Gas Sensing with Iridium Oxide Nanoparticle Decorated Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Material Characterization
2.3. Sensor Fabrication
2.4. Gas Sensing Studies
3. Results
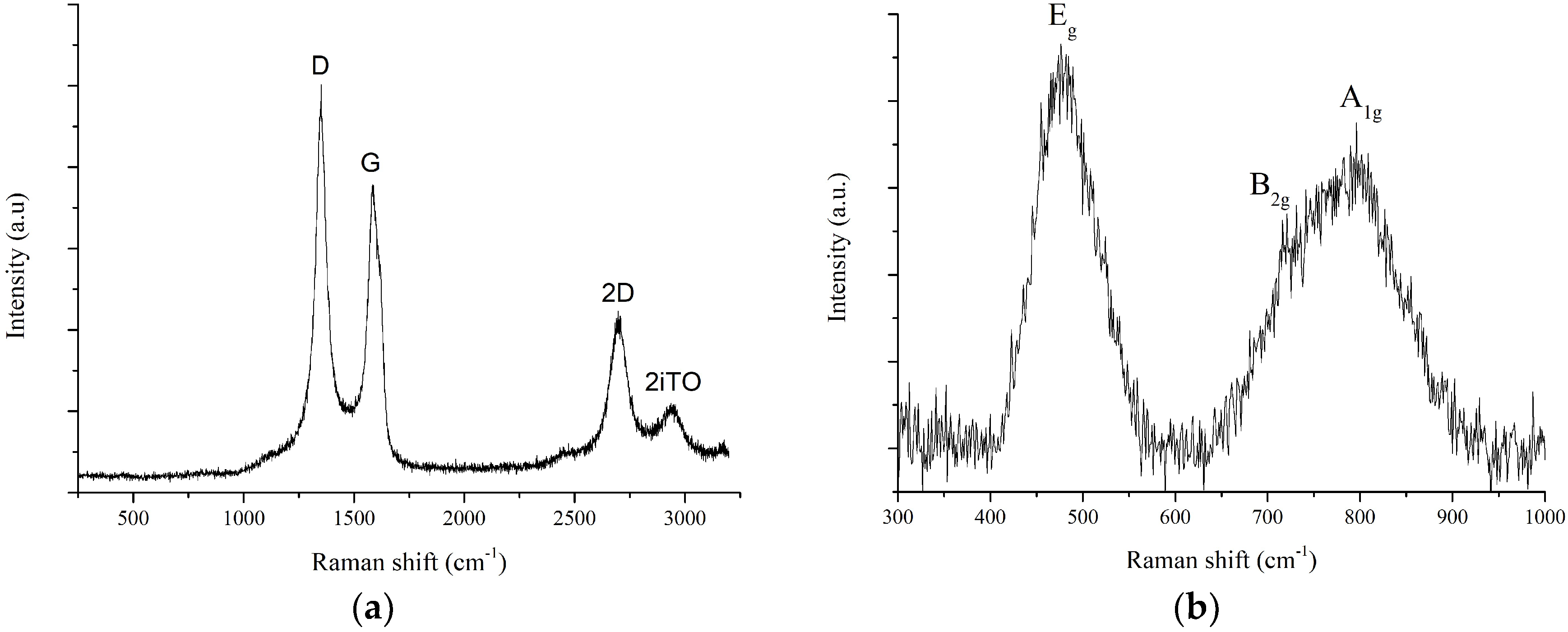
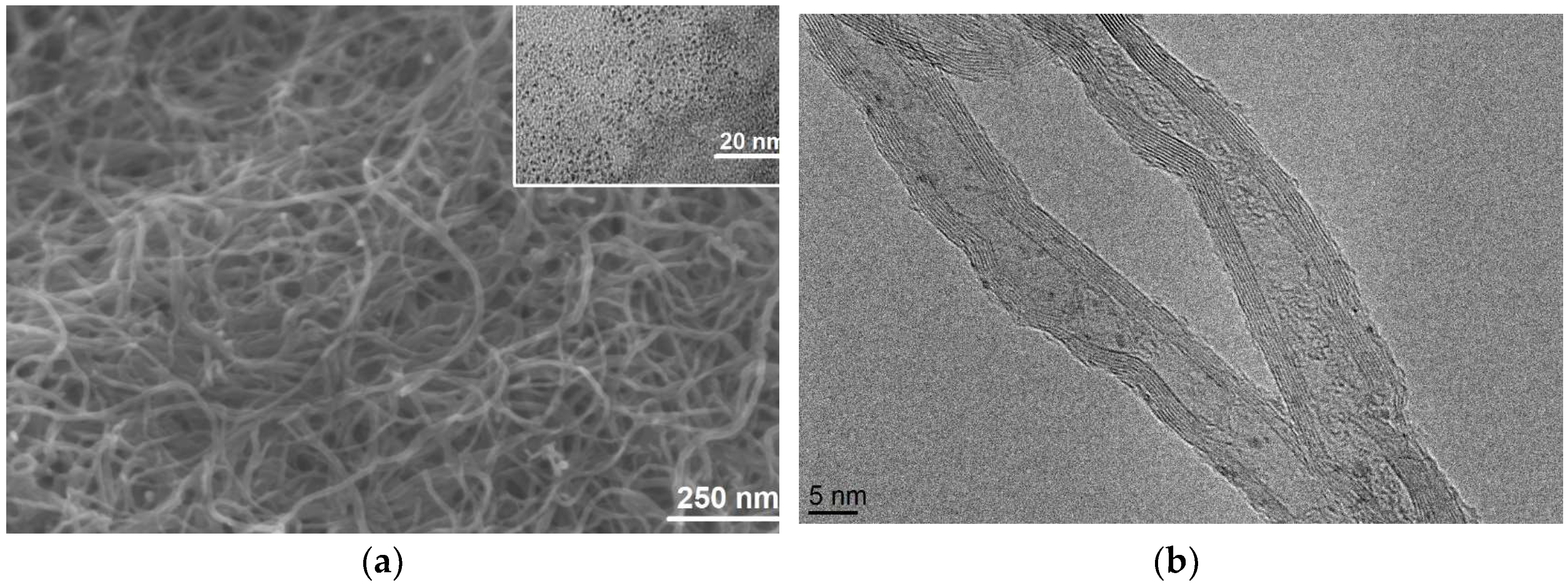
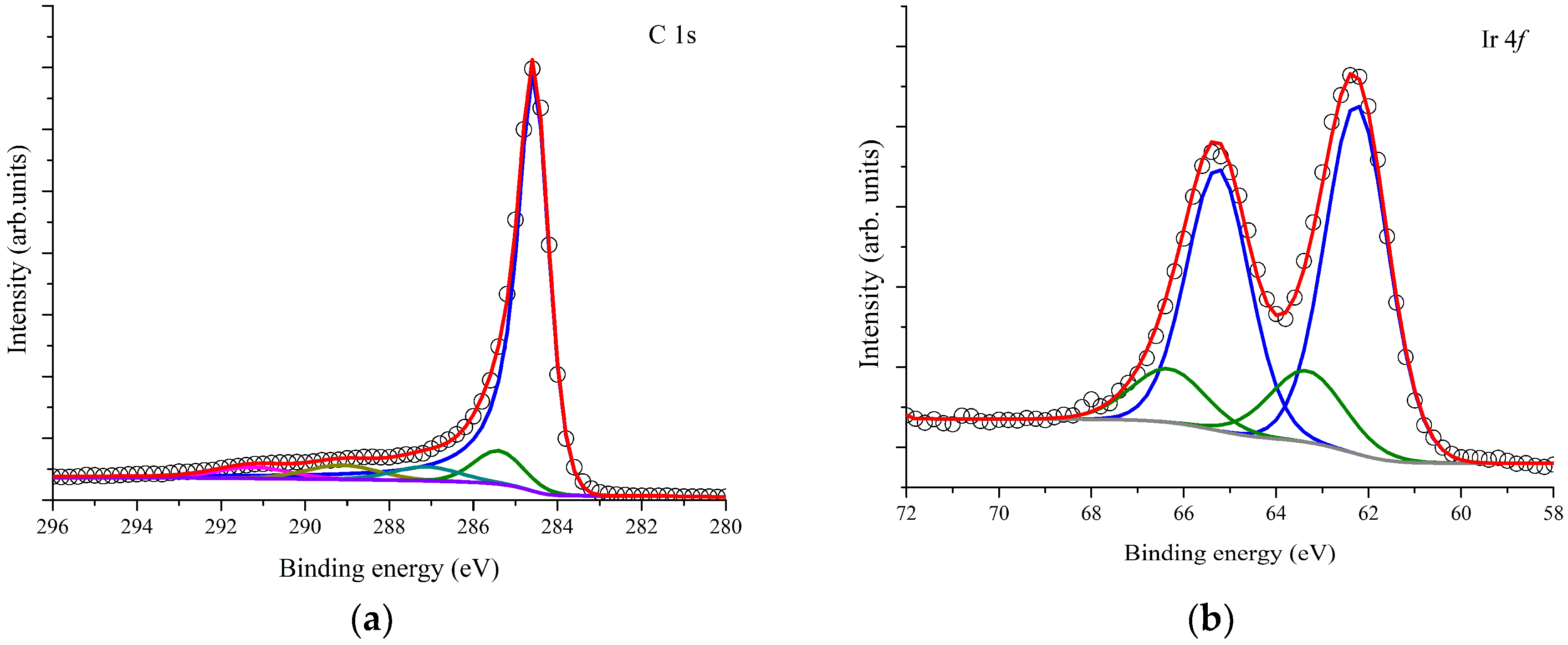
3.1. Material Characterization Results
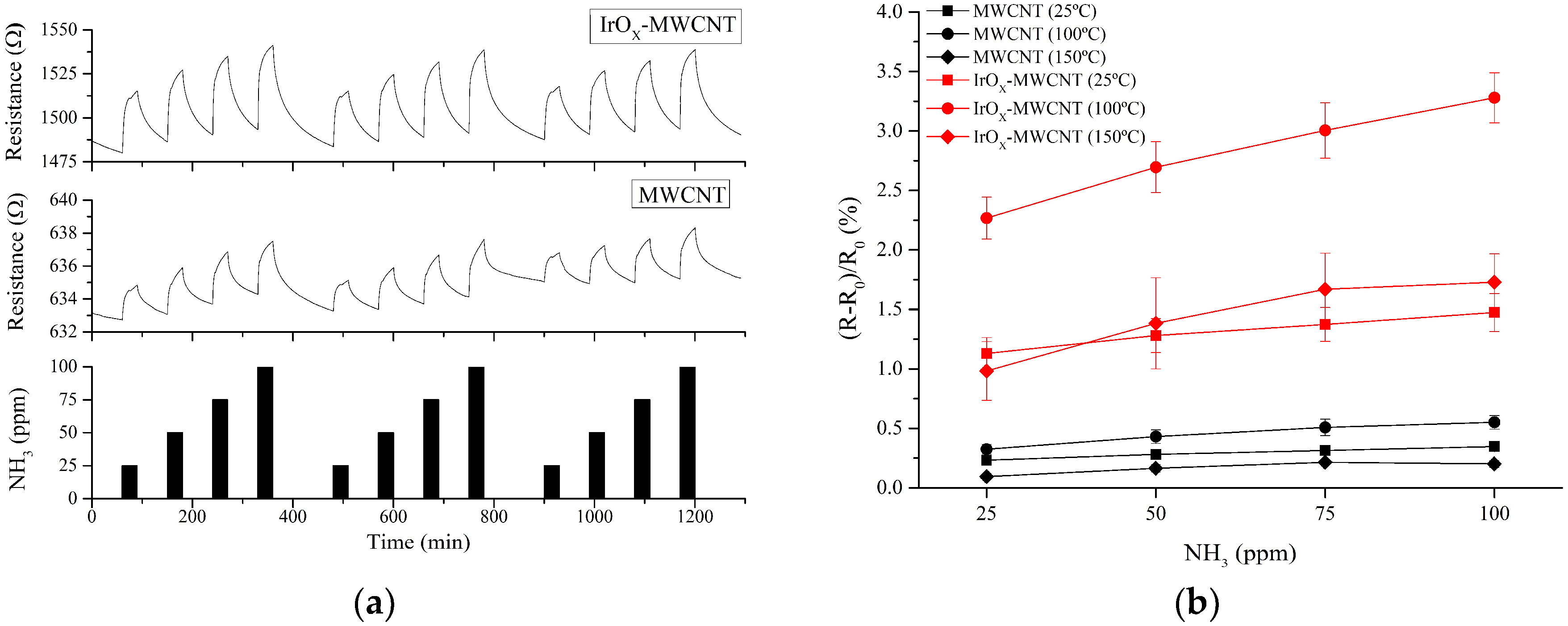
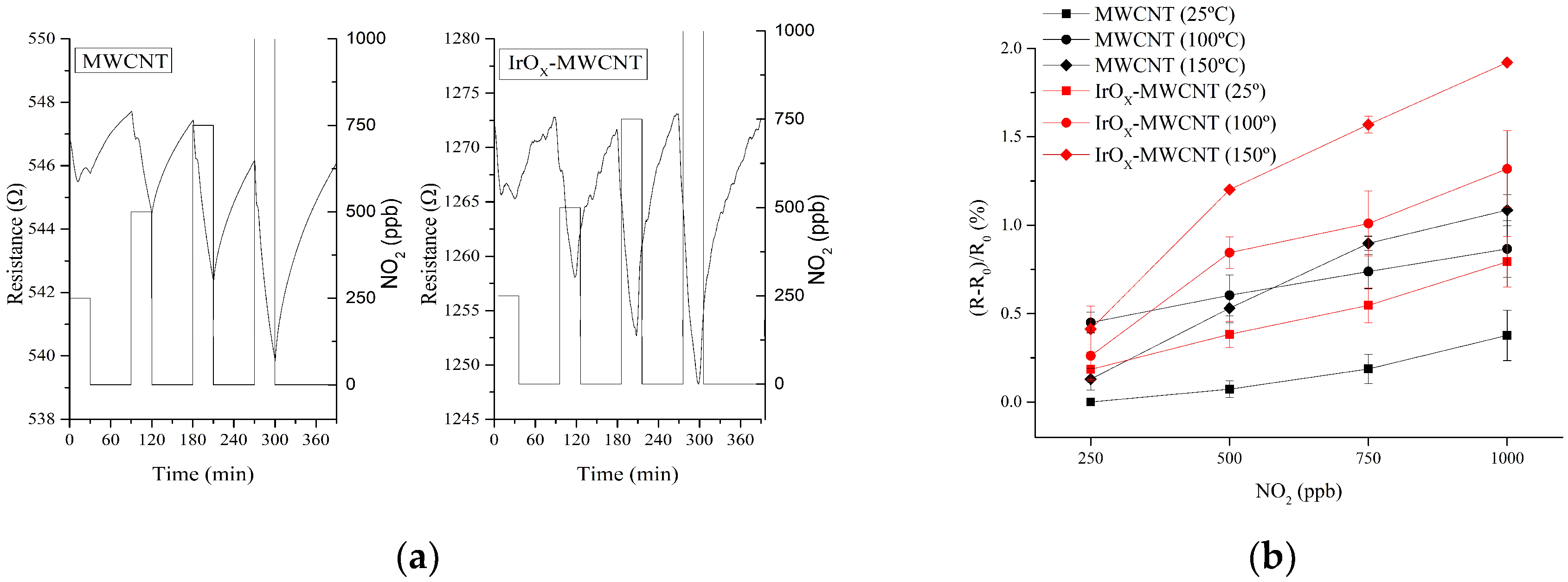
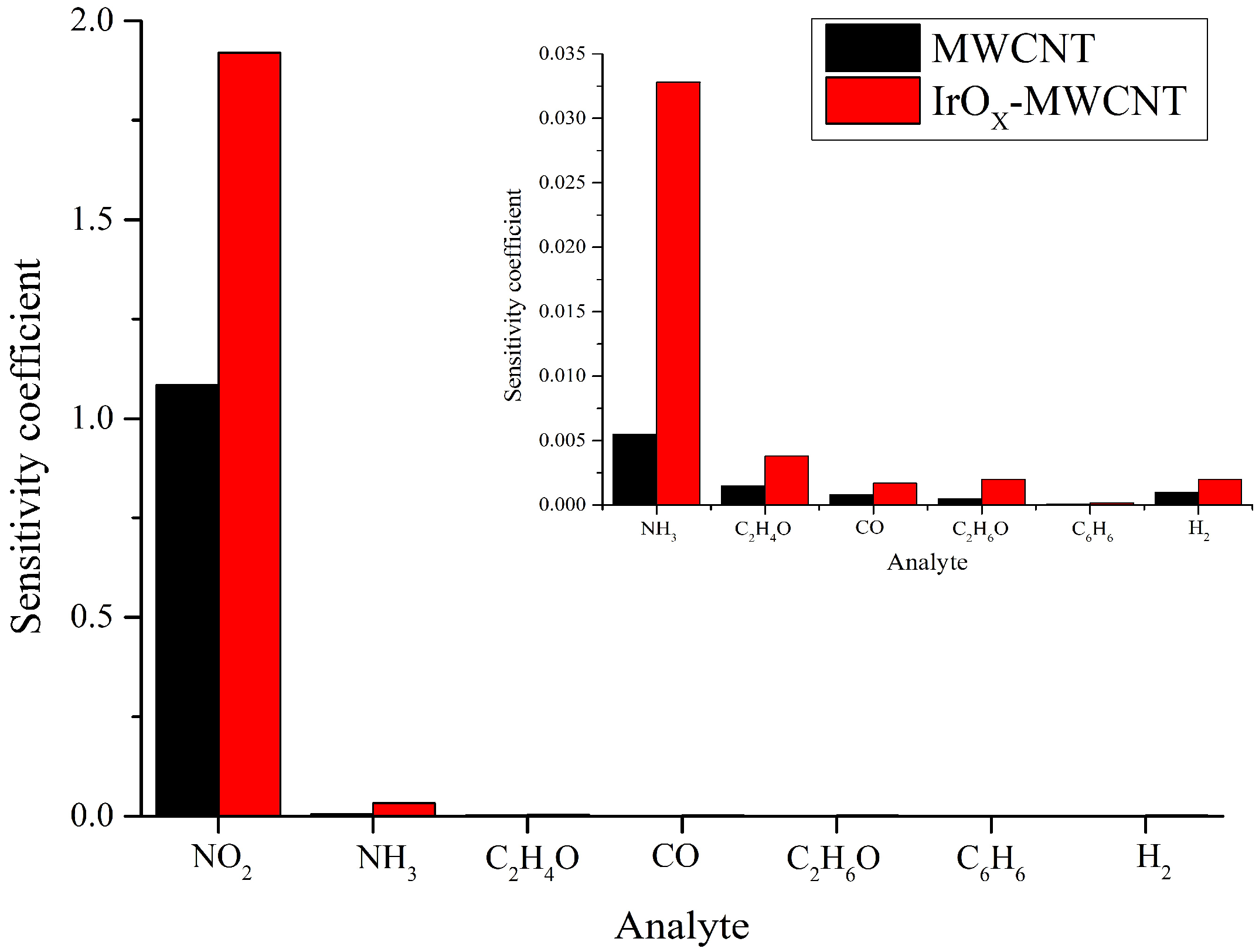
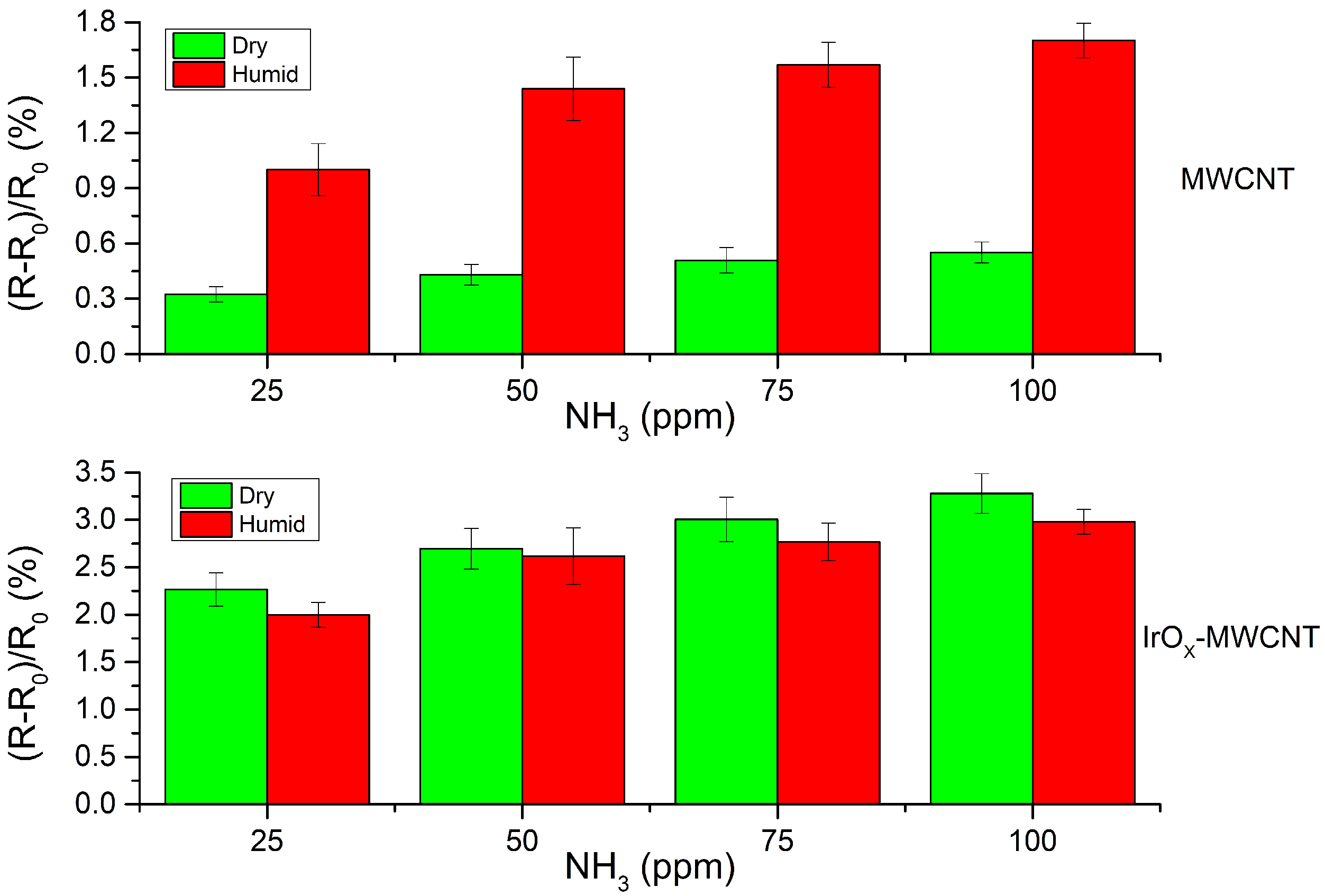
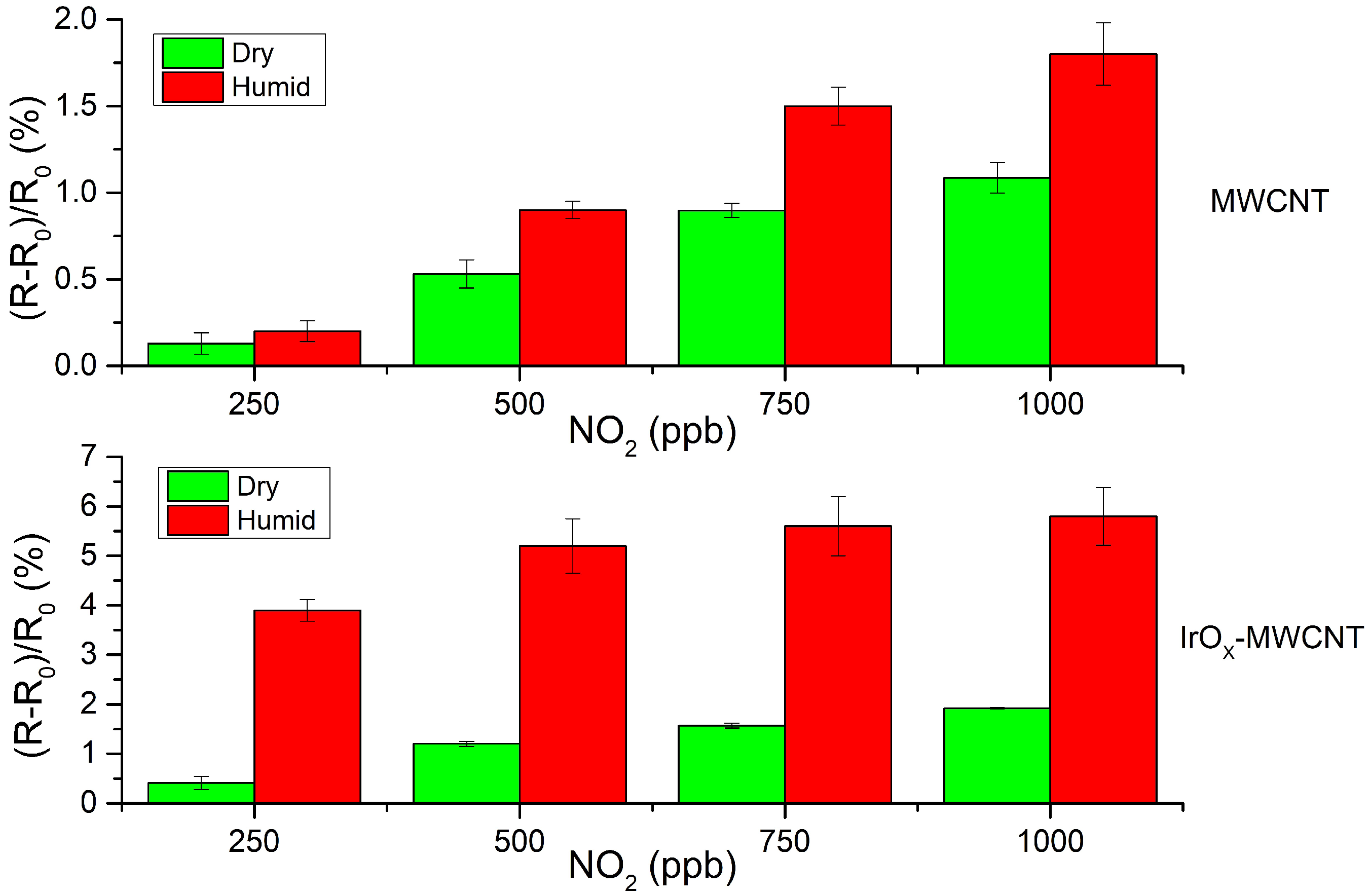
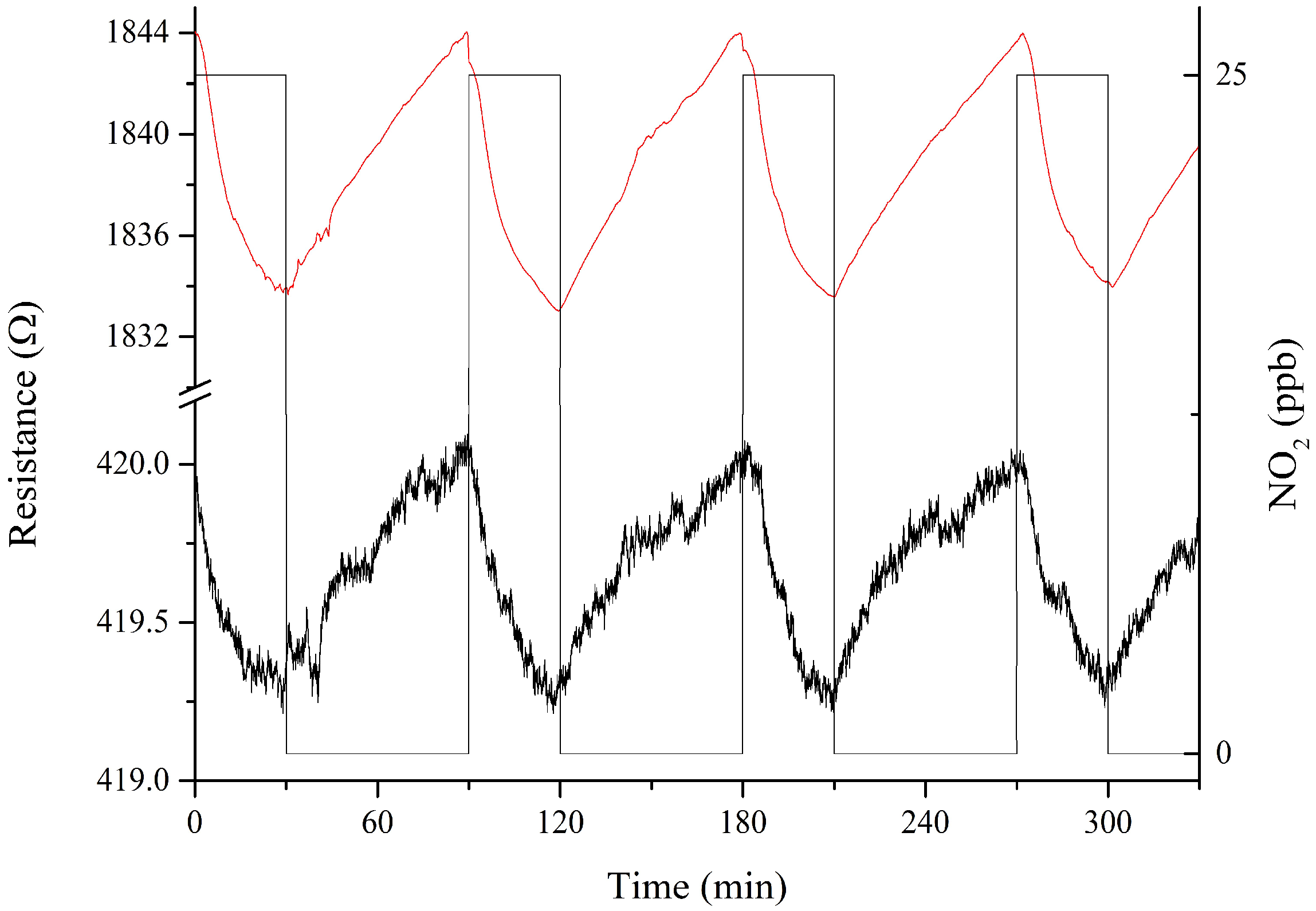
3.2. Gas Sensing Results
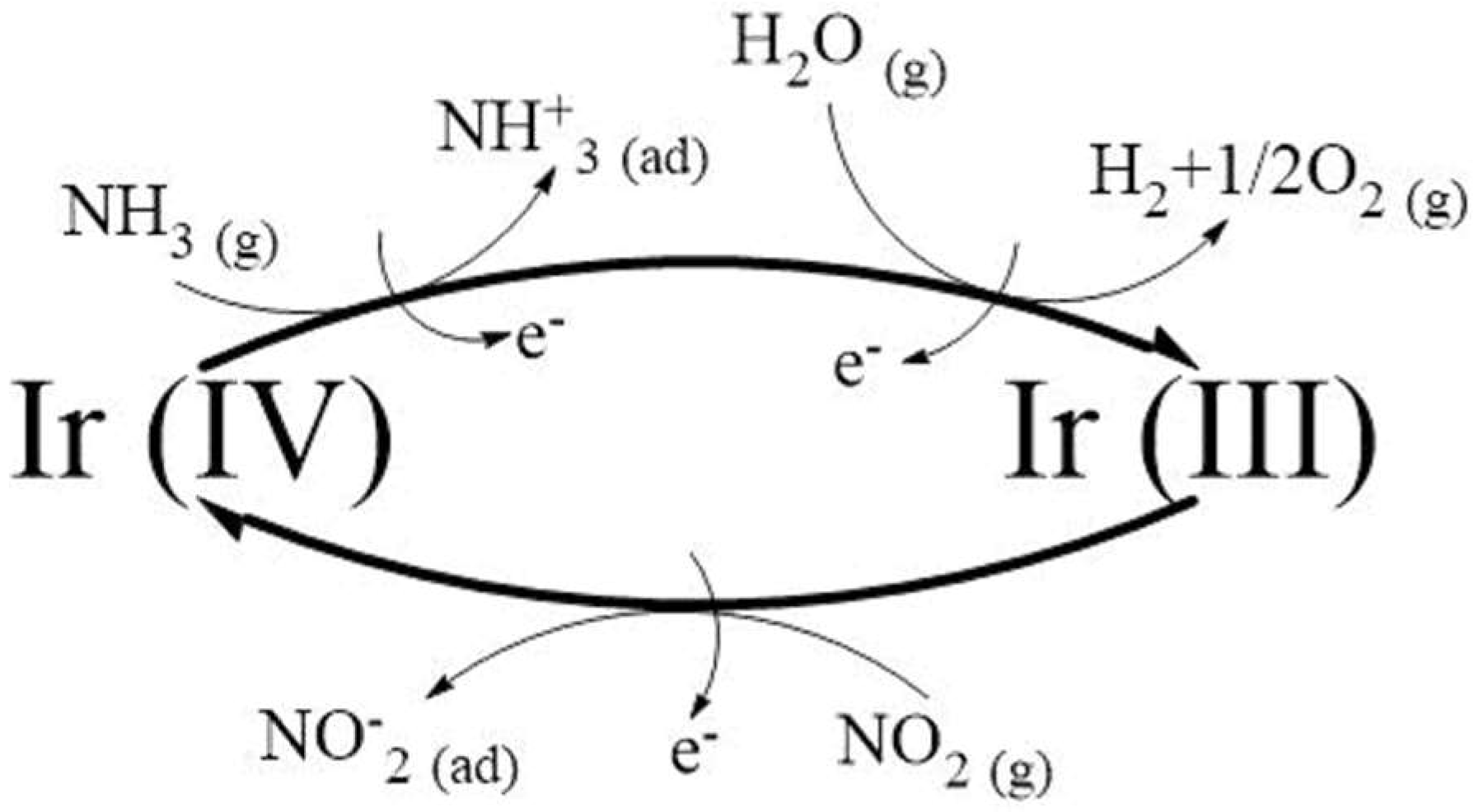
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Holzinger, M.; Vostrowsky, O.; Hirsch, A.; Hennrich, F.; Kappes, M.; Weiss, R.; Jellen, F. Sidewall Functionalization of Carbon Nanotubes. Angew. Chem. Int. Ed. 2001, 40, 4002–4005. [Google Scholar] [CrossRef]
