Highly Sensitive Voltammetric Glucose Biosensor Based on Glucose Oxidase Encapsulated in a Chitosan/Kappa-Carrageenan/Gold Nanoparticle Bionanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
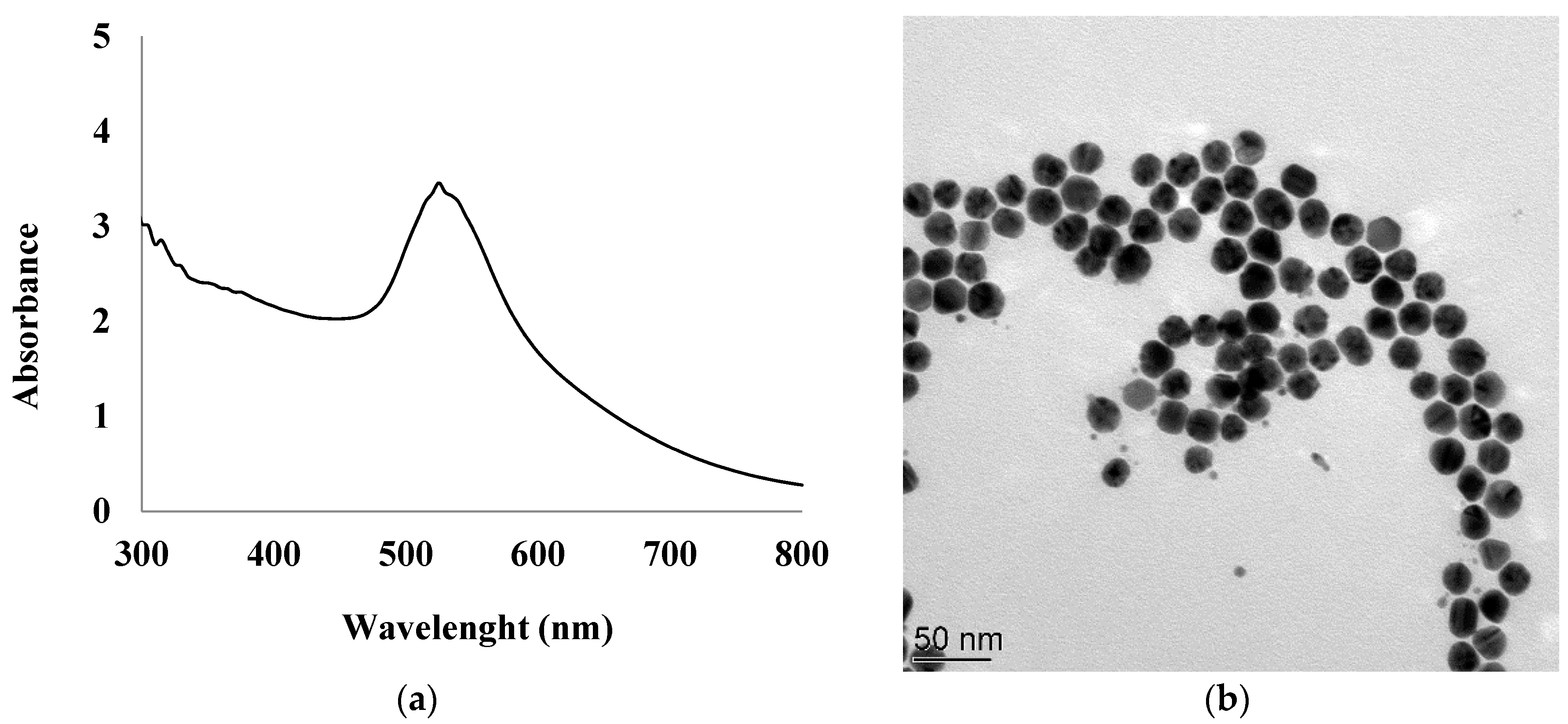
2.2. Synthesis of Gold Nanoparticles
2.3. Instrumentation
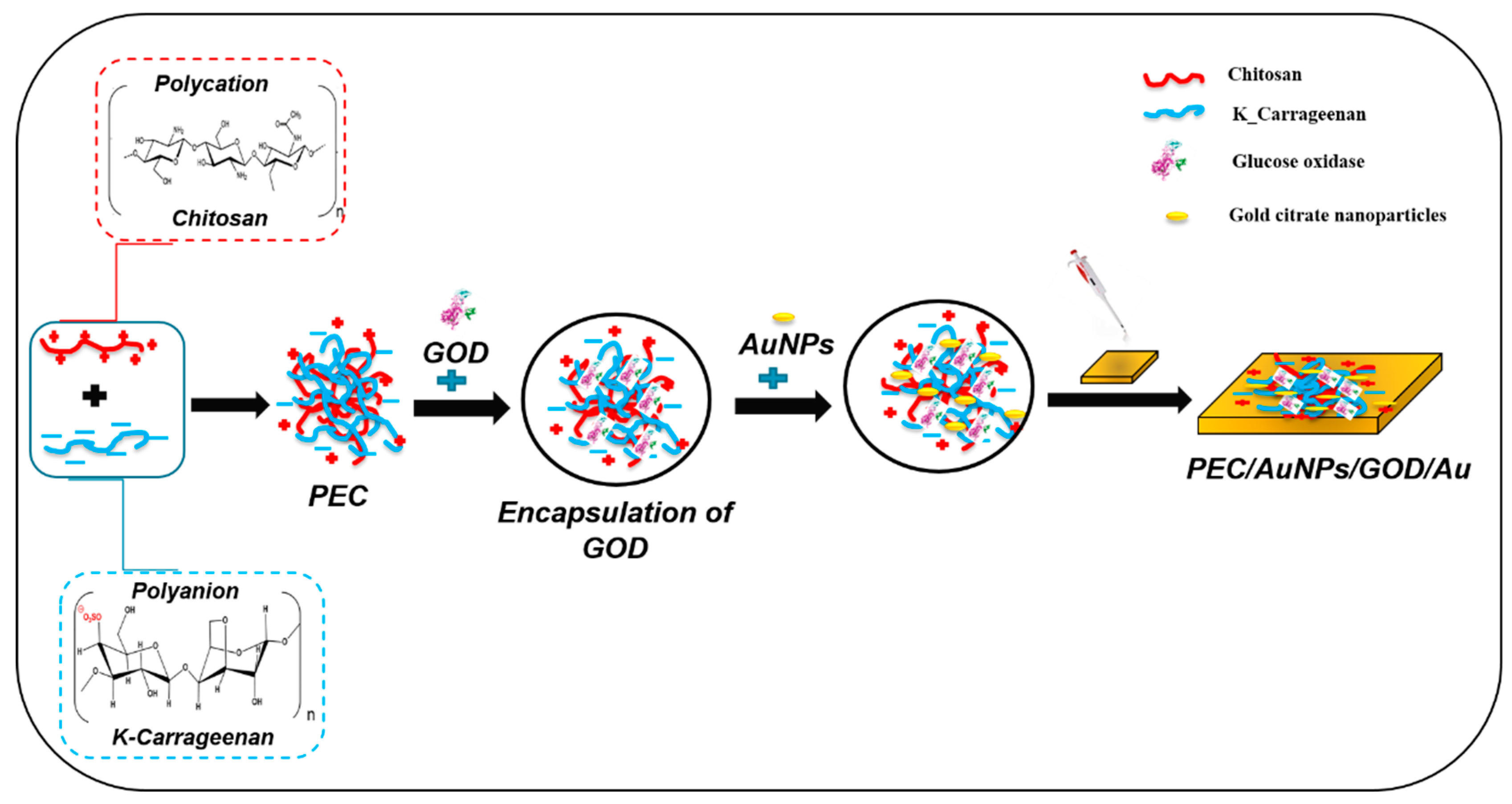
2.4. Preparation of Polysaccharide Complex PEC/AuNPs/GOD Mixture
2.5. Elaboration of the Glucose Biosensor
3. Results
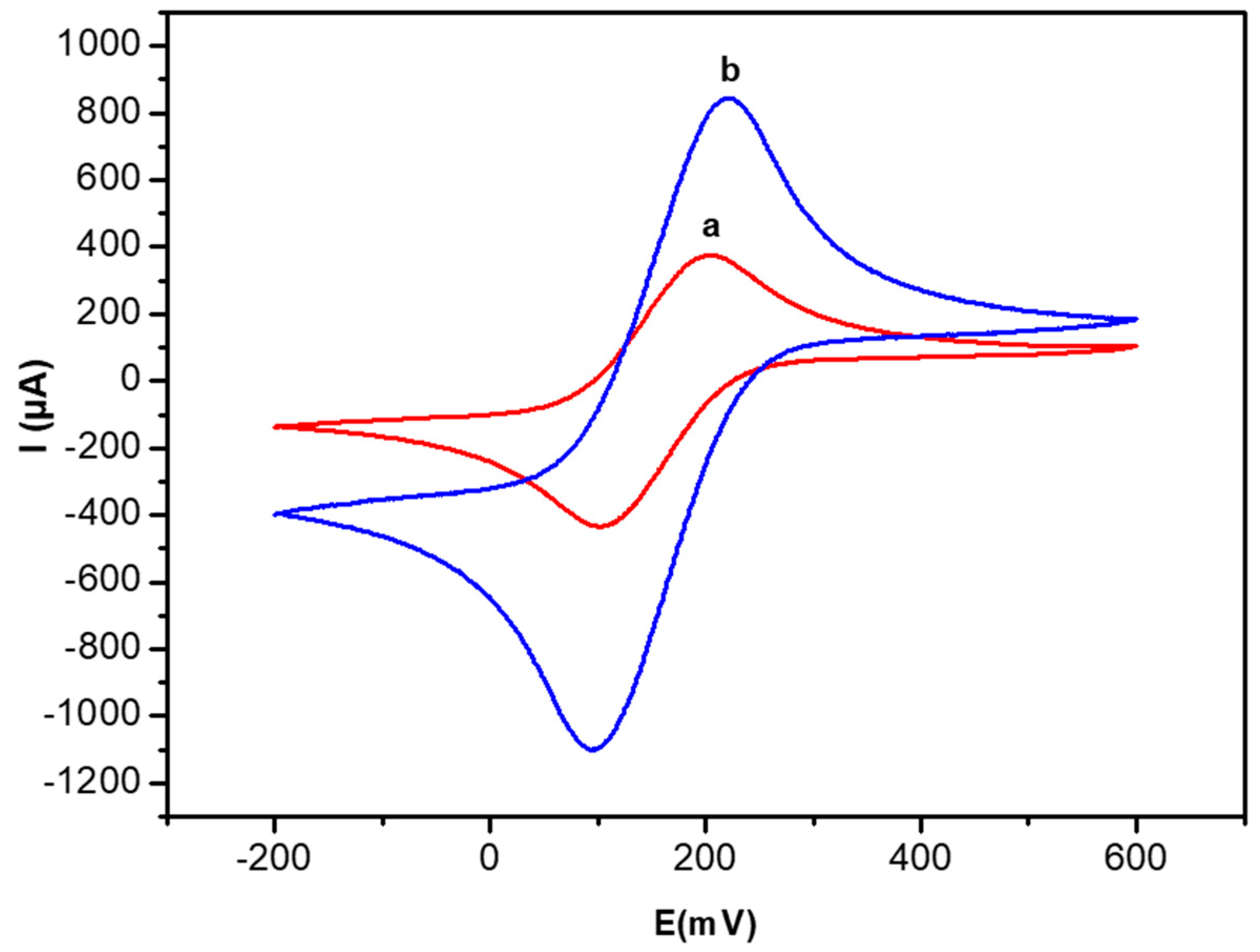
3.1. Electrochemical Characterization of the Modified Gold Electrode
3.2. SWV Response of the Glucose Biosensor
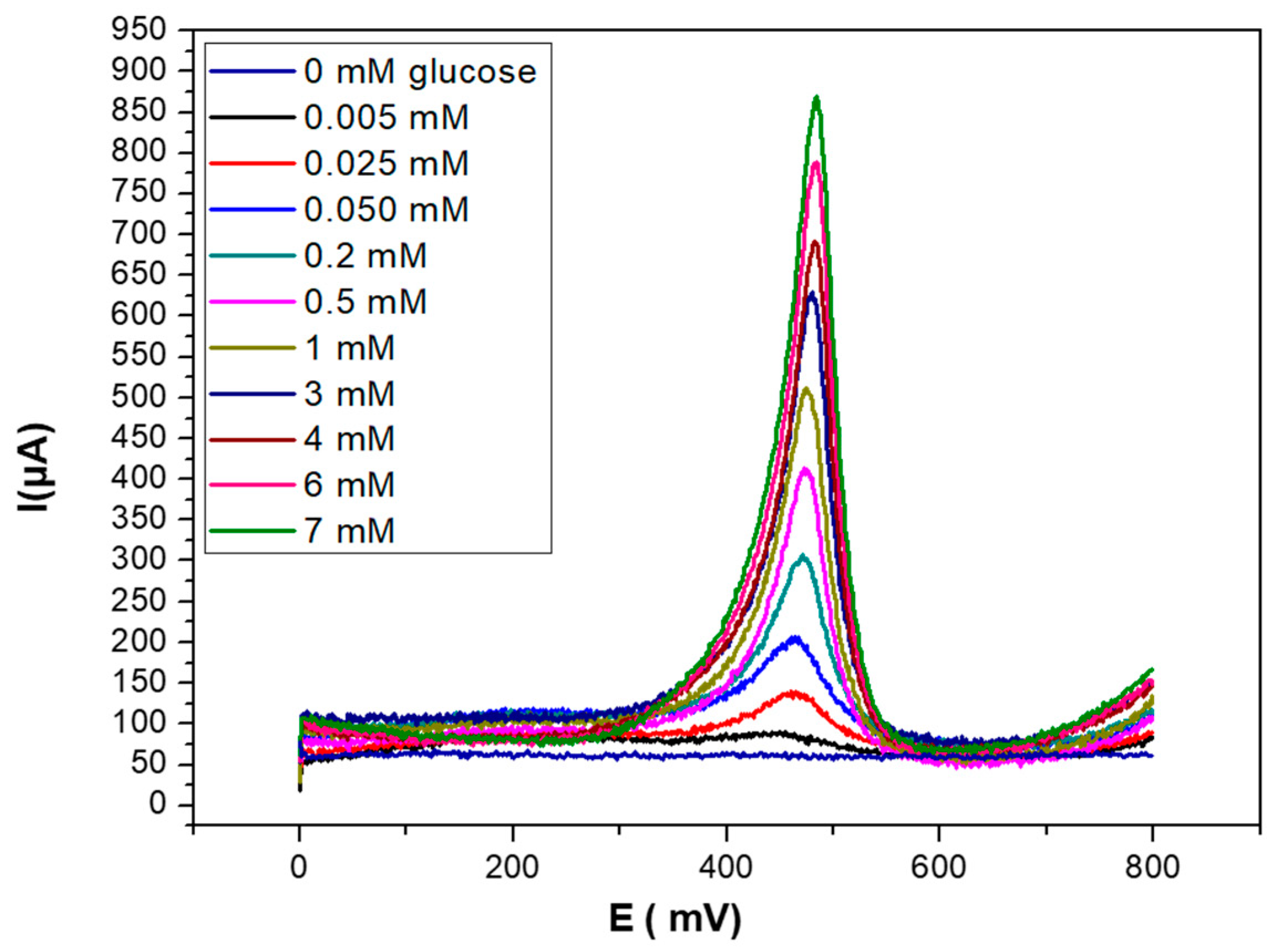
3.3. SWV Response of PEC/AuNPs/GOD/Au Biosensor when the Concentration of Glucose Increases
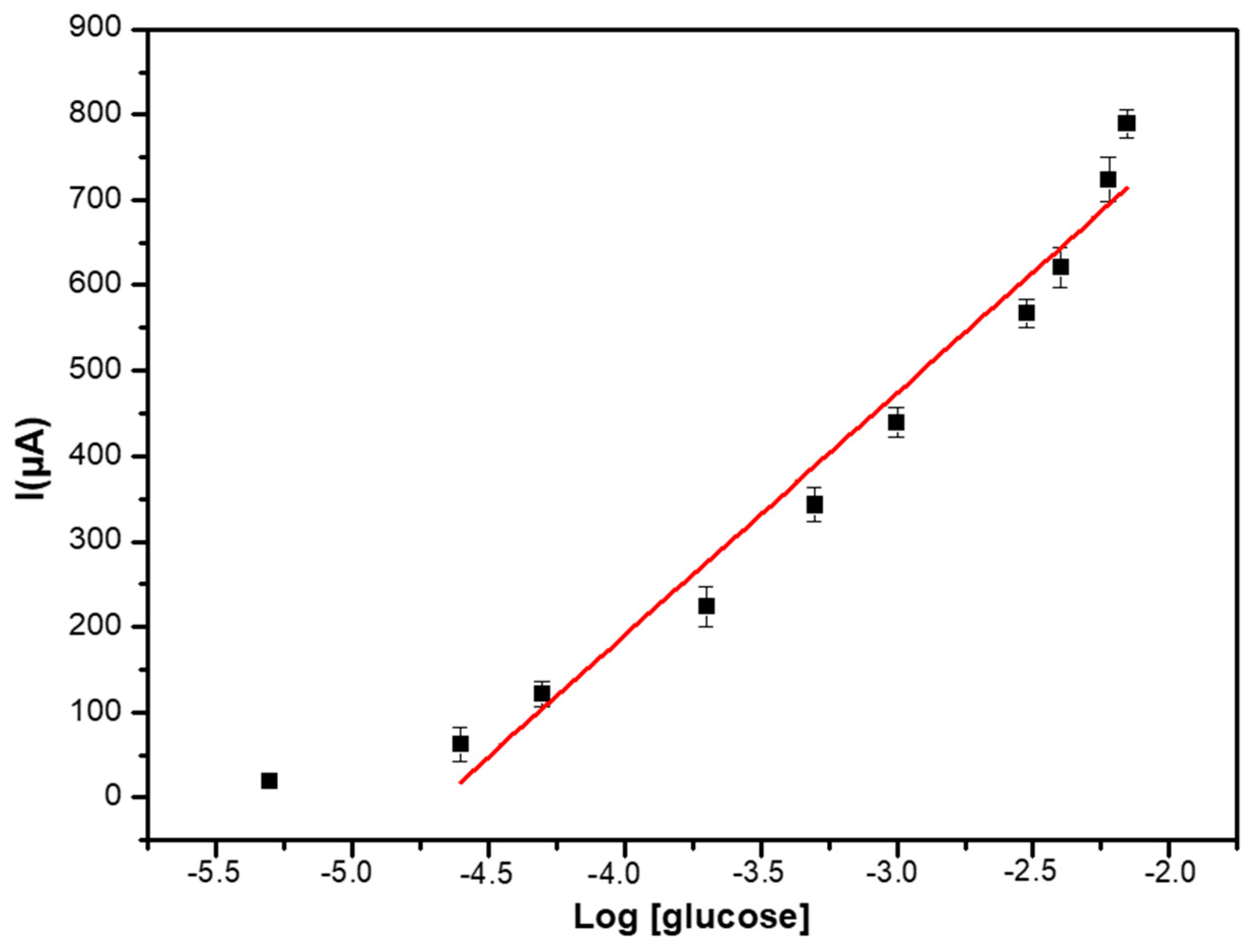
3.4. Analytical Performance of the Glucose Biosensor
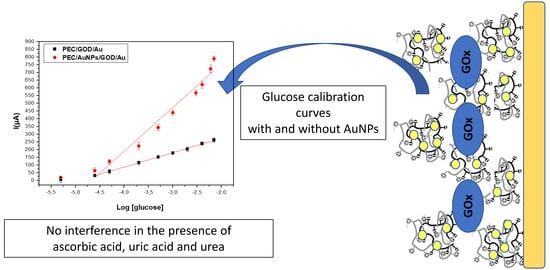
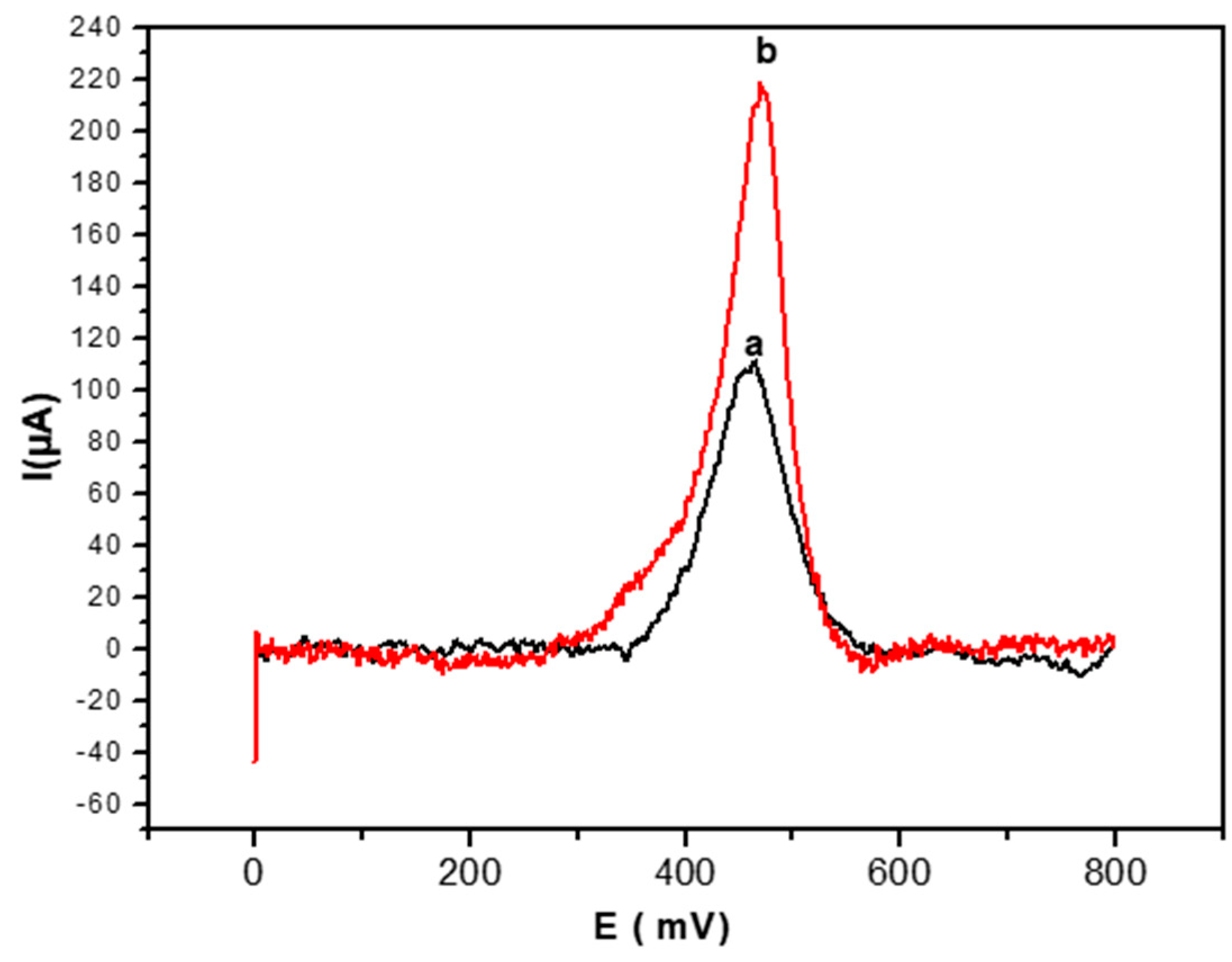
3.4.1. Comparison of Biosensor Response Recorded for Glucose in Absence and Presence of AuNPs
3.4.2. Reproducibility and Shelf Life of the PEC/AuNPs/GOD Based Glucose Biosensor
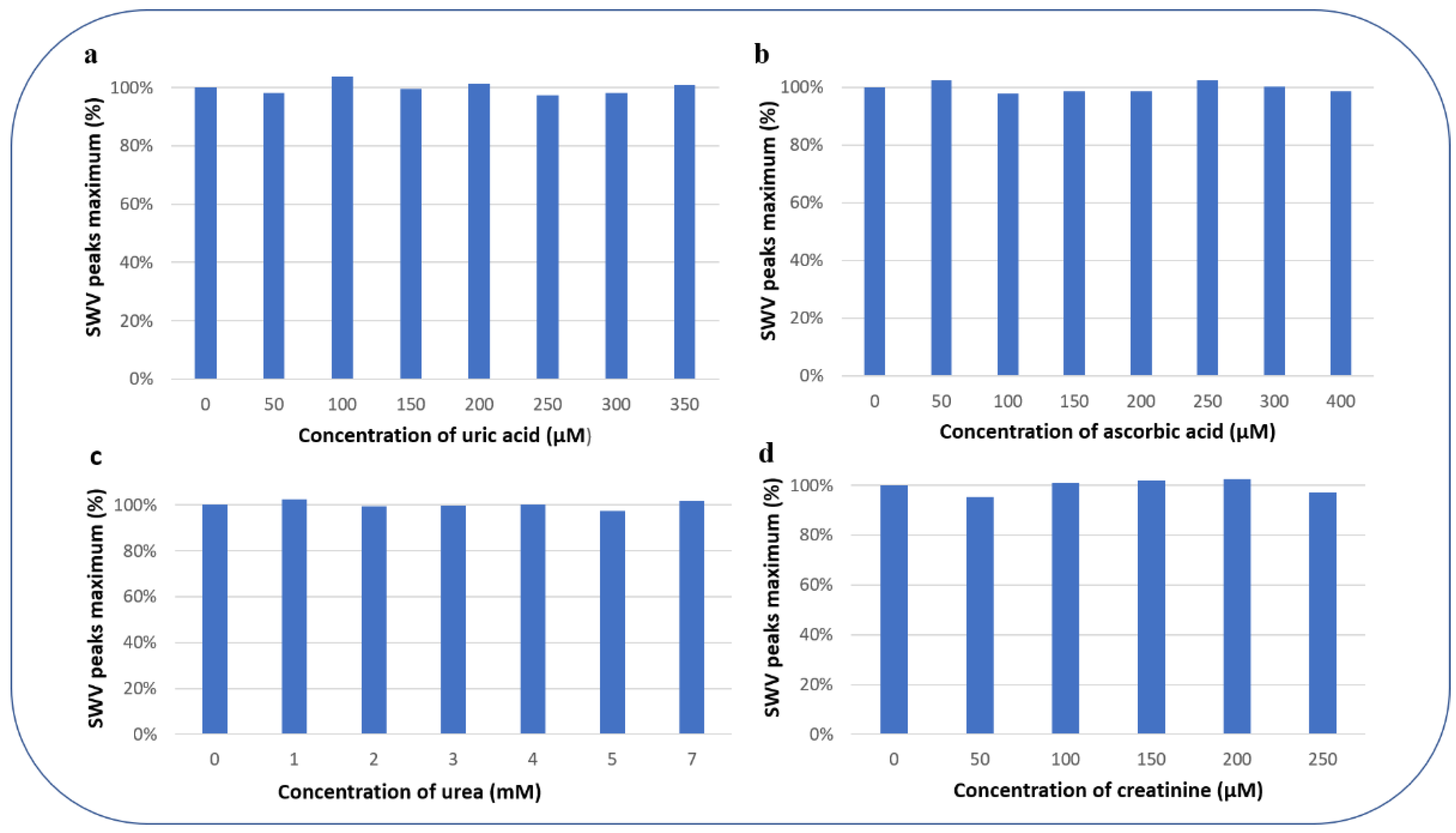
3.4.3. Selectivity
