Portable System for Real-Time Detection of Stress Level
Abstract
1. Introduction
2. Materials and Methods
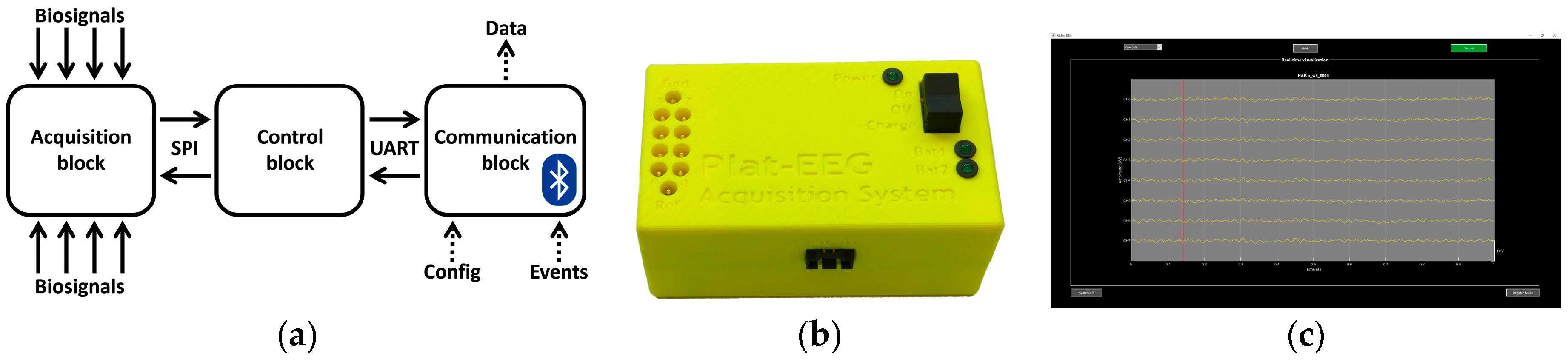
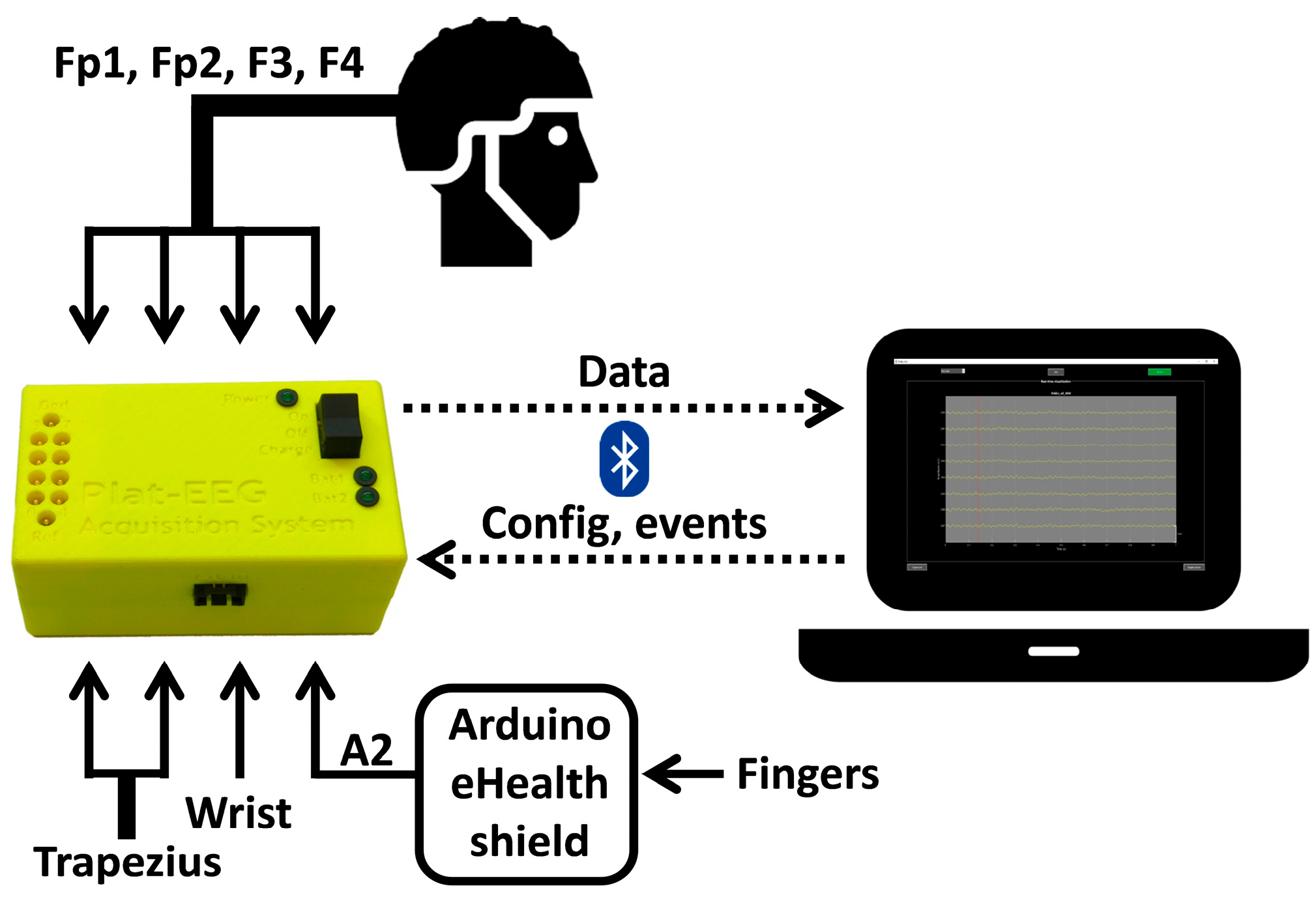
2.1. Description of the System
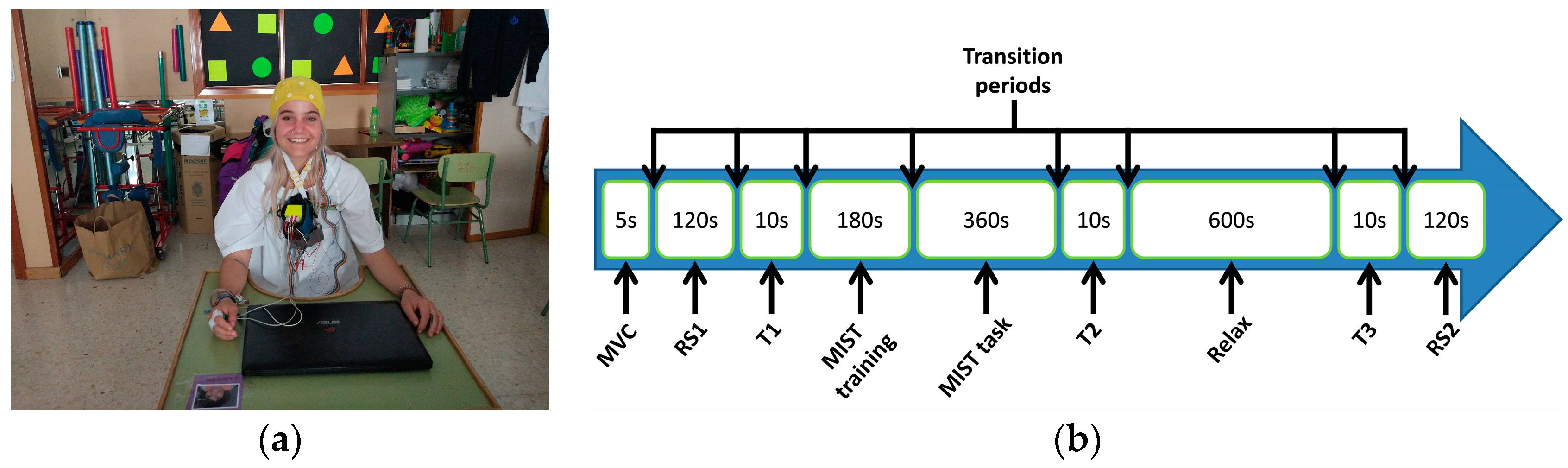
2.2. Experimental Procedure
2.3. Signal Processing
2.3.1. EEG
2.3.2. ECG
2.3.3. EMG
2.3.4. GSR
2.4. Statistical Analysis
2.5. Three-Level Stress Classification
3. Results
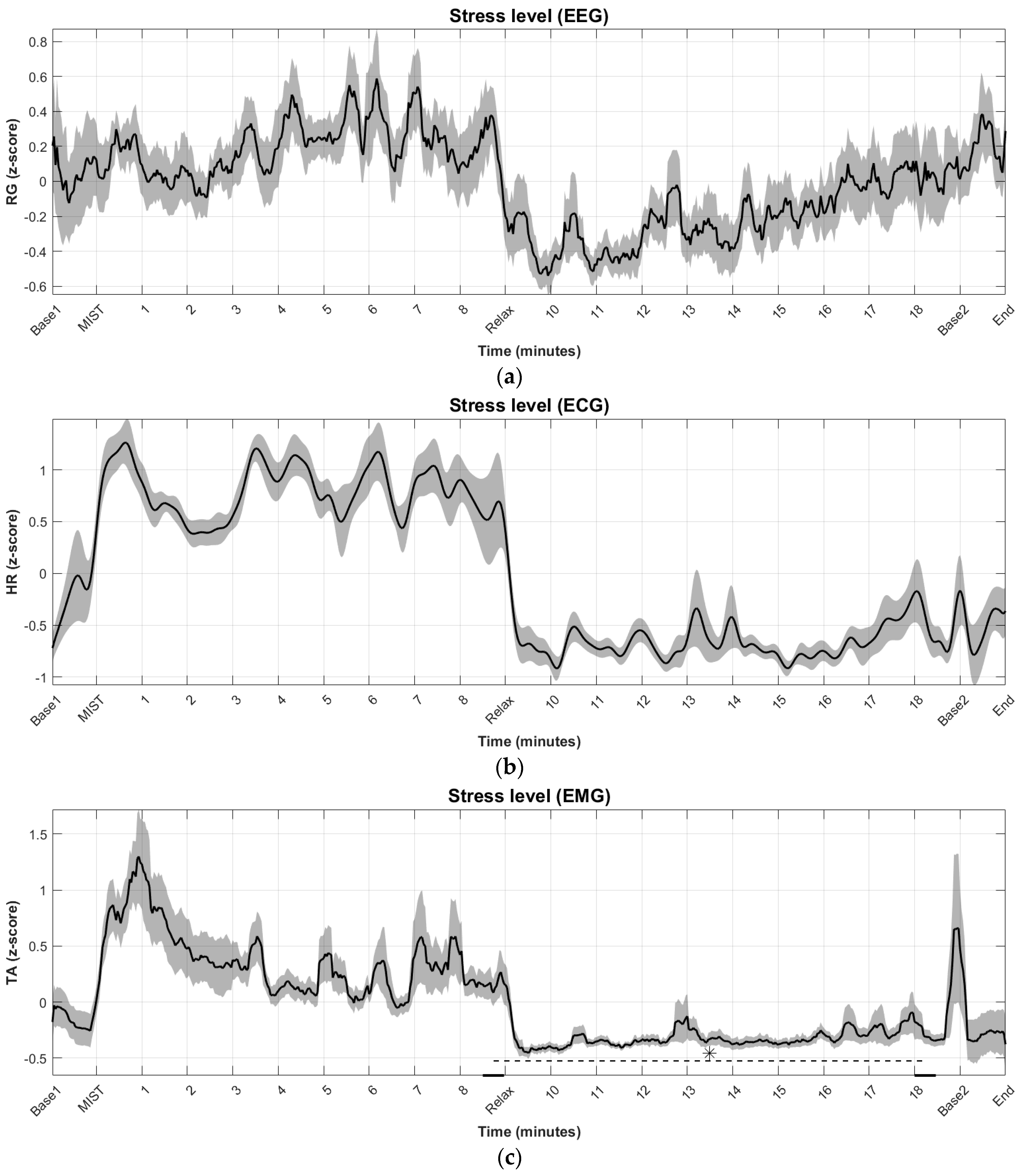
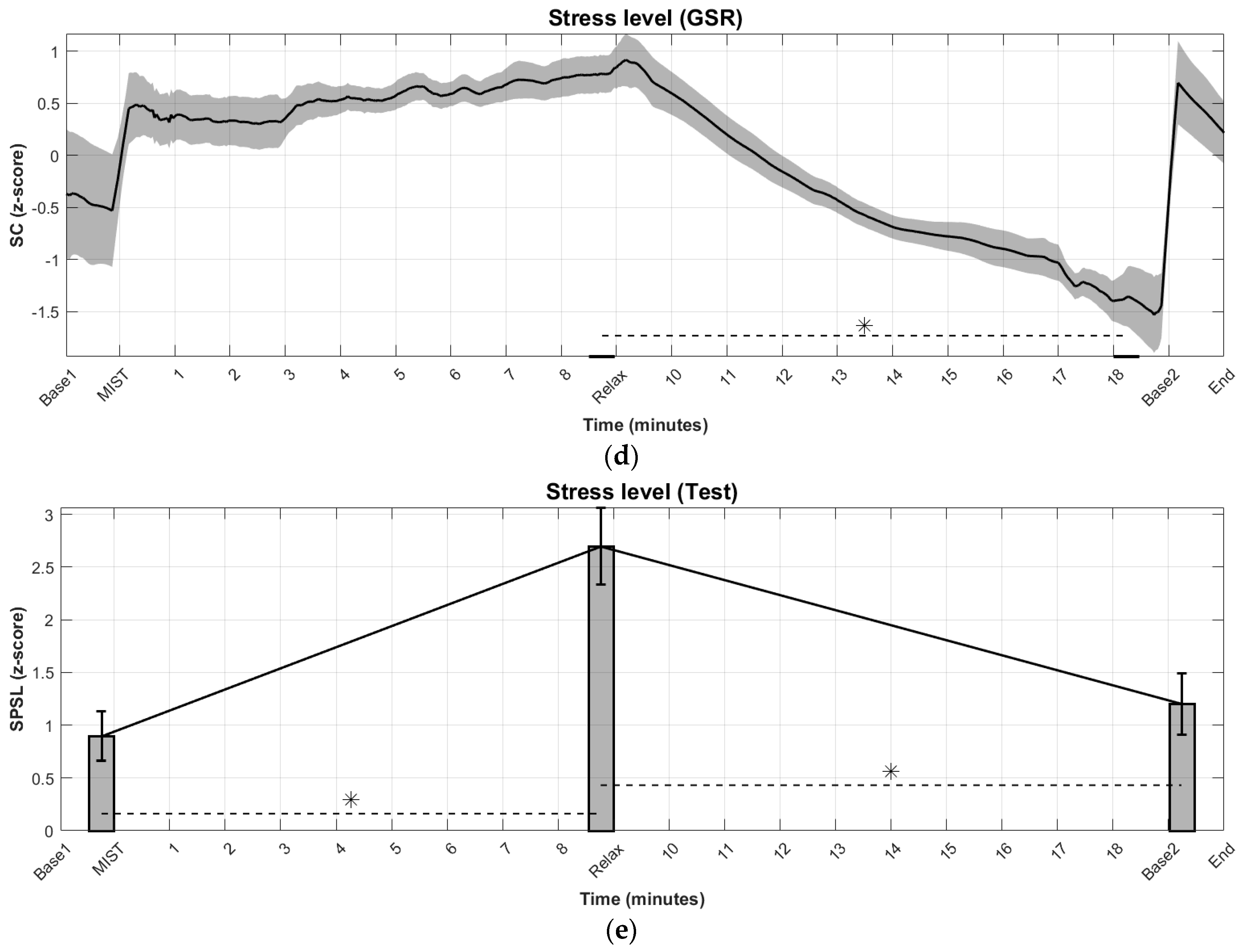
3.1. Time Evolution of Biosignal-Based Markers
3.2. Stress Level Detection
4. Discussion
4.1. Stress and Biosignals
4.2. Real-Time Detection of Stress Level
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef] [PubMed]
- Minguillon, J.; Lopez-Gordo, M.A.; Pelayo, F. Trends in EEG-BCI for daily-life: Requirements for artifact removal. Biomed. Signal Process. Control 2017, 31, 407–418. [Google Scholar] [CrossRef]
- Black, A.D.; Car, J.; Pagliari, C.; Anandan, C.; Cresswell, K.; Bokun, T.; McKinstry, B.; Procter, R.; Majeed, A.; Sheikh, A. The impact of ehealth on the quality and safety of health care: A systematic overview. PLoS Med. 2011, 8, e1000387. [Google Scholar] [CrossRef] [PubMed]
- Blaya, J.A.; Fraser, H.S.F.; Holt, B. E-health technologies show promise in developing countries. Health Aff. 2010, 29, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Barello, S.; Triberti, S.; Graffigna, G.; Libreri, C.; Serino, S.; Hibbard, J.; Riva, G. eHealth for patient engagement: A Systematic Review. Front. Psychol. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Muellmann, S.; Forberger, S.; Möllers, T.; Bröring, E.; Zeeb, H.; Pischke, C.R. Effectiveness of eHealth interventions for the promotion of physical activity in older adults: A systematic review. Prev. Med. 2018, 108, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Morland, L.A.; Greene, C.J.; Rosen, C.S.; Kuhn, E.; Hoffman, J.; Sloan, D.M. Telehealth and eHealth interventions for posttraumatic stress disorder. Curr. Opin. Psychol. 