- Zhang, Z.; Pfefferle, L.; Haller, G.L. Characterization of functional groups on oxidized multi-wall carbon nanotubes by potentiometric titration. Catal. Today 2015, 249, 23–29. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Kharissova, O.V.; Ortiz Méndez, U.; De La Fuente, I.G. Decoration of Carbon Nanotubes with Metal Nanoparticles: Recent Trends. Synth. React. Inorg. Met. Nano Met. Chem. 2016, 46, 55–76. [Google Scholar] [CrossRef]
- Irfan, M.; Pham, X.-H.; Han, K.N.; Li, C.A.; Hong, M.H.; Seong, G.H. Decoration of carbon nanotube films with iridium nanoparticles and their electrochemical characterization. BioChip J. 2014, 8, 129–136. [Google Scholar] [CrossRef]
- Blakemore, J.D.; Crabtree, R.H.; Brudvig, G.W. Molecular Catalysts for Water Oxidation. Chem. Rev. 2015, 115, 12974–13005. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Rauh, R.D. Novel amperometric immunosensors based on iridium oxide matrices. Biosens. Bioelectron. 2004, 19, 693–699. [Google Scholar] [CrossRef]
- Irhayem, E.A.; Elzanowska, H.; Jhas, A.S.; Skrzynecka, B.; Birss, V. Glucose detection based on electrochemically formed Ir oxide films. J. Electroanal. Chem. 2002, 538–539, 153–164. [Google Scholar] [CrossRef]
- Marzouk, S.A.M.; Ufer, S.; Buck, R.P.; Johnson, T.A.; Dunlap, L.A.; Cascio, W.E. Electrodeposited iridium oxide pH electrode for measurement of extracellular myocardial acidosis during Acute Ischemia. Anal. Chem. 1998, 70, 5054–5061. [Google Scholar] [CrossRef]
- Huang, W.D.; Cao, H.; Deb, S.; Chiao, M.; Chiao, J.C. A flexible pH sensor based on the iridium oxide sensing film. Sens. Actuators A Phys. 2011, 169, 1–11. [Google Scholar] [CrossRef]
- Brewer, S.H.; Wicaksana, D.; Maria, J.P.; Kingon, A.I.; Franzen, S. Investigation of the electrical and optical properties of iridium oxide by reflectance FTIR spectroscopy and density functional theory calculations. Chem. Phys. 2005, 313, 25–31. [Google Scholar] [CrossRef]
