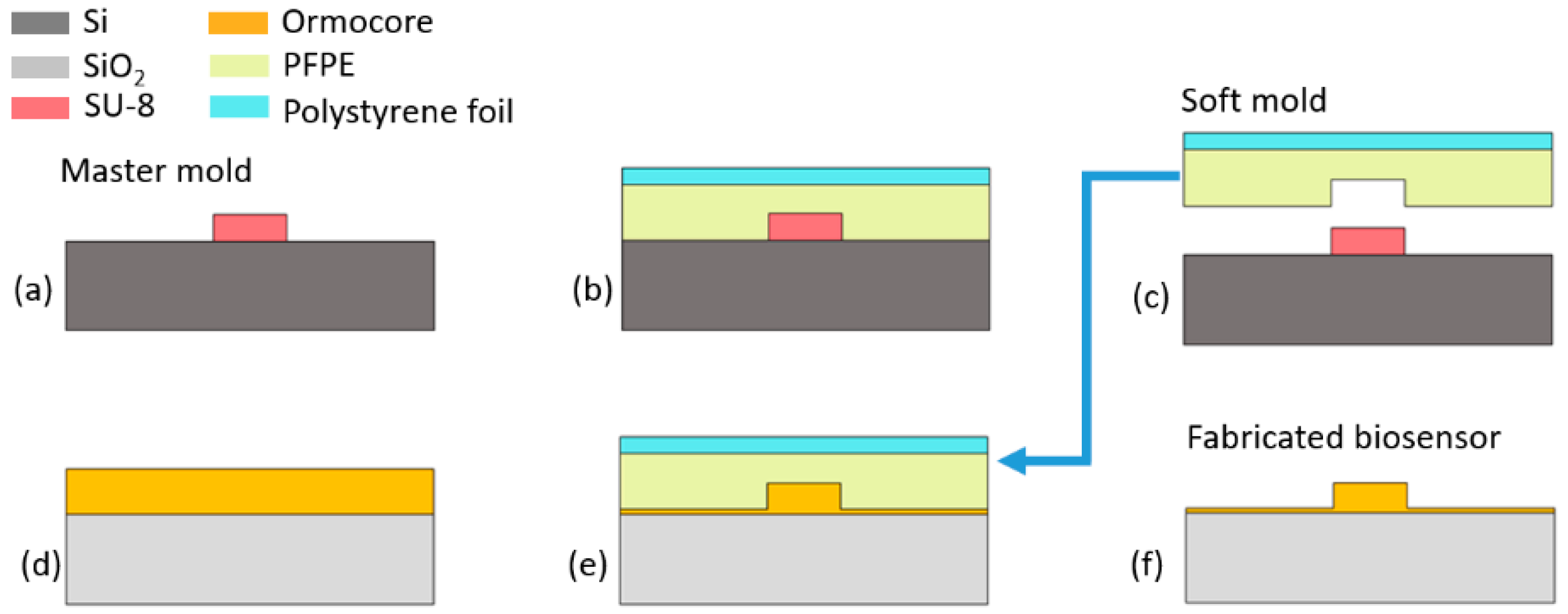
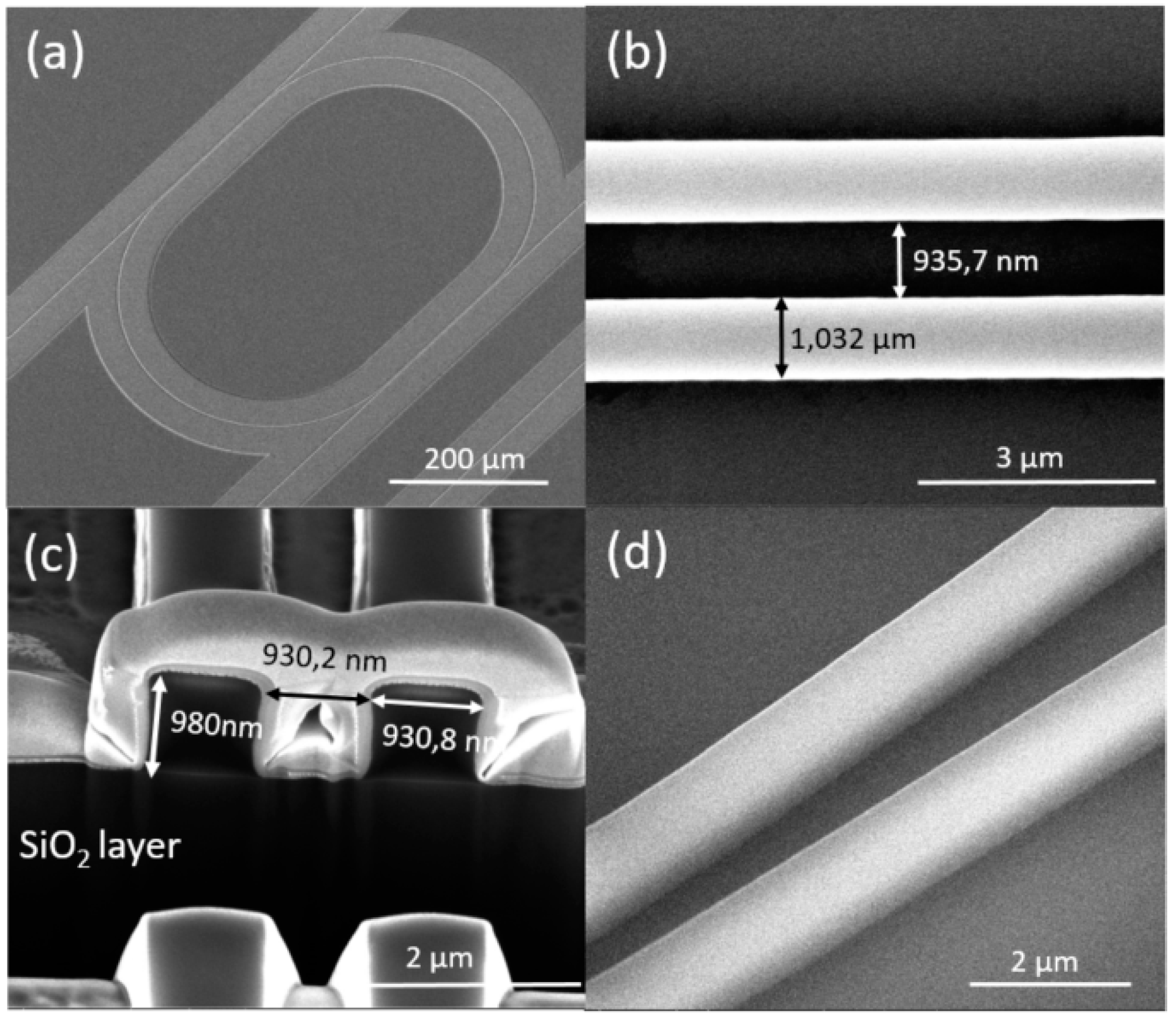
3. Fabrication
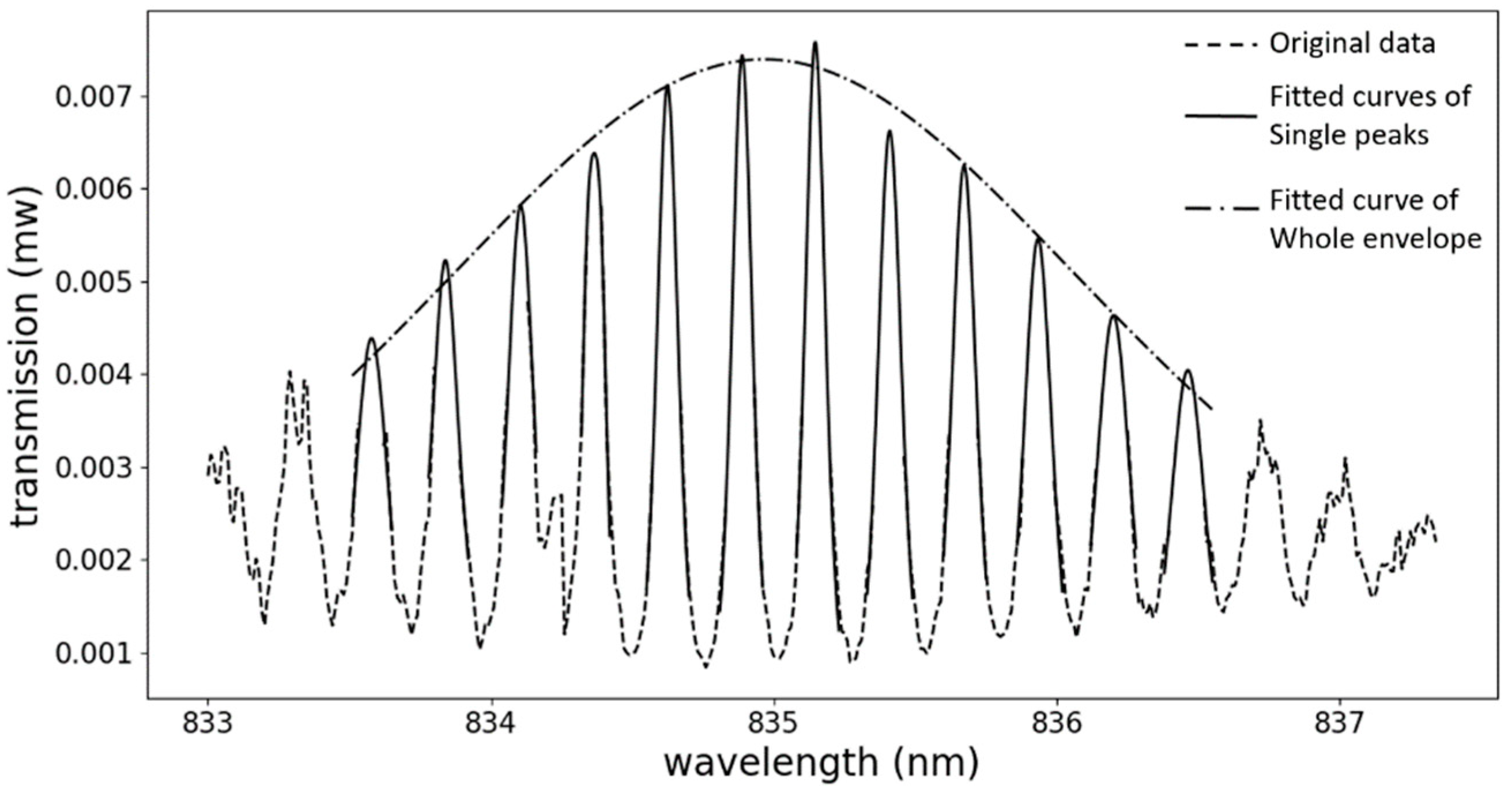
4. Measurement and Result Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pinhas, H.; Malka, D.; Danan, Y.; Sinvani, M.; Zalevsky, Z. Design of fiber-integrated tunable thermo-optic C-band filter based on coated silicon slab. J. Eur. Opt. Soc.-Rapid Publ. 2017, 13, 32. [Google Scholar] [CrossRef]
- Katz, O.; Malka, D. Design of novel SOI 1 × 4 optical power splitter using seven horizontally slotted waveguides. Photonics Nanostruct. Fundam. Appl. 2017, 25, 9–13. [Google Scholar] [CrossRef]
- Malka, D.; Cohen, M.; Turkiewicz, J.; Zalevsky, Z. Optical micro-multi-racetrack resonator filter based on SOI waveguides. Photonics Nanostruct. Fundam. Appl. 2015, 16, 16–23. [Google Scholar] [CrossRef]
- Rizal, C.S.; Niraula, B. Compact Si-based asymmetric MZI waveguide on SOI as a thermo-optical switch. Opt. Commun. 2018, 410, 947–955. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.C.; Alvarez, M.; Lechuga, L.M. Integrated optical devices for lab-on-a-chip biosensing applications. Laser Photonics Rev. 2012, 6, 463–487. [Google Scholar] [CrossRef]
- Luff, B.J.; Wilkinson, J.S.; Piehler, J.; Hollenbach, U.; Ingenhoff, J.; Fabricius, N. Integrated optical mach-zehnder biosensor. J. Light. Technol. 1998, 16, 583. [Google Scholar] [CrossRef]
- Hiltunen, M.; Hiltunen, J.; Stenberg, P.; Aikio, S.; Kurki, L.; Vahimaa, P.; Karioja, P. Polymeric slot waveguide interferometer for sensor applications. Opt. Express 2014, 22, 7229–7237. [Google Scholar] [CrossRef]