2017, 14, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, A.; Oppermann, R. Mobile health monitoring for the elderly: Designing for diversity. Pervasive Mob. Comput. 2009, 5, 478–495. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Silva, J.N.A. Use of smartphone technology in cardiology. Trends Cardiovasc. Med. 2016, 26, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.M.; Nowostawski, M.; Franz, E.A.; Purvis, M. Parkinson’s disease and essential tremor classification on mobile device. Pervasive Mob. Comput. 2014, 13, 1–12. [Google Scholar] [CrossRef]
- Lakshminarayan, K.; Westberg, S.; Northuis, C.; Fuller, C.C.; Ikramuddin, F.; Ezzeddine, M.; Scherber, J.; Speedie, S. A mHealth-based care model for improving hypertension control in stroke survivors: Pilot RCT. Contemp. Clin. Trials 2018, 70, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Han, H.; Du, J.; Zhao, M.; Hser, Y.I. A pilot study of a smartphone application supporting recovery from drug addiction. J. Subst. Abuse Treat. 2018, 88, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Picard, R.W. Affective Computing: From laughter to IEEE. IEEE Trans. Affect. Comput. 2010, 1, 11–17. [Google Scholar] [CrossRef]
- Dimitriev, D.A.; Saperova, E.V. Heart rate variability and blood pressure during mental stress. Ross Fiziol Zh Im I M Sechenova 2015, 101, 98–107. [Google Scholar] [PubMed]
- Ranganathan, G.; Rangarajan, R.; Bindhu, V. Estimation of heart rate signals for mental stress assessment using neuro fuzzy technique. Appl. Soft Comput. 2012, 12, 1978–1984. [Google Scholar] [CrossRef]
- Chandiramani, S.; Cohorn, L.C.; Chandiramani, S. Heart rate changes during acute mental stress with closed loop stimulation: Report on two single-blinded, pacemaker studies. Pacing Clin. Electrophysiol. 2007, 30, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Regula, M.; Socha, V.; Kutilek, P.; Socha, L.; Hana, K.; Hanakova, L.; Szabo, S. Study of heart rate as the main stress indicator in aircraft pilots. In Proceedings of the 16th IEEE International Conference on Mechatronics—Mechatronika, Brno, Czech Republic, 3–5 December 2014; pp. 639–643. [Google Scholar]