3.4.4. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tomida, H.; Nakamura, C.; Kiryu, S. A novel method for the preparation of controlled-release theophylline capsules coated with a polyelectrolyte complex of k-carrageenan and chitosan. Chem. Pharm. Bull. 1994, 42, 979–981. [Google Scholar] [CrossRef]
- Tapia, C.; Escobar, Z.; Costa, E.; Sapag-Hagar, J.; Valenzuela, F.; Basualto, C.; Gai, M.N.; Yazdami-Pedram, M. Comparative studies on polyelectrolyte complexes and mixtures of chitosan–alginate and chitosan–carrageenan as prolonged diltiazem clorhydrate release systems. Eur. J. Pharm. Biopharm. 2004, 57, 65–75. [Google Scholar] [CrossRef]
- Piyakulawat, P.; Praphairaksit, N.; Chantarasiri, N.; Muangsin, N. Preparation and evaluation of chitosan/carrageenan beads for controlled release of sodium diclofenac. Am. Assoc. Pharm. Sci. 2007, 8, 97. [Google Scholar] [CrossRef]
- Briones, A.V.; Sato, T. Encapsulation of glucose oxidase (GOD) in polyelectrolyte complexes of chitosan–carrageenan. React. Funct. Polym. 2010, 70, 19–27. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Liu, B.; Shen, G.; Yu, R. Amperometric glucose biosensor based on chitosan with improved selectivity and stability. Sens. Actuators B Chem. 2004, 101, 269–276. [Google Scholar] [CrossRef]
