Sensor Selection to Improve Estimates of Particulate Matter Concentration from a Low-Cost Network
Abstract
1. Introduction
2. Methods and Materials
2.1. Sensor Selection Methods and Rationale
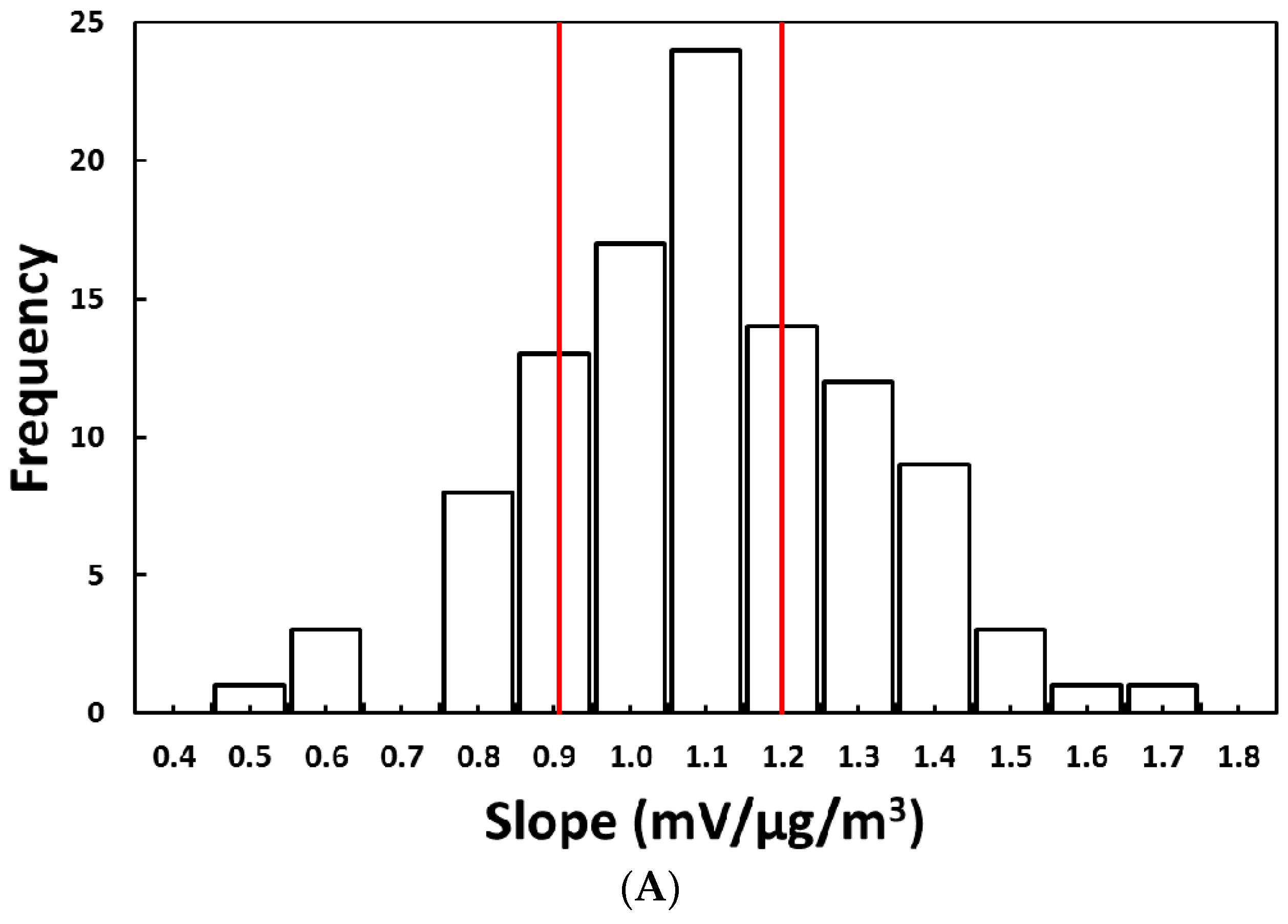
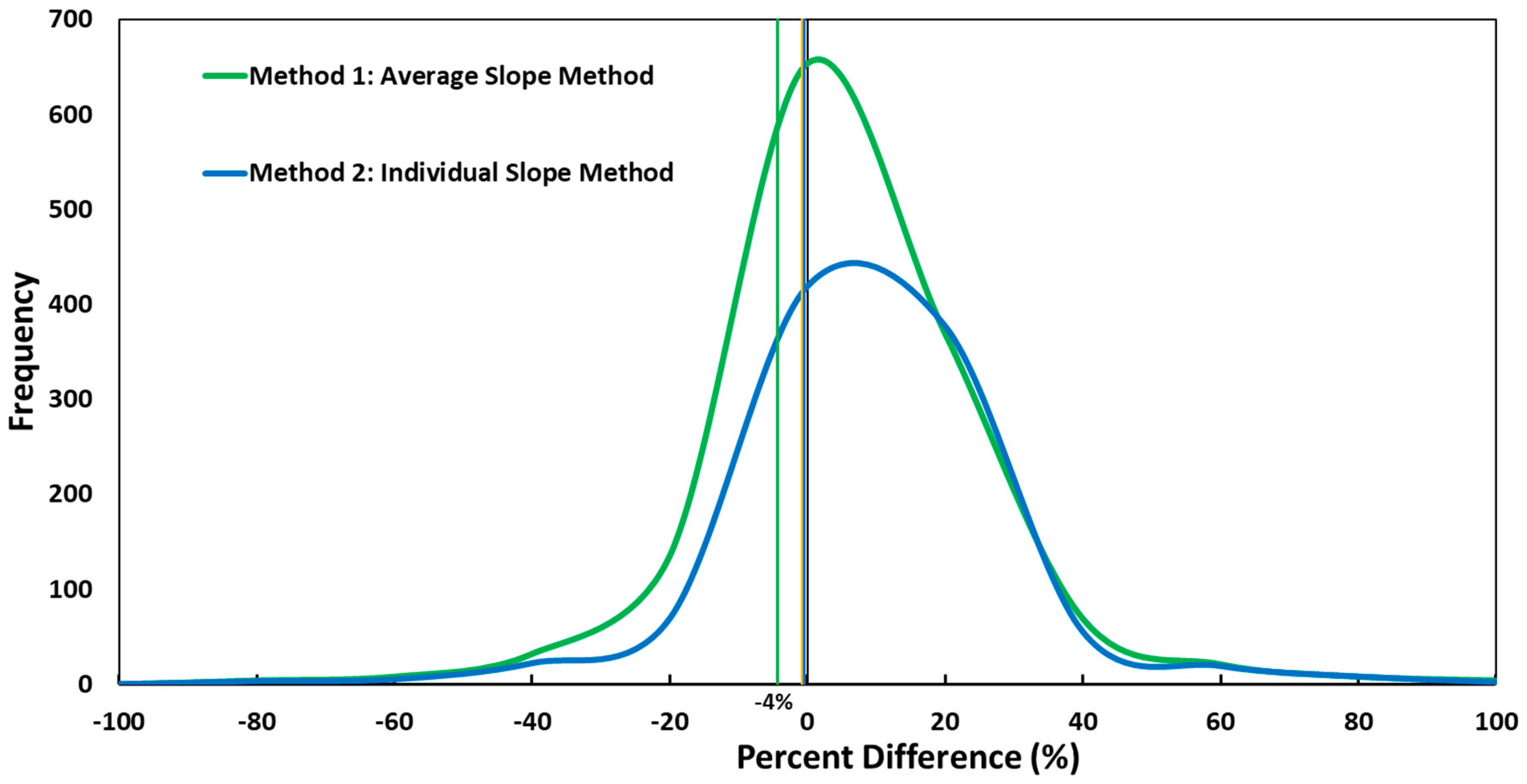
2.1.1. Method 1: Average Slope Method