- Vos, K.D.; Bartolozzi, I.; Schacht, E.; Bienstman, P.; Baets, R. Silicon-on-Insulator microring resonator for sensitive and label-free biosensing. Opt. Express 2007, 15, 7610–7615. [Google Scholar] [CrossRef]
- Morarescu, R.; Pal, P.; Beneitez, N.; Missinne, J.; Steenberge, G.; Bienstman, P.; Morthier, G. Fabrication and characterization of high-optical-quality-factor hybrid polymer microring resonators operating at very near infrared wavelengths. IEEE Photonics J. 2016, 8, 1–9. [Google Scholar] [CrossRef]
- Jahns, S.; Bräu, M.; Meyer, B.-O.; Karrock, T.; Gutekunst, S.B.; Blohm, L.; Selhuber-Unkel, C.; Buhmann, R.; Nazirizadeh, Y.; Gerken, M. Handheld imaging photonic crystal biosensor for multiplexed, label-free protein detection. Biomed. Opt. Express 2015, 6, 3724–3736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Jiang, J.-H.; Rashid, A.A.; John, S. Biosensor architecture for enhanced disease diagnostics: Lab-in-a-photonic-crystal. Opt. Express 2016, 24, 12166–12191. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xin, Z.; Gan, Q.; Cheng, X.; Bartoli, F.J. Plasmonic interferometers for label-free multiplexed sensing. Opt. Express 2013, 21, 5859–5871. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-T.; Lai, Y.-C.; Li, C.-T.; Chen, C.-K.; Yen, T.-J. A multi-functional plasmonic biosensor. Opt. Express 2010, 18, 9561–9569. [Google Scholar] [CrossRef] [PubMed]
- Rizal, C.; Pisana, S.; Hrvoic, I. Improved Magneto-Optic Surface Plasmon Resonance Biosensors. Photonics 2018, 5, 15. [Google Scholar] [CrossRef]