- Dai, H.; Wu, X.; Xu, H.; Wang, Y.; Chi, Y.; Chen, G. A highly performing electrochemiluminescent biosensor for glucose based on a polyelectrolyte-chitosan modified electrode. Electrochim. Acta 2009, 54, 4582–4586. [Google Scholar] [CrossRef]
- Rassas, I.; Braiek, M.; Bonhomme, A.; Bessueille, F.; Rafin, G.; Majdoub, H.; Jaffrezic-Renault, N. Voltammetric glucose biosensor based on glucose oxidase encapsulation in a chitosan-kappa-carrageenan polyelectrolyte complex. Mater. Sci. Eng. C 2019, 95, 152–159. [Google Scholar] [CrossRef]
- Haes, A.J.; Van Duyne, R.P. A nanoscale optical biosensor: Sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. J. Am. Chem. Soc. 2002, 124, 10596–10604. [Google Scholar] [CrossRef]
- Huang, M.; Shao, Y.; Sun, X.; Chen, H.; Liu, B.; Dong, S. Alternate assemblies of platinum nanoparticles and metalloporphyrins as tunable electrocatalysts for dioxygen reduction. Langmuir 2005, 21, 323–329. [Google Scholar] [CrossRef]
- Wang, L.; Wang, E. A novel hydrogen peroxide sensor based on horseradish peroxidase immobilized on colloidal Au modified ITO electrode. Electrochem. Commun. 2004, 6, 225–229. [Google Scholar] [CrossRef]