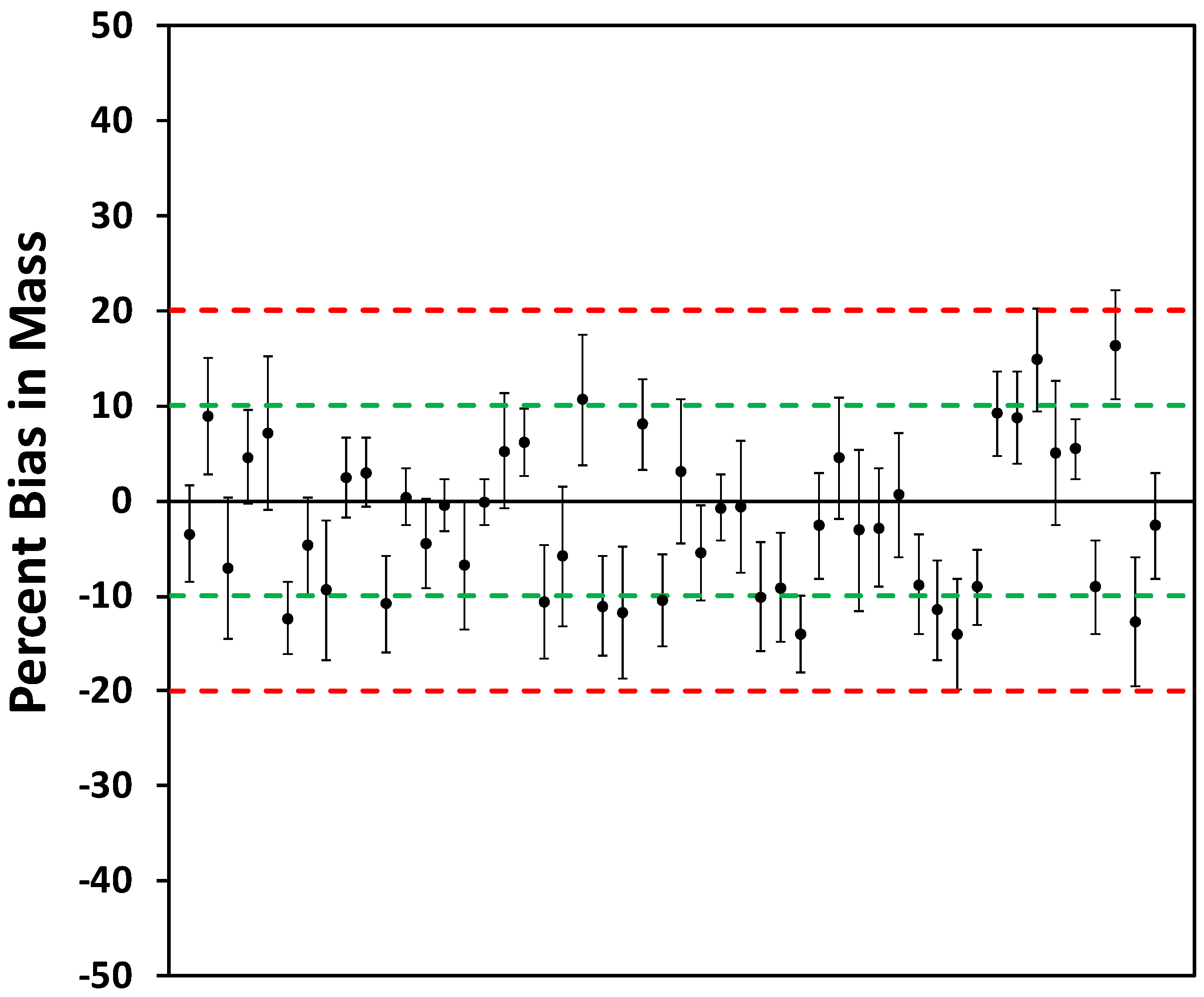
2.1.2. Method 2: Individual Slope Method
2.2. Laboratory Evaluation of PM Sensors
2.3. Application in Field
3. Results and Discussion
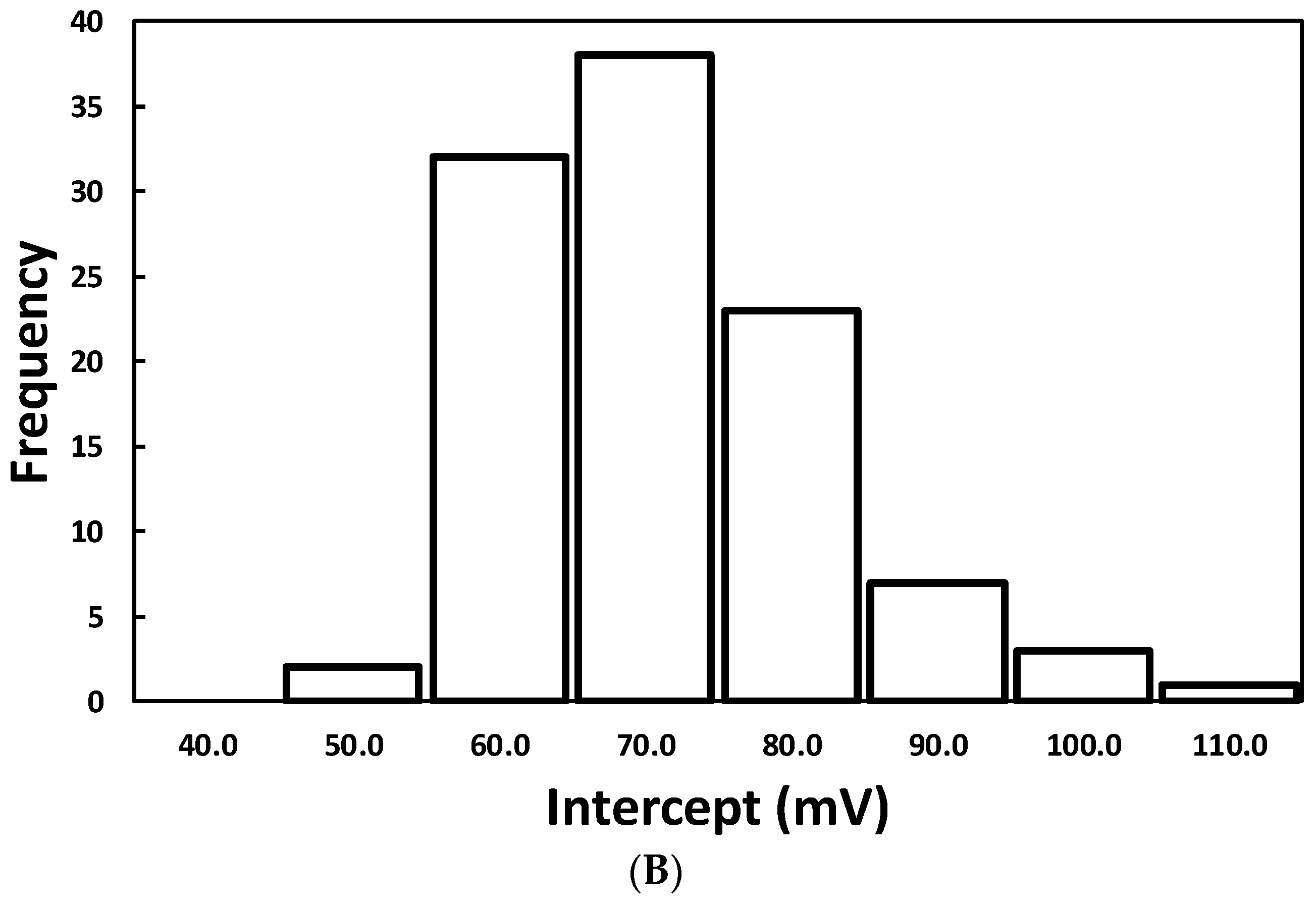
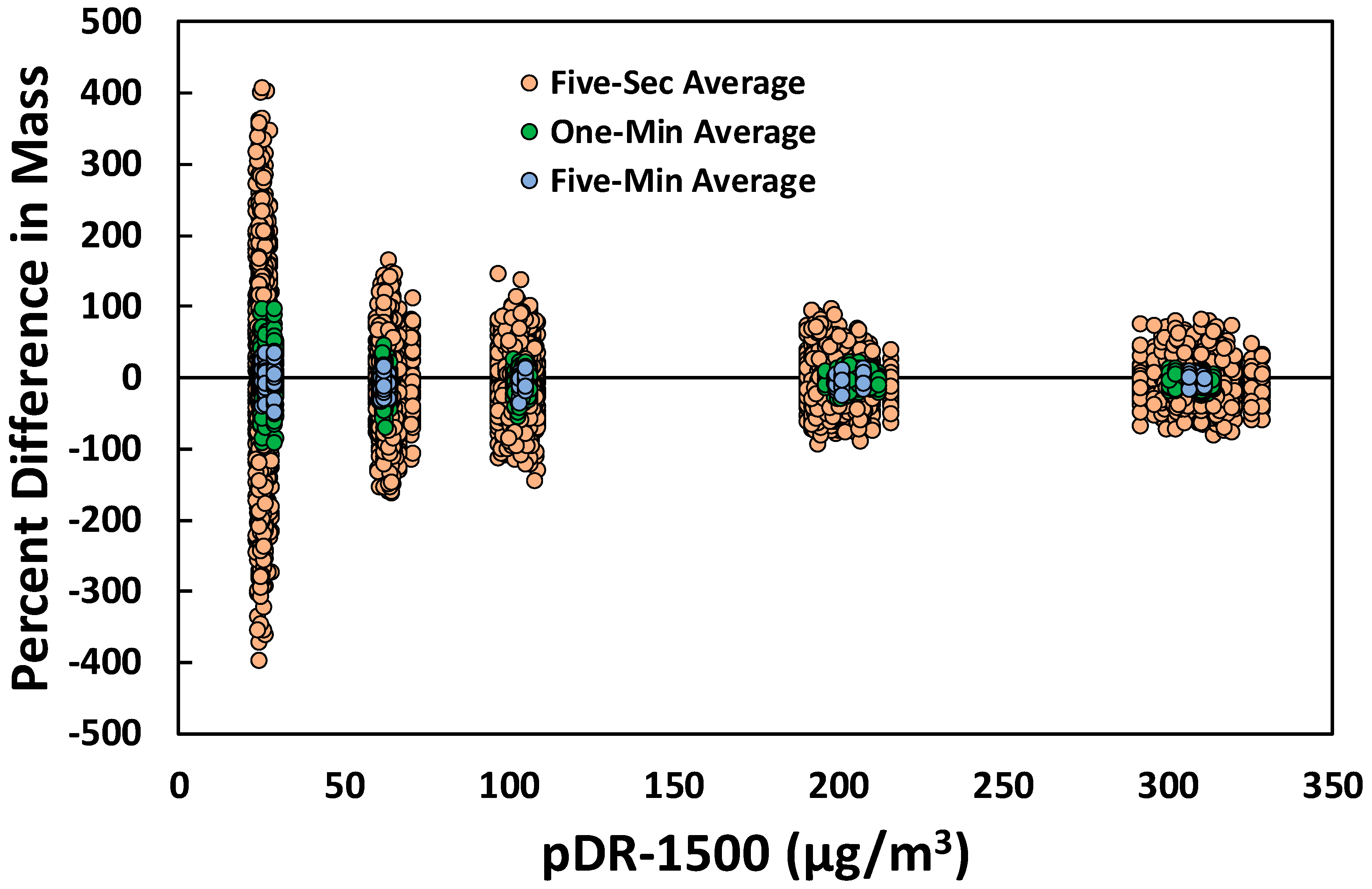
3.1. Laboratory Selection of PM Sensors