- Sayette, M.A. Heart rate as an index of stress response in alcohol administration research: A critical review. Alcohol Clin. Exp. Res. 1993, 17, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Michels, N.; Sioen, I.; Clays, E.; De Buyzere, M.; Ahrens, W.; Huybrechts, I.; Vanaelst, B.; De Henauw, S. Children’s heart rate variability as stress indicator: Association with reported stress and cortisol. Biol. Psychol. 2013, 94, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, U.; Kadefors, R.; Melin, B.; Palmerud, G.; Hassmén, P.; Engström, M.; Dohns, I.E. Psychophysiological stress and EMG activity of the trapezius muscle. Int. J. Behav. Med. 1994, 1, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Wijsman, J.; Grundlehner, B.; Penders, J.; Hermens, H. Trapezius muscle EMG as predictor of mental stress. ACM Trans. Embed. Comput. Syst. 2013, 12. [Google Scholar] [CrossRef]
- Larsson, S.E.; Larsson, R.; Zhang, Q.; Cai, H.; Åke Öberg, P. Effects of psychophysiological stress on trapezius muscles blood flow and electromyography during static load. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, L.M.; Spalding, T.W.; Kerick, S.E.; Cram, J.R.; Ley, R.; Hatfield, B.D. Mental stress and trapezius muscle activation under psychomotor challenge: A focus on EMG gaps during computer work. Psychophysiology 2008, 45, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Papousek, I.; Weiss, E.M.; Schulter, G.; Fink, A.; Reiser, E.M.; Lackner, H.K. Prefrontal EEG alpha asymmetry changes while observing disaster happening to other people: Cardiac correlates and prediction of emotional impact. Biol. Psychol. 2014, 103, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Lee, J. Stress and EEG. In Convergence and Hybrid Information Technologies; Crisan, M., Ed.; IntechOpen: London, UK, 2010; pp. 413–426. [Google Scholar]
