Abstract
In this paper, an investigation into detecting immunosuppressive medicine in aqueous solutions using a spectrometry-based technique is described. Using optical transmissive spectrometry, absorbance measurements in the spectra range from 250 nm to 1000 nm were carried out for different cyclosporine A (CsA) concentrations in aqueous solutions. The experiment was conducted for samples both with and without interferent substances—glucose and sodium chloride. Using a dedicated algorithm, the measured data was analyzed and a high correlation coefficient R2 = 0.8647 was achieved. The experiment showed that the described technique allowed for the detection of various CsA concentration levels in a selective, label-free and simple way. This method could be used in medicine, veterinary medicine and laboratory diagnostics.
1. Introduction
Cyclosporine A (CsA) is an inert, hydrophobic chemical compound. It is a cyclic polypeptide of 11 amino acids, whose molecular weight is 1203 [1]. It blocks humoral and cellular immune reactions, thus affecting the inflammatory reactions. CsA is used in the treatment of chronic inflammatory diseases in rheumatology, dermatology and ophthalmology [2,3,4]. However, long-term intravenous administration leads to serious side effects, including an increase of creatinine level, hypertension and the increased risk of cancer [5,6,7,8,9]. CsA contributes to water retention in the body resulting in the formation of edema and significant weight gains in people consuming this drug [10,11].
The use of cyclosporine in aquaculture animals, as well as its presence in water reservoirs, may contribute to an increase in mortality. Due to the legal regulations in force in the European Union, it is forbidden to use drugs that may contribute to the increase of the risk of disease and transmission of non-exotic diseases and the spread of exotic diseases in aquaculture animals [12,13]. The CsA presence in water will result in its accumulation in aquatic organisms. This can lead to various diseases due to the immunosuppressive action, causing e.g., nephrotoxicity, hepatotoxicity, among others. Consumption of aquaculture animals contaminated by CsA may lead to abnormalities in birds and mammals [14,15,16,17,18].
The main goal of this research is to detect CsA in water in general. It is possible to use the proposed method for measurements of any kind of aqueous solutions. It could be used for investigation of drinking water for animals, for water in reservoirs where the water animals live, for the waste water. This method allows for the detection of e.g., if the drinking water for animals contains CsA (this could be used for illegally caused gain of weight) or if the water in reservoirs is contaminated with this drug. In the future this method could also be used for testing drinking water for humans.
Out of several optical measurements methods, we chose the optical spectroscopy as the most promising [19]. In this paper, we present the optical spectrometry-based method for label-free detection of cyclosporine concentration in aqueous solutions.
2. Materials and Methods
2.1. Materials
A set of different aqueous solutions of cyclosporine was prepared in order to investigate if the proposed technique allows for the detection of CsA concentrations. Cyclaid®, produced by Apotex, and deionized water were used. Different weight percent concentrations [%] of CsA in deionized water were prepared according to Table 1.

Table 1.
Concentrations of CsA in prepared aqueous solutions.
2.2. Methods
A UV-9000 UV-VIS Double Scanning Spectrophotometer by Shanghai Metash Instruments Co. LTD was used for performing the measurements. In this spectrophotometer, a Deuterium Lamp and a Tungsten Halogen Lamp are used as light sources, a double beam optical system with 1200 lines/mm grating is implemented, and silicon photodiodes play the role of detectors. In this set-up, the light produced by the light source incidents on the diffraction grating and a spectrum is created. A part of it is directed to the beam-splitter. One of the beams passes thorough the sample and the other passes through the reference. The photodetectors measure the signal and the data is analyzed on a PC.
The spectrophotometer enables measurements in the spectra range from 190 nm to 1100 nm, with a bandwidth of 1.8 nm. The wavelength repeatability is about 0.2 nm, and the wavelength accuracy is equal to ±0.3 nm. Photometric accuracy is equal about ±0.3%T and photometric repeatability is equal to 0.2%T, where %T is percent transmittance. 10 series of measurements in the spectra range of 250–1000 nm were carried out with the increment of 1 nm.