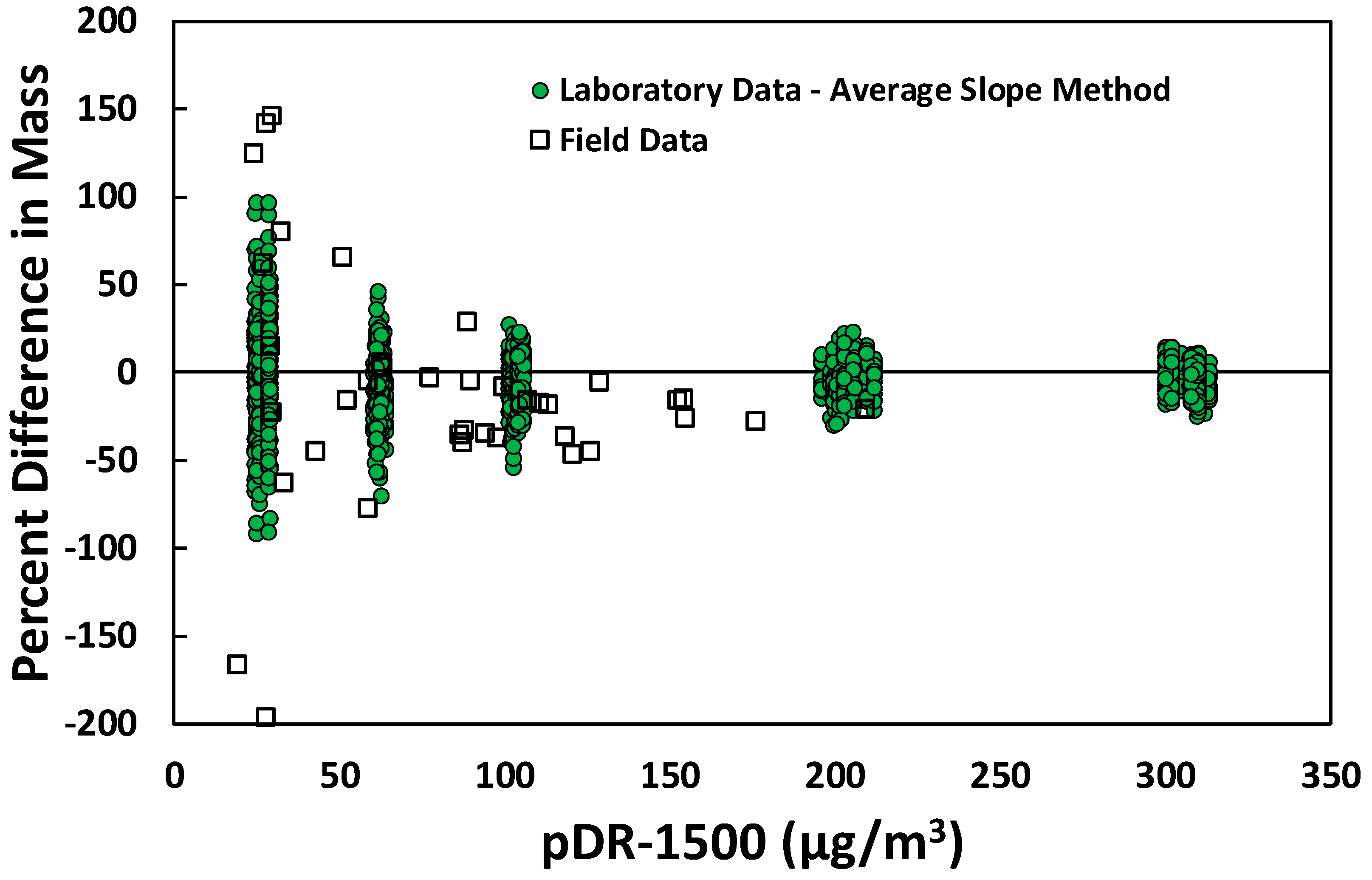
3.2. Application in the Field
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ACGIH. Threshold Limit Values and Biological Exposure Indicies. In Proceedings of the American Conference of Governmental Industrial Hygienists, Cincinnati, OH, USA, 8 July 2017. [Google Scholar]
- NIOSH. Method 0600, Issue 3: Particulates not otherwise regulated, respirable. In NIOSH Manual of Analytical Methods; Eller, P.M., Ed.; National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 1998. [Google Scholar]
- NIOSH. Components for Evaluation of Direct-Reading Monitors for Gases and Vapors; DHHS (NIOSH) Publication No. 2012–162; National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2012. [Google Scholar]
- White, W.H. Considerations in the use of ozone and PM(2.5) data for exposure assessment. Air Qual. Atmos. Health 2009, 2, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M. The rules of the game: An analysis of OSHA’s enforcement strategy. Am. J. Ind. Med. 1984, 6, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Thermo. Model PDM3700 Personal Dust Monitor. Thermo Fisher Scientific. Available online: http://tools.thermofisher.com/content/sfs/manuals/EPM-manual-PDM3700.pdf (accessed on 7 September 2018).