- Lang, A.C.; Fleischer, M.; Meixner, H. Surface modifications of Ga2O3 thin film sensors with Rh, Ru and Ir clusters. Sens. Actuators B Chem. 2000, 66, 80–84. [Google Scholar] [CrossRef]
- Bastuck, M.; Puglisi, D.; Huotari, J.; Sauerwald, T.; Lappalainen, J.; Lloyd Spetz, A.; Andersson, M.; Schütze, A. Exploring the selectivity of WO3 with iridium catalyst in an ethanol/naphthalene mixture using multivariate statistics. Thin Solid Films 2016, 618, 263–270. [Google Scholar] [CrossRef]
- Castañeda, L.; Maldonado, A.; Olvera, M.L. Sensing properties of chemically sprayed TiO2 thin films using Ni, Ir, and Rh as catalysts. Sens. Actuators B Chem. 2008, 133, 687–693. [Google Scholar] [CrossRef]
- Ozaki, Y.; Suzuki, S.; Morimitsu, M.; Matsunaga, M. Temperature and humidity dependence of SnO2-based CO gas sensors modified with iridium and ruthenium. Electrochem. Solid State Lett. 2000, 3, 297–299. [Google Scholar] [CrossRef]
- Pisal, S.H.; Harale, N.S.; Bhat, T.S.; Dshmukh, H.P.; Patil, P.S. Functionalized Multi-Walled Carbon Nanotubes for Nitrogen Sensor. IOSR J. Appl. Chem. 2014, 7, 49–52. [Google Scholar] [CrossRef]
- Peng, N.; Zhang, Q.; Chow, C.L.; Tan, O.K.; Marzari, N. Sensing Mechanisms for Carbon Nanotube Based NH3 Gas Detection. Nano Lett. 2009, 9, 1626–1630. [Google Scholar] [CrossRef]
- Karthigeyan, A.; Gupta, R.P.; Scharnagl, K.; Burgmair, M.; Sharma, S.K.; Eisele, I. A room temperature HSGFET ammonia sensor based on iridium oxide thin filmsmall size electronic nose. Sens. Actuators B Chem. 2002, 85, 145–153. [Google Scholar] [CrossRef]
- Zhao, Y.; Hernandez-Pagan, E.A.; Vargas-Barbosa, N.M.; Dysart, J.L.; Mallouk, T.E. A high yield synthesis of ligand-free iridium oxide nanoparticles with high electrocatalytic activity. J. Phys. Chem. Lett. 2011, 2, 402–406. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Korotcov, A.V.; Huang, Y.-S.; Tiong, K.-K.; Tsai, D.-S. Raman scattering characterization of well-aligned RuO2 and IrO2 nanocrystals. J. Raman Spectrosc. 2007, 38, 1538–1553. [Google Scholar] [CrossRef]