- Zamora, V.; Lützow, P.; Weiland, M.; Pergande, D. Investigation of cascaded SiN microring resonators at 1.3 µm and 1.5 µm. Opt. Express 2013, 21, 27550–27557. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ren, J.; Han, X.; Claes, T.; Jian, X.; Bienstman, P.; Baets, R.; Zhao, M.; Morthier, G. A label-free optical biosensor built on a low-cost polymer platform. IEEE Photonics J. 2012, 4, 920–930. [Google Scholar] [CrossRef]
- Jiang, J.; Callender, C.L.; Noad, J.P.; Walker, R.B.; Mihailov, S.J.; Ding, J.; Day, M. All-polymer photonic devices using excimer laser micromachining. IEEE Photonics Technol. Lett. 2004, 16, 509–511. [Google Scholar] [CrossRef]
- Salleh, M.H.M.; Andrew, G.; Marc, S.; Julien, R.; Cooper, J.M. Polymer dual ring resonators for label-free optical biosensing using microfluidics. Chem. Commun. 2013, 49, 3095–3097. [Google Scholar] [CrossRef]
- Hiltunen, M.; Hiltunen, J.; Stenberg, P.; Petäjä, J.; Heinonen, E.; Vahimaa, P.; Karioja, P. Polymeric slot waveguide at visible wavelength. Opt. Lett. 2012, 37, 4449–4451. [Google Scholar] [CrossRef]
- Robi, B.; Jaeger, N.A.; Nicolas, R.; Lukas, C. Series-coupled silicon racetrack resonators and the Vernier effect: Theory and measurement. Opt. Express 2010, 18, 25151. [Google Scholar]
- He, J.-J.; Liu, D. Wavelength switchable semiconductor laser using half-wave V-coupled cavities. Opt. Express 2008, 16, 3896–3911. [Google Scholar] [CrossRef] [PubMed]
- Daoxin, D. Highly sensitive digital optical sensor based on cascaded high-Q ring-resonators. Opt. Express 2009, 17, 23817–23822. [Google Scholar]