- Halterman, A.; Sousan, S.; Peters, T.M. Comparison of Respirable Mass Concentrations Measured by a Personal Dust Monitor and a Personal DataRAM to Gravimetric Measurements. Ann. Work. Expo. Health 2017, 62, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Görner, P.; Bemer, D.; Fabriés, J.F. Photometer measurement of polydisperse aerosols. J. Aerosol Sci. 1995, 26, 1281–1302. [Google Scholar] [CrossRef]
- Steinle, S.; Reis, S.; Sabel, C.E.; Semple, S.; Twigg, M.M.; Braban, C.F.; Leeson, S.R.; Heal, M.R.; Harrison, D.; Lin, C.; et al. Personal exposure monitoring of PM2.5 in indoor and outdoor microenvironments. Sci. Total. Environ. 2015, 508, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Holstius, D.M.; Pillarisetti, A.; Smith, K.R.; Seto, E. Field calibrations of a low-cost aerosol sensor at a regulatory monitoring site in California. Atmos. Meas. Tech. Discuss. 2014, 7, 605–632. [Google Scholar] [CrossRef]
- Gao, M.; Cao, J.; Seto, E. A distributed network of low-cost continuous reading sensors to measure spatiotemporal variations of PM2.5 in Xi’an, China. Environ. Pollut. 2015, 199, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Anthony, T.R.; Sousan, S.; Altmaier, R.; Park, J.H.; Peters, T.M. Evaluation of a Low-Cost Aerosol Sensor to Assess Dust Concentrations in a Swine Building. Ann. Occup. Hyg. 2016, 60, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Dausman, T.B.C. Low cost air quality monitors in agriculture. Available from Dissertations & Theses @ CIC Institutions; Dissertations & Theses @ University of Iowa; ProQuest Dissertations & Theses Global. (1929896237). Available online: http://proxy.lib.uiowa.edu/login?url=https://search.proquest.com/docview/1929896237?accountid=14663 (accessed on 7 September 2018).
- Unger, R.L. Compact, Low Cost Particle Sensor. Google Patents, U.S. Patent No. 8,009,290, 2011. [Google Scholar]
- Semple, S.; Apsley, A.; Maccalman, L. An inexpensive particle monitor for smoker behaviour modification in homes. Tob Control. 2013, 22, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Sharp. Application Note of SHARP Dust Sensor GP2Y1010AU0F [Online Resource]. Available online: http://www.sharp-world.com/products/device/lineup/data/pdf/datasheet/gp2y1010au_appl_e.pdf (accessed on 7 September 2018).
- Patel, S.; Li, J.; Pandey, A.; Pervez, S.; Chakrabarty, R.K.; Biswas, P. Spatio-temporal measurement of indoor particulate matter concentrations using a wireless network of low-cost sensors in households using solid fuels. Environ. Res. 2017, 152, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sridevi, S.; Pitchiah, R. Indoor air quality monitoring using wireless sensor network. In Proceedings of the 2012 Sixth International Conference on Sensing Technology (ICST), Kolkata, India, 18–21 December 2012; pp. 422–427. [Google Scholar]
- Kim, J.-J.; Jung, S.K.; Kim, J.T. Wireless Monitoring of Indoor Air Quality by a Sensor Network. Indoor Built Environ. 2010, 19, 145–150. [Google Scholar] [CrossRef]
- Rajasegarar, S.; Zhang, P.; Zhou, Y.; Karunasekera, S.; Leckie, C.; Palaniswami, M. High resolution spatio-temporal monitoring of air pollutants using wireless sensor networks. In Proceedings of the 2014 IEEE Ninth International Conference on Intelligent Sensors, Sensor Networks and Information Processing (ISSNIP), Singapore, 21–24 April 2014; pp. 1–6. [Google Scholar]