3. Results
To establish whether optical spectroscopy can be used for detecting CsA in an aqueous solution, we performed a series of measurements for various CsA concentrations in water. A dedicated algorithm for measured data analysis was developed. A relationship between the drug concentration levels and the value of measured absorbance was established. Furthermore, the metrological parameters and selectivity of the method were established. Results of the aforementioned steps are shown in the following subsections.
3.1. Aqueous CsA Solutions
In Figure 1, the acquired data for CsA concentrations varying from 0.09% to 13.61% in water is shown, using the measurement setup described in Section 2.2.
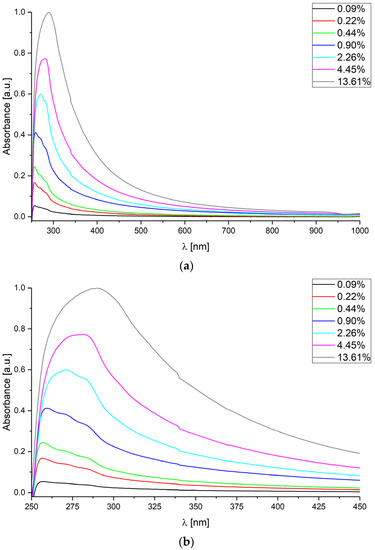
Figure 1.
Absorbance spectra measured for various concentrations of cyclosporine A in deionized water in spectra range: (a) from 250 nm to 1000 nm; (b) from 250 nm to 450 nm.
It is clearly visible that absorbance of CsA solutions increases, due to the increase of the CsA concentration level. The absorbance value changes rapidly in spectra range from 250 nm to 450 nm, which is shown in Figure 1b. The shapes of the measured absorbance curves are similar, regardless of CsA concentration, and the value of absorbance depends on CsA concentration. Furthermore, the wavelength of the observed peak λpeak, defined as the value of the wavelength for the highest value of absorbance, increases with the increase of CsA concentration level.
3.2. Selectivity of the Proposed Method
The next step was to investigate the effect of glucose C6H12O6 and sodium chloride NaCl presence in the measured samples on the measurements of CsA. We chose sodium chloride because it is the main ionic compound in water [20] and glucose because it impacts the aqueous animals in water ecosystems [21,22].
Measurements for constant CsA concentration equal to 0.44%, and two glucose levels: 0.8‰ and 1.3‰ were performed. The measured absorbance spectra are shown in Figure 2.
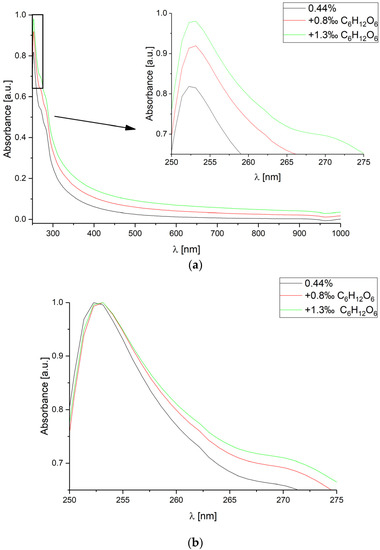
Figure 2.
Absorbance spectra measured for various glucose C6H12O6 concentrations in deionized water, for constant cyclosporine A concentration level equal to 0.44%. The spectra range is: (a) from 250 nm to 1000 nm; (b) from 250 nm to 275 nm (each curve has been normalized separately).
For increasing glucose concentration level, the value of absorbance increases and the slope of the absorbance spectrum curve is smaller. Despite various glucose concentrations, the wavelength of the observed peak λpeak is almost constant—slightly shifted by <1 nm.
Similarly, measurements for the same CsA concentration and two sodium chloride (NaCl) concentration levels: 7‰ and 36‰ were performed. The measured absorbance spectra are shown in Figure 3.
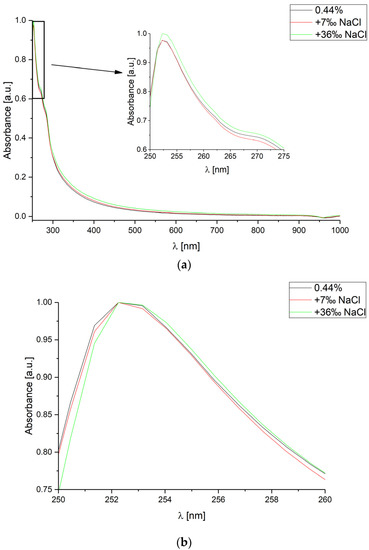
Figure 3.