- D’Acunto, G.; Ripanti, F.; Postorino, P.; Betti, M.G.; Scardamaglia, M.; Bittencourt, C.; Mariani, C. Channelling and induced defects at ion-bombarded aligned multiwall carbon nanotubes. Carbon 2018, 139, 768–775. [Google Scholar] [CrossRef]
- Bittencourt, C.; Felten, A.; Douhard, B.; Ghijsen, J.; Johnson, R.L.; Drube, W.; Pireaux, J.-J. Photoemission studies of gold clusters thermally evaporated on multiwall carbon nanotubes. Chem. Phys. 2006, 328, 385–391. [Google Scholar] [CrossRef]
- Chen, C.; Liang, B.; Ogino, A.; Wang, X.; Nagatsu, M. Oxygen Functionalization of Multiwall Carbon Nanotubes by Microwave-Excited Surface-Wave Plasma Treatment. J. Phys. Chem. C 2009, 113, 7659–7665. [Google Scholar] [CrossRef]
- Casella, I.G.; Contursi, M.; Toniolo, R. Anodic electrodeposition of iridium oxide particles on glassy carbon surfaces and their electrochemical/SEM/XPS characterization. J. Electroanal. Chem. 2015, 736, 147–152. [Google Scholar] [CrossRef]
- Kötz, R.; Neff, H.; Stucki, S. Anodic Iridium Oxide Films. J. Electrochem. Soc. 1984, 131, 72. [Google Scholar] [CrossRef]
- Liu, L.; Ye, X.; Wu, K.; Han, R.; Zhou, Z.; Cui, T. Humidity Sensitivity of Multi-Walled Carbon Nanotube Networks Deposited by Dielectrophoresis. Sensors 2009, 9, 1714–1721. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Ammonia. Available online: https://www.cdc.gov/niosh/pel88/7664-41.html (accessed on 7 August 2018).
- Forootan, A.; Sjöback, R.; Björkman, J.; Sjögreen, B.; Linz, L.; Kubista, M. Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR). Biomol. Detect. Quantif. 2017, 12, 1–6. [Google Scholar] [CrossRef]
- Air Quality Standards. Available online: http://ec.europa.eu/environment/air/quality/standards.htm (accessed on 7 August 2018).
- NAAQS Table. Available online: https://www.epa.gov/criteria-air-pollutants/naaqs-table (accessed on 7 August 2018).