- Xu, S.; Han, X. A novel method to construct a third-generation biosensor: Self-assembling gold nanoparticles on thiol-functionalized poly (styreneco-acrylic acid) nanospheres. Biosens. Bioelectron. 2004, 19, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, T.; Cheng, F.; Zhang, J.; Zhu, J. Toward the Early Evaluation of Therapeutic Effects: An Electrochemical Platform for Ultrasensitive Detection of Apoptotic Cells. Anal. Chem. 2011, 83, 7902–7909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gu, M.; Zheng, T.; Zhu, J. Synthesis of Gelatin-Stabilized Gold Nanoparticles and Assembly of Carboxylic Single-Walled Carbon Nanotubes/Au Composites for Cytosensing and Drug Uptake. Anal. Chem. 2009, 81, 6641–6648. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yuan, R.; Chai, Y.; Tang, D.; Zhang, Y.; Wang, N.; Li, X.; Zhu, Q. A reagentless amperometric immunosensor based on gold nanoparticles/thionine/Nafion-membrane-modified gold electrode for determination of α-1-fetoprotein. Electrochem. Commun. 2005, 7, 355–360. [Google Scholar] [CrossRef]
- Niazov, T.; Pavlov, V.; Xiao, Y.; Gill, R.; Willner, I. DNAzyme-functionalized Au nanoparticles for the amplified detection of DNA or telomerase activity. Nano Lett. 2004, 4, 1683–1687. [Google Scholar] [CrossRef]
- Gole, A.; Dash, C.; Ramakrishnan, V.; Sainkar, S.R.; Mandale, A.B.; Rao, M.; Sastry, M. Pepsin-gold colloid conjugates: Preparation, characterization, and enzymatic activity. Langmuir 2001, 17, 1674–1679. [Google Scholar] [CrossRef]
- Xiao, Y.; Ju, H.X.; Chen, H.Y. Hydrogen peroxide sensor based on horseradish peroxidase-labeled Au colloids immobilized on gold electrode surface by cysteamine monolayer. Anal. Chim. Acta 1999, 391, 73–82. [Google Scholar] [CrossRef]
- Jia, J.; Wang, B.; Wu, A.; Cheng, G.; Li, Z.; Dong, S. A method to construct a third-generation horseradish peroxidase biosensor: Self-assembling gold nanoparticles to three-dimensional sol-gel network. Anal. Chem. 2002, 74, 2217–2223. [Google Scholar] [CrossRef]
- Shchipunov, Y.A. Bionanocomposites: Green sustainable materials for the near future. Pure Appl. Chem. 2012, 84, 2499–2675. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Chitosan mediated assembly of gold nanoparticles multiplayer. Colloids Surf. A 2003, 226, 77–86. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A. Study of structural and electro-catalytic behaviour of amperometric biosensor based on chitosan/polypyrrole nanotubes-gold nanoparticles nanocomposites. Synth. Met. 2016, 220, 551–559. [Google Scholar] [CrossRef]
- Du, Y.; Luo, X.-L.; Xu, J.-J.; Chen, H.-Y. A simple method to fabricate a chitosan-gold nanoparticles film and its application in glucose biosensor. Bioelectrochemistry 2007, 70, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, N.; Yu, H.; Niu, Y.; Sun, C. Covalent attachment of glucose oxidase to an Au electrode modified with gold nanoparticles for use as glucose biosensor. Bioelectrochemistry 2004, 67, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.I.; Xu, J.J.; Du, Y.; Den, H.Y. A glucose biosensor based on chitosan–glucose oxidase–gold nanoparticles biocomposite formed by one-step electrodeposition. Anal. Biochem. 2004, 334, 284–289. [Google Scholar] [CrossRef]
- Wang, B.; Ji, X.; Zhao, H.; Wang, N.; Li, X.; Ni, R.; Liu, Y. An amperometric β-glucan biosensor based on the immobilization of bi-enzyme on Prussian blue–chitosan and gold nanoparticles–chitosan nanocomposite films. Biosens. Bioelectron. 