- Hu, B.; Peng, H.; Zhao, Q.; Hu, B.; Majoe, D.; Zheng, F.; Moore, P. Signal Quality Assessment Model for Wearable EEG Sensor on Prediction of Mental Stress. IEEE Trans. Nanobiosci. 2015, 14, 553–561. [Google Scholar] [CrossRef]
- Brouwer, A.-M.; Neerincx, M.A.; Kallen, V.; van der Leer, L.; ten Brinke, M. EEG alpha asymmetry, heart rate variability and cortisol in response to Virtual Reality induced stress. J. Cyberther. Rehabil. 2011, 4, 27–40. [Google Scholar]
- Minguillon, J.; Lopez-Gordo, M.A.; Pelayo, F. Stress Assessment by Prefrontal Relative Gamma. Front. Comput. Neurosci. 2016, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R.; Wineinger, N.E.; Patel, S.; Boeldt, D.L.; Mackellar, G.; Porter, V.; Redmond, J.T.; Muse, E.D.; Nicholson, L.; Chopra, D.; et al. Cardiovascular and nervous system changes during meditation. Front. Hum. Neurosci. 2015, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.; Greischar, L.L.; Rawlings, N.B.; Ricard, M.; Davidson, R.J. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proc. Natl. Acad. Sci. USA 2004, 101, 16369–16373. [Google Scholar] [CrossRef] [PubMed]
- Minguillon, J.; Lopez-Gordo, M.A.; Renedo-Criado, D.A.; Sanchez-Carrion, M.J.; Pelayo, F. Blue lighting accelerates post-stress relaxation: Results of a preliminary study. PLoS ONE 2017, 12, e0186399. [Google Scholar] [CrossRef] [PubMed]
- Sriramprakash, S.; Prasanna, V.D.; Murthy, O.V.R. Stress Detection in Working People. Procedia Comput. Sci. 2017, 115, 359–366. [Google Scholar] [CrossRef]
- Villarejo, M.V.; Zapirain, B.G.; Zorrilla, A.M. A stress sensor based on galvanic skin response (GSR) controlled by ZigBee. Sensors 2012, 12, 6075–6101. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Messina, G.; Valenzano, A.; Messina, A.; Salerno, M.; Marsala, G.; Bertozzi, G.; Daniele, A.; Monda, V.; Russo, R. Sports training and adaptive changes. Sport Sci. Health 2018, 1–4. [Google Scholar] [CrossRef]
- Seoane, F.; Mohino-Herranz, I.; Ferreira, J.; Alvarez, L.; Buendia, R.; Ayllón, D.; Llerena, C.; Gil-Pita, R. Wearable biomedical measurement systems for assessment of mental stress of combatants in real time. Sensors 2014, 14, 7120–7141. [Google Scholar] [CrossRef] [PubMed]