- Zikova, N.; Masiol, M.; Chalupa, D.; Rich, D.; Ferro, A.; Hopke, P. Estimating Hourly Concentrations of PM2.5 across a Metropolitan Area Using Low-Cost Particle Monitors. Sensors 2017, 17, 1922. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Castell, N.; Vogt, M.; Dauge, F.R.; Lahoz, W.A.; Bartonova, A. Mapping urban air quality in near real-time using observations from low-cost sensors and model information. Environ. Int. 2017, 106, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Sousan, S.; Koehler, K.; Thomas, G.; Park, J.H.; Hillman, M.; Halterman, A.; Peters, T.M. Inter-comparison of low-cost sensors for measuring the mass concentration of occupational aerosols. Aerosol Sci. Technol. 2016, 50, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Mohajer, N.; Zuidema, C.; Sousan, S.; Hallett, L.; Tatum, M.; Rule, A.M.; Thomas, G.; Peters, T.M.; Koehler, K. Evaluation of low-cost electro-chemical sensors for environmental monitoring of ozone, nitrogen dioxide and carbon monoxide. J. Occup. Environ. Hyg. 2017, 15, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Hallett, L.; Tatum, M.; Thomas, G.; Sousan, S.; Koehler, K.; Peters, T. An inexpensive sensor for noise. J. Occup. Environ. Hyg. 2018, 15, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.W.; Sousan, S.; Tatum, M.; Liu, X.; Zuidema, C.; Fitzpatrick, M.; Koehler, K.A.; Peters, T.M. Low-Cost, Distributed Environmental Monitors for Factory Worker Health. Sensors 2018, 18, 1411. [Google Scholar] [CrossRef] [PubMed]
- EPA. 40 CFR Parts 58-Ambient Air Quality Surveillance (Subchapter C); EPA: Washington, DC, USA, 2016. [Google Scholar]
- Hughes, I.; Hase, T. Measurements and Their Uncertainties: A Practical Guide to Modern Error Analysis; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Zikova, N.; Hopke, P.K.; Ferro, A.R. Evaluation of new low-cost particle monitors for PM2.5 concentrations measurements. J. Aerosol Sci. 2017, 105, 24–34. [Google Scholar] [CrossRef]
- Wallace, L.A.; Wheeler, A.J.; Kearney, J.; Van Ryswyk, K.; You, H.; Kulka, R.H.; Rasmussen, P.E.; Brook, J.R.; Xu, X. Validation of continuous particle monitors for personal, indoor, and outdoor exposures. J. Expo. Sci. Environ. Epidemiol. 2010, 21, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Sousan, S.; Koehler, K.; Hallett, L.; Peters, T.M. Evaluation of the Alphasense optical particle counter (OPC-N2) and the Grimm portable aerosol spectrometer (PAS-1.108). Aerosol Sci. Technol. 2016, 50, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- Budde, M.; Busse, M.; Beigl, M. Investigating the use of commodity dust sensors for the embedded measurement of particulate matter. In Proceedings of the 2012 Ninth International Conference on Networked Sensing Systems (INSS), Antwerp, Belgium, 11–12 June 2012; pp. 1–4. [Google Scholar]
| Sensor Number | Experiment 1 | Experiment 2 | Experiment 3 | Mean | Standard Deviation | CV (%) |
|---|---|---|---|---|---|---|
| 1 | 0.92 | 0.81 | 0.92 | 0.88 | 0.06 | 7 |
| 2 | 0.74 | 0.75 | 0.71 | 0.73 | 0.02 | 3 |
| 3 | 1.07 | 1.05 | 1.04 | 1.05 | 0.02 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousan, S.; Gray, A.; Zuidema, C.; Stebounova, L.; Thomas, G.; Koehler, K.; Peters, T. Sensor Selection to Improve Estimates of Particulate Matter Concentration from a Low-Cost Network. Sensors 2018, 18, 3008. https://doi.org/10.3390/s18093008
Sousan S, Gray A, Zuidema C, Stebounova L, Thomas G, Koehler K, Peters T. Sensor Selection to Improve Estimates of Particulate Matter Concentration from a Low-Cost Network. Sensors. 2018; 18(9):3008. https://doi.org/10.3390/s18093008
Chicago/Turabian StyleSousan, Sinan, Alyson Gray, Christopher Zuidema, Larissa Stebounova, Geb Thomas, Kirsten Koehler, and Thomas Peters. 2018. "Sensor Selection to Improve Estimates of Particulate Matter Concentration from a Low-Cost Network" Sensors 18, no. 9: 3008. https://doi.org/10.3390/s18093008
APA StyleSousan, S., Gray, A., Zuidema, C., Stebounova, L., Thomas, G., Koehler, K., & Peters, T. (2018). Sensor Selection to Improve Estimates of Particulate Matter Concentration from a Low-Cost Network. Sensors, 18(9), 3008. https://doi.org/10.3390/s18093008