2013, 55, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Wang, F.; Chen, Z. Electrochemical glucose sensor based on one-step construction of gold nanoparticle–chitosan composite film. Sens. Actuators B Chem. 2009, 138, 539–544. [Google Scholar] [CrossRef]


| Biosensor | LOD for Glucose | Sensitivity µA/log[glucose] | RSD |
|---|---|---|---|
| PEC/GOD/Au (Biosensor without AuNPs) | 5 µM | 93.7 | 6% |
| PEC/AuNPs/GOD/Au (Biosensor with AuNPs) | 5 µM | 283.9 | 4.5% |
| Detection Limit | Dynamic Range | References | |
|---|---|---|---|
| PEC/AuNPs/GOD/Au | 5 µM | 10 µM–7 mM | This work |
| Chitosan/PPy/AuNPs/ITO | 3.1 µM | 0–3.2 mM | [22] |
| Chitosan-GOD/AuNPs/GC | 13 µM | 50 µM–1.3 mM | [23] |
| Au/dithiol/Au/cystamine/GOx | 8.2 µM | 20 µM–5.7 mM | [24] |
| Amperometric detection (GOx-AuNP)n/Au | 2.7 mM | 0.005–2.4 mM | [25] |
| CS/β-G-GOD/AuNPs–CS/PB–CS/Au | 1.56 μM | 6.25–93.75 μM | [26] |
| (GCE/CHI–AuNPs) | 370 µM | 400 µL to 10.7 mM | [27] |
| Added Concentration of Glucose | Recovery % |
|---|---|
| 50 µM | 106 |
| 0.2 mM | 97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rassas, I.; Braiek, M.; Bonhomme, A.; Bessueille, F.; Raffin, G.; Majdoub, H.; Jaffrezic-Renault, N. Highly Sensitive