- Kikhia, B.; Stavropoulos, T.G.; Andreadis, S.; Karvonen, N.; Kompatsiaris, I.; Sävenstedt, S.; Pijl, M.; Melander, C. Utilizing a wristband sensor to measure the stress level for people with dementia. Sensors 2016, 16, 1989. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Yamabe, S.; Nakano, K.; Suda, Y. Biosignal analysis to assess mental stress in automatic driving of trucks: Palmar perspiration and masseter electromyography. Sensors 2015, 15, 5136–5150. [Google Scholar] [CrossRef] [PubMed]
- Ollander, S.; Godin, C.; Charbonnier, S. Feature and Sensor Selection for Detection of Driver Stress. In Proceedings of the 3rd International Conference on Physiological Computing Systems, Lisbon, Portugal, 27–28 July 2016; pp. 115–122. [Google Scholar]
- Keshan, N.; Parimi, P.V.; Bichindaritz, I. Machine learning for stress detection from ECG signals in automobile drivers. In Proceedings of the 2015 IEEE International Conference on Big Data, Santa Clara, CA, USA, 29 October–1 November 2015; pp. 2661–2669. [Google Scholar] [CrossRef]
- Minguillon, J.; Lopez-Gordo, M.A.; Morillas, C.; Pelayo, F. A Mobile Brain-Computer Interface for Clinical Applications: From the Lab to the Ubiquity. In Proceedings of the 7th International Work-Conference on the Interplay between Natural and Artificial Computation; Ferrández Vicente, J.M., Álvarez-Sánchez, J.R., De la Paz López, F., Toledo Moreo, J., Adeli, H., Eds.; Springer International Publishing: Corunna, Spain, 2017; pp. 68–76. [Google Scholar] [CrossRef]
- Dedovic, K.; Renwick, R.; Mahani, N.K.; Engert, V.; Lupien, S.J.; Pruessner, J.C. The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J. Psychiatry Neurosci. 2005, 30, 319–325. [Google Scholar] [PubMed]