Absorbance spectra measured for various concentrations of sodium chloride (NaCl) in deionized water, for constant cyclosporine concentration level equal to 0.44%. The spectra range is: (a) from 250 nm to 1000 nm; (b) from 250 nm to 260 nm (each curve has been normalized separately).
It can be observed that the investigated NaCl concentrations only slightly change measurement value of absorbance and could be omitted. Nevertheless, their influence might be significant for very low CsA concentrations. What is the most important, changes in the concentration level of NaCl do not affect the measured spectra shift.
As the next step, measurements for CsA concentration level equal to 0.44%, NaCl concentration level 36‰, and glucose concentration level 1.3‰ were performed. Similarly, the same measurements for CsA concentration equal to 4.45% were performed. The wavelengths of the observed peaks λpeak for both CsA concentration levels are summarized in Table 2.

Table 2.
The wavelengths of the observed peaks λpeak for various concentrations of sodium chloride NaCl and glucose C6H12O6 in deionized water, for constant cyclosporine concentration levels equal to 0.44% and 4.45%.
As it can observed, for different solutions the wavelength of the observed peak is almost constant—for selected solutions it is slightly shifted. This proves that the proposed method is insensitive to the influence of sodium chloride and glucose.
4. Discussion
4.1. Data Analysis Algorithm
A dedicated data analysis algorithm was developed for measuring data analysis. It is illustrated in Figure 4.
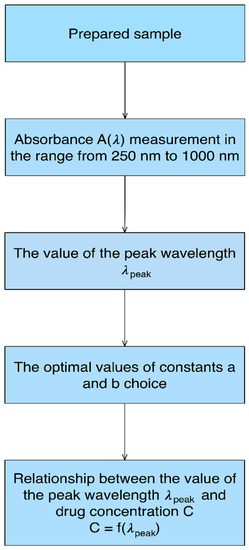
Figure 4.
Data analysis algorithm schema. C is the drug concentration, λpeak is the wavelength of the observed peak.
For each CsA concentration, measurements of absorbance in the spectra range from 250 nm to 1000 nm were performed. Next, the wavelength of the observed peak λpeak for various CsA concentrations was found. Those values were described by mathematical curves, using parameters a and b.
4.2. Relationship between the Value of the Peak Wavelength λpeak and CsA Concentration
The relationship between the wavelength of the observed peak λpeak and CsA concentration C was calculated. It is shown in Figure 5.
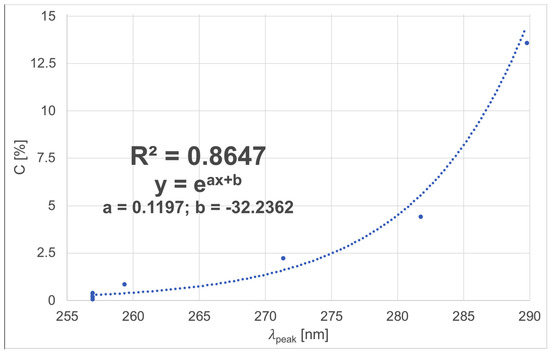
Figure 5.
Relationship between the wavelength of the observed peak λpeak and cyclosporine A concentration C for aqueous solutions.
We can observe that the relationship between the value of the peak wavelength λpeak and CsA concentration C, shown in Figure 5 can be described by an exponential curve with parameters a and b. The obtained value of the coefficient of correlation R2 is equal to 0.8647. It proves that the selected mathematical model fits well with the measurements.
The obtained relationship enables us to calculate the value of the CsA concentration based on the wavelength of the observed peak λpeak.
5. Conclusions
In this paper, we presented the optical spectrometry-based method for detection of different concentrations of the immunosuppressive medicine—CsA—in aqueous solutions. The investigation showed that the correlation between CsA level and the value of absorbance can be found. Moreover, the proposed method is insensitive to the presence of investigated interferent substances, such as glucose and sodium chloride. The use of optical transmissive spectrometry assures many advantages, e.g., short measurement time, label-free measurements, simplicity. This technique could be successfully used in biosensors, e.g., for veterinary medicine, where CsA could be illegally administrated to farm animals in order to increase their weight.