- Claes, T.; Bogaerts, W.; Bienstman, P. Experimental characterization of a silicon photonic biosensor consisting of two cascaded ring resonators based on the Vernier-effect and introduction of a curve fitting method for an improved detection limit. Opt. Express 2010, 18, 22747–22761. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Yuying, T.; Rothstein, J.P.; Watkins, J.J.; Carter, K.R. Large-area, continuous roll-to-roll nanoimprinting with PFPE composite molds. Nanotechnology 2013, 24, 505307. [Google Scholar]
- Jin, L.; Li, M.; He, J.-J. Optical waveguide double-ring sensor using intensity interrogation with a low-cost broadband source. Opt. Lett. 2011, 36, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Rongsheng, L.; Seokwoo, J.; Hee Hyun, L.; Joana, M.; Anshu, G.; Feng, H.; Ines, M.; Rogers, J.A. Soft lithography using acryloxy perfluoropolyether composite stamps. Langmuir ACS J. Surf. Colloids 2007, 23, 2898–2905. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Liu, Q.; Wu, Z.; Morthier, G.; Zhao, M. Cascaded-Microrings Biosensors Fabricated on a Polymer Platform. Sensors 2019, 19, 181. https://doi.org/10.3390/s19010181
Liang Y, Liu Q, Wu Z, Morthier G, Zhao M. Cascaded-Microrings Biosensors Fabricated on a Polymer Platform. Sensors. 2019; 19(1):181. https://doi.org/10.3390/s19010181
Chicago/Turabian StyleLiang, Yuxin, Qi Liu, Zhenlin Wu, Geert Morthier, and Mingshan Zhao. 2019. "Cascaded-Microrings Biosensors Fabricated on a Polymer Platform" Sensors 19, no. 1: 181. https://doi.org/10.3390/s19010181
APA StyleLiang, Y., Liu, Q., Wu, Z., Morthier, G., & Zhao, M. (2019). Cascaded-Microrings Biosensors Fabricated on a Polymer Platform. Sensors, 19(1), 181. https://doi.org/10.3390/s19010181