- Kirschbaum, C.; Pirke, K.-M.; Hellhammer, D.H. The “Trier Social Stress Test”—A Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Dedovic, K.; D’Aguiar, C.; Pruessner, J.C. What stress does to your brain: A review of neuroimaging studies. Can. J. Psychiatry 2009, 54, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Zschucke, E.; Renneberg, B.; Dimeo, F.; Wüstenberg, T.; Ströhle, A. The stress-buffering effect of acute exercise: Evidence for HPA axis negative feedback. Psychoneuroendocrinology 2015, 51, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Nagano-Saito, A.; Dagher, A.; Booij, L.; Gravel, P.; Welfeld, K.; Casey, K.F.; Leyton, M.; Benkelfat, C. Stress-induced dopamine release in human medial prefrontal cortex—18F-fallypride/PET study in healthy volunteers. Synapse 2013, 67, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Bali, A.; Jaggi, A.S. Clinical experimental stress studies: Methods and assessment. Rev. Neurosci. 2015, 26, 555–579. [Google Scholar] [CrossRef] [PubMed]
- Bichindaritz, I.; Breen, C.; Cole, E.; Keshan, N.; Parimi, P. Feature Selection and Machine Learning Based Multilevel Stress Detection from ECG Signals. In Innovation in Medicine and Healthcare 2017; Chen, Y.-W., Tanaka, S., Howlett, R.J., Jain, L.C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 202–213. [Google Scholar]
- Lopez-Gordo, M.A.; Pelayo, F. A Binary Phase-Shift Keying Receiver for the Detection of Attention to Human Speech. Int. J. Neural Syst. 2013, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Minguillon, J.; Lopez-Gordo, M.A.; Pelayo, F. Detection of Attention in Multi-Talker Scenarios: A Fuzzy Approach. Expert Syst. Appl. 2016, 64, 261–268. [Google Scholar] [CrossRef]
- Lopez-Gordo, M.A.; Pelayo, F.; Fernandez, E.; Padilla, P. Phase-shift keying of EEG signals: Application to detect attention in multitalker scenarios. Signal Process. 2015, 117, 165–173. [Google Scholar] [CrossRef]
- Lopez-Gordo, M.A.; Sanchez Morillo, D.; Pelayo Valle, F. Dry EEG electrodes. Sensors 2014, 14, 12847–12870. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Bin Queyam, A. A Novel Method of Stress Detection Using Physiological Measurements of Automobile Drivers. Int. J. Electron. Eng. 2013, 5, 13–20. [Google Scholar]