- Tang, R.; Shi, Y.; Hou, Z.; Wei, L. Carbon nanotube-based chemiresistive sensors. Sensors 2017, 17, 882. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.; Bosco, J.; Rayappan, B. Gas sensing mechanism of metal oxides: The role of ambient atmosphere, type of semiconductor and gases—A review. Sci. Lett. J. 2015, 4, 126. [Google Scholar]
- Rout, C.S.; Hegde, M.; Govindaraj, A.; Rao, C.N.R. Ammonia sensors based on metal oxide nanostructures. Nanotechnology 2007, 18, 205504. [Google Scholar] [CrossRef]
- Leghrib, R.; Dufour, T.; Demoisson, F.; Claessens, N.; Reniers, F.; Llobet, E. Gas sensing properties of multiwall carbon nanotubes decorated with rhodium nanoparticles. Sens. Actuators B Chem. 2011, 160, 974–980. [Google Scholar] [CrossRef]
- Sayago, I.; Santos, H.; Horrillo, M.C.; Aleixandre, M.; Fernández, M.J.; Terrado, E.; Tacchini, I.; Aroz, R.; Maser, W.K.; Benito, A.M.; et al. Carbon nanotube networks as gas sensors for NO2 detection. Talanta 2008, 77, 758–764. [Google Scholar] [CrossRef]
- Rahmani, M.B.; Breedon, M.; Lau, D.; Campbell, J.L.; Moafi, A.; McCulloch, D.G.; Wlodarski, W.; Kalantar-Zadeh, K. Gas sensing properties of interconnected ZnO nanowires. Sens. Lett. 2011, 9, 1–7. [Google Scholar] [CrossRef]
- Han, J.W.; Kim, B.; Li, J.; Meyyappan, M. Carbon nanotube based humidity sensor on cellulose paper. J. Phys. Chem. C 2012, 116, 22094–22097. [Google Scholar] [CrossRef]
- Chen, W.-P.; Zhao, Z.-G.; Liu, X.-W.; Zhang, Z.-X.; Suo, C.-G. A Capacitive Humidity Sensor Based on Multi-Wall Carbon Nanotubes (MWCNTs). Sensors 2009, 9, 7431–7444. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Liu, X.W.; Chen, W.P.; Li, T. Carbon nanotubes humidity sensor based on high testing frequencies. Sens. Actuators A Phys. 2011, 168, 10–13. [Google Scholar] [CrossRef]
- Roso, S.; Degler, D.; Llobet, E.; Barsan, N.; Urakawa, A. Temperature-Dependent NO2 Sensing Mechanisms over Indium Oxide. ACS Sens. 2017, 2, 1272–1277. [Google Scholar] [CrossRef]
- Mudimela, P.R.; Scardamaglia, M.; González-León, O.; Reckinger, N.; Snyders, R.; Llobet, E.; Bittencourt, C.; Colomer, J.F. Gas sensing with gold-decorated vertically aligned carbon nanotubes. Beilstein J. Nanotechnol. 2014, 5, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Baccar, H.; Thamri, A.; Clément, P.; Llobet, E.; Abdelghani, A. Pt- and Pd-decorated MWCNTs for vapour and gas detection at room temperature. Beilstein J. Nanotechnol. 2015, 6, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Leghrib, R.; Llobet, E.; Pavelko, R.; Vasiliev, A.A.; Felten, A.; Pireaux, J.J. Gas sensing properties of MWCNTs decorated with gold or tin oxide nanoparticles. Procedia Chem. 2009, 1, 168–171. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Mirzaei, A.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Synthesis, characterization and gas sensing properties of ZnO-decorated MWCNTs. Appl. Surf. Sci. 2017, 413, 242–252. [Google Scholar] [CrossRef]
- Nguyen, L.Q.; Phan, P.Q.; Duong, H.N.; Nguyen, C.D.; Nguyen, L.H. Enhancement of NH3 gas sensitivity at room temperature by carbon nanotube-based sensor coated with Co nanoparticles. Sensors 2013, 13, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Young, S.J.; Lin, Z.D. Ammonia gas sensors with Au-decorated carbon nanotubes. Microsyst. Technol. 2018, 24, 4207–4210. [Google Scholar] [CrossRef]
- Choi, H.H.; Lee, J.; Dong, K.Y.; Ju, B.K.; Lee, W. Noxious gas detection using carbon nanotubes with Pd nanoparticles. Nanoscale Res. Lett. 2011, 6, 1–6. [Google Scholar] [CrossRef]
- Lich, N.Q.; Thanh, T.P.; Truong, D.V.; Kien, P.T.; Tu, N.C.; Bac, L.H.; Vuong, D.D.; Chien, N.D.; Lam, N.H. Pt- and Ag-Decorated Carbon Nanotube Network Layers for Enhanced NH3 Gas Sensitivity at Room Temperature. Mater. Trans. 2015, 56, 1399–1402. [Google Scholar] [CrossRef]
| CNT | IrOx-MWCNT | |
|---|---|---|
| LOD | 17.8 ppb | 1 ppb |
| LOQ | 59.1 ppb | 3.2 ppb |
| CNT Decoration | Sensitivity | Reference |
|---|---|---|
| IrOx | 3.2 | TW |
| Au | 8 | [44] |
| Rh | 5 | [37] |
| Pt | 0.094 | [45] |
| Pd | 0.069 | [45] |
| SnO2 | 4.8 | [46] |
| ZnO2 | 0.25 | [47] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova-Cháfer, J.; Navarrete, E.; Noirfalise, X.; Umek, P.; Bittencourt, C.; Llobet, E. Gas Sensing with Iridium Oxide Nanoparticle Decorated Carbon Nanotubes. Sensors 2019, 19, 113. https://doi.org/10.3390/s19010113
Casanova-Cháfer J, Navarrete E, Noirfalise X, Umek P, Bittencourt C, Llobet E. Gas Sensing with Iridium Oxide Nanoparticle Decorated Carbon Nanotubes. Sensors. 2019; 19(1):113. https://doi.org/10.3390/s19010113
Chicago/Turabian StyleCasanova-Cháfer, Juan, Eric Navarrete, Xavier Noirfalise, Polona Umek, Carla Bittencourt, and Eduard Llobet. 2019. "Gas Sensing with Iridium Oxide Nanoparticle Decorated Carbon Nanotubes" Sensors 19, no. 1: 113. https://doi.org/10.3390/s19010113
APA StyleCasanova-Cháfer, J., Navarrete, E., Noirfalise, X., Umek, P., Bittencourt, C., & Llobet, E. (2019). Gas Sensing with Iridium Oxide Nanoparticle Decorated Carbon Nanotubes. Sensors, 19(1), 113. https://doi.org/10.3390/s19010113