Voltammetric Glucose Biosensor Based on Glucose Oxidase Encapsulated in a Chitosan/Kappa-Carrageenan/Gold Nanoparticle Bionanocomposite. Sensors 2019, 19, 154. https://doi.org/10.3390/s19010154
Rassas I, Braiek M, Bonhomme A, Bessueille F, Raffin G, Majdoub H, Jaffrezic-Renault N. Highly Sensitive Voltammetric Glucose Biosensor Based on Glucose Oxidase Encapsulated in a Chitosan/Kappa-Carrageenan/Gold Nanoparticle Bionanocomposite. Sensors. 2019; 19(1):154. https://doi.org/10.3390/s19010154
Chicago/Turabian StyleRassas, Ilhem, Mohamed Braiek, Anne Bonhomme, Francois Bessueille, Guy Raffin, Hatem Majdoub, and Nicole Jaffrezic-Renault. 2019. "Highly Sensitive Voltammetric Glucose Biosensor Based on Glucose Oxidase Encapsulated in a Chitosan/Kappa-Carrageenan/Gold Nanoparticle Bionanocomposite" Sensors 19, no. 1: 154. https://doi.org/10.3390/s19010154
APA StyleRassas, I., Braiek, M., Bonhomme, A., Bessueille, F., Raffin, G., Majdoub, H., & Jaffrezic-Renault, N. (2019). Highly Sensitive Voltammetric Glucose Biosensor Based on Glucose Oxidase Encapsulated in a Chitosan/Kappa-Carrageenan/Gold Nanoparticle Bionanocomposite. Sensors, 19(1), 154. https://doi.org/10.3390/s19010154