- Alić, B.; Sejdinović, D.; Gurbeta, L.; Badnjevic, A. Classification of stress recognition using Artificial Neural Network. In Proceedings of the 2016 5th Mediterranean Conference on Embedded Computing (MECO), Bar, Montenegro, 12–16 June 2016; pp. 297–300. [Google Scholar]
| Pair | PCC | CI Low | CI Up |
|---|---|---|---|
| RG, HR | 0.7296 | 0.6909 | 0.7642 |
| RG, TA | 0.5753 | 0.5206 | 0.6253 |
| RG, SC | 0.3293 | 0.2579 | 0.3972 |
| HR, TA | 0.8338 | 0.8083 | 0.8561 |
| HR, SC | 0.6327 | 0.5834 | 0.6773 |
| TA, SC | 0.4632 | 0.3995 | 0.5224 |
| Participant | RG | HR | TA | SC |
|---|---|---|---|---|
| 1 | 72 ± 7 | 74 ± 6 | 31 ± 7 | 49 ± 7 |
| 2 | 61 ± 7 | 57 ± 7 | 28 ± 7 | 69 ± 7 |
| 3 | 61 ± 7 | 45 ± 7 | 29 ± 7 | 84 ± 5 |
| 4 | 51 ± 7 | 60 ± 7 | 61 ± 7 | 51 ± 7 |
| 5 | 28 ± 7 | 93 ± 4 | 22 ± 6 | 69 ± 7 |
| 6 | 44 ± 7 | 94 ± 3 | 45 ± 7 | 61 ± 7 |
| 7 | 47 ± 7 | 82 ± 6 | 66 ± 7 | 60 ± 7 |
| 8 | 33 ± 7 | 77 ± 6 | 21 ± 6 | 61 ± 7 |
| 9 | 67 ± 7 | 77 ± 6 | 52 ± 7 | 18 ± 6 |
| 10 | 33 ± 7 | 62 ± 7 | 62 ± 7 | 76 ± 6 |
| Mean ± Std | 50 ± 15 | 72 ± 16 | 42 ± 18 | 60 ± 18 |
| Participant | RG, HR | RG, TA | RG, SC | HR, TA | HR, SC | TA, SC |
|---|---|---|---|---|---|---|
| 1 | 76 ± 6 | 83 ± 6 | 69 ± 7 | 86 ± 5 | 71 ± 7 | 64 ± 7 |
| 2 | 73 ± 6 | 82 ± 6 | 73 ± 6 | 61 ± 7 | 70 ± 7 | 78 ± 6 |
| 3 | 77 ± 6 | 60 ± 7 | 81 ± 6 | 52 ± 7 | 92 ± 4 | 90 ± 4 |
| 4 | 59 ± 7 | 64 ± 7 | 72 ± 7 | 70 ± 7 | 68 ± 7 | 87 ± 5 |
| 5 | 92 ± 4 | 46 ± 7 | 54 ± 7 | 93 ± 4 | 84 ± 5 | 76 ± 6 |
| 6 | 94 ± 3 | 69 ± 7 | 64 ± 7 | 93 ± 4 | 96 ± 3 | 71 ± 7 |
| 7 | 84 ± 5 | 66 ± 7 | 64 ± 7 | 86 ± 5 | 86 ± 5 | 66 ± 7 |
| 8 | 74 ± 6 | 48 ± 7 | 61 ± 7 | 76 ± 6 | 71 ± 7 | 64 ± 7 |
| 9 | 82 ± 6 | 72 ± 7 | 64 ± 7 | 78 ± 6 | 73 ± 6 | 49 ± 7 |
| 10 | 67 ± 7 | 54 ± 7 | 67 ± 7 | 73 ± 6 | 77 ± 6 | 81 ± 6 |
| Mean ± Std | 78 ± 11 | 64 ± 13 | 67 ± 7 | 77 ± 14 | 79 ± 10 | 73 ± 12 |
| Participant | RG, HR, TA | RG, HR, SC | RG, TA, SC | HR, TA, SC | RG, HR, TA, SC |
|---|---|---|---|---|---|
| 1 | 91 ± 4 | 79 ± 6 | 84 ± 5 | 92 ± 4 | 92 ± 4 |
| 2 | 82 ± 6 | 78 ± 6 | 83 ± 6 | 75 ± 6 | 82 ± 6 |
| 3 | 77 ± 6 | 93 ± 4 | 82 ± 6 | 92 ± 4 | 93 ± 4 |
| 4 | 68 ± 7 | 69 ± 7 | 78 ± 6 | 82 ± 6 | 83 ± 6 |
| 5 | 93 ± 4 | 84 ± 5 | 73 ± 7 | 84 ± 5 | 84 ± 5 |
| 6 | 93 ± 4 | 97 ± 3 | 72 ± 7 | 98 ± 2 | 98 ± 2 |
| 7 | 86 ± 5 | 87 ± 5 | 67 ± 7 | 89 ± 4 | 90 ± 4 |
| 8 | 74 ± 6 | 75 ± 6 | 64 ± 7 | 71 ± 7 | 74 ± 6 |
| 9 | 81 ± 6 | 80 ± 6 | 67 ± 7 | 76 ± 6 | 81 ± 6 |
| 10 | 72 ± 7 | 77 ± 6 | 79 ± 6 | 79 ± 6 | 78 ± 6 |
| Mean ± Std | 82 ± 9 | 82 ± 8 | 75 ± 7 | 84 ± 9 | 86 ± 8 |
| Participant | RG, HR, TA | RG, HR, SC | RG, TA, SC | HR, TA, SC | RG, HR, TA, SC |
|---|---|---|---|---|---|
| 1 | 33 ± 7 | 33 ± 7 | 36 ± 7 | 33 ± 7 | 33 ± 7 |
| 2 | 67 ± 7 | 37 ± 7 | 58 ± 7 | 64 ± 7 | 65 ± 7 |
| 3 | 33 ± 7 | 41 ± 7 | 36 ± 7 | 33 ± 7 | 36 ± 7 |
| 4 | 47 ± 7 | 36 ± 7 | 33 ± 7 | 49 ± 7 | 34 ± 7 |
| 5 | 66 ± 7 | 41 ± 7 | 37 ± 7 | 64 ± 7 | 38 ± 7 |
| 6 | 36 ± 7 | 34 ± 7 | 33 ± 7 | 34 ± 7 | 34 ± 7 |
| 7 | 33 ± 7 | 33 ± 7 | 39 ± 7 | 33 ± 7 | 33 ± 7 |
| 8 | 34 ± 7 | 53 ± 7 | 51 ± 7 | 54 ± 7 | 54 ± 7 |
| 9 | 41 ± 7 | 60 ± 7 | 56 ± 7 | 51 ± 7 | 66 ± 7 |
| 10 | 48 ± 7 | 48 ± 7 | 48 ± 7 | 36 ± 7 | 42 ± 7 |
| Mean ± Std | 44 ± 13 | 42 ± 9 | 43 ± 10 | 45 ± 13 | 44 ± 13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minguillon, J.; Perez, E.; Lopez-Gordo, M.A.; Pelayo, F.; Sanchez-Carrion, M.J. Portable System for Real-Time Detection of Stress Level. Sensors 2018, 18, 2504. https://doi.org/10.3390/s18082504
Minguillon J, Perez E, Lopez-Gordo MA, Pelayo F, Sanchez-Carrion MJ. Portable System for Real-Time Detection of Stress Level. Sensors. 2018; 18(8):2504. https://doi.org/10.3390/s18082504
Chicago/Turabian StyleMinguillon, Jesus, Eduardo Perez, Miguel Angel Lopez-Gordo, Francisco Pelayo, and Maria Jose Sanchez-Carrion. 2018. "Portable System for Real-Time Detection of Stress Level" Sensors 18, no. 8: 2504. https://doi.org/10.3390/s18082504
APA StyleMinguillon, J., Perez, E., Lopez-Gordo, M. A., Pelayo, F., & Sanchez-Carrion, M. J. (2018). Portable System for Real-Time Detection of Stress Level. Sensors, 18(8), 2504. https://doi.org/10.3390/s18082504