Author Contributions
Conceptualization, D.M. and M.J.-S.; Methodology, D.M. and M.J.-S.; Validation, M.M. and M.K., Formal Analysis, M.J.-S. and M.M.; Investigation, M.M. and M.K.; Resources, B.B.-P. and M.W.; Data Curation, M.J.-S. and M.M.; Writing-Original Draft Preparation, Writing-Review & Editing, all authors; Visualization, M.M. and M.K.; Supervision, M.J.-S.; Funding Acquisition, D.M, M.W. and M.J.-S.
Funding
This research was funded by NATO under the research grant SPS G5147 and by Polish National Science Centre (NCN) under the Grants No. 2016/22/E/ST7/00102, 2017/25/N/ST7/01610 and 2016/21/B/ST7/01430, and by the Polish National Centre for Research and Development (NCBiR) under the project Techmatstrateg Diamsec 347324, and DS Programs of the Faculty of Electronics, Telecommunications and Informatics of the Gdańsk University of Technology. The author M.W. acknowledges the financial support from the Leading National Research Centre Scientific Consortium “Healthy Animal-Safe Food” Faculty of Veterinary Medicine, Warsaw University of Life Sciences, Poland, decision of Ministry of Science and Higher Education No. 05-1/KNOW2/2015”.
Acknowledgments
The authors want to acknowledge Jerzy Pluciński for valuable comments on the manuscript and suggested improvements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haschek, W.M.; Rousseaux, C.G.; Wallig, M.A. Chapter 15—Immune System. In Fundamentals of Toxicologic Pathology, 2nd ed.; Academic Press: San Diego, CA, USA, 2010; pp. 451–489. ISBN 978-0-12-370469-6. [Google Scholar]
- Hernández-Martín, A.; Noguera-Morel, L.; Bernardino-Cuesta, B.; Torrelo, A.; Pérez-Martin, M.A.; Aparicio-López, C.; de Lucas-Collantes, C. Cyclosporine A for severe atopic dermatitis in children. efficacy and safety in a retrospective study of 63 patients. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Chew, A.-L.; Bashir, S.J.; Johnston, A.; Barker, J.N.W.N.; Smith, C.H. The value of monitoring ciclosporin concentration 2 hours post-dose (C2) in dermatology: A prospective cohort study. J. Dermatol. Treat. 2011, 22, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M.; Katsambas, A.; Dijkmans, B.A.C.; Finlay, A.Y.; Ho, V.C.; Johnston, A.; Luger, T.A.; Mrowietz, U.; Thestrup-Pedersen, K. Update on the use of ciclosporin in immune-mediated dermatoses. Br. J. Dermatol. 2006, 155 (Suppl. 2), 1–16. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.B.; Xu, J.; Xu, H.; Kurundkar, A.R.; Maheshwari, A.; Grizzle, W.E.; Timares, L.; Huang, C.C.; Kopelovich, L.; Elmets, C.A.; et al. Cyclosporine a mediates pathogenesis of aggressive cutaneous squamous cell carcinoma by augmenting epithelial-mesenchymal transition: Role of TGFβ signaling pathway. Mol. Carcinog. 2011, 50, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Abikhair, M.; Mitsui, H.; Yanofsky, V.; Roudiani, N.; Ovits, C.; Bryan, T.; Oberyszyn, T.M.; Tober, K.L.; Gonzalez, J.; Krueger, J.G.; et al. Cyclosporine A immunosuppression drives catastrophic squamous cell carcinoma through IL-22. JCI Insight 2016, 1, e86434. [Google Scholar] [CrossRef] [PubMed]
- Van der Schaft, J.; van Zuilen, A.D.; Deinum, J.; Bruijnzeel-Koomen, C.A.F.M.; de Bruin-Weller, M.S. Serum creatinine levels during and after long-term treatment with cyclosporine a in patients with severe atopic dermatitis. Acta Derm. Venereol. 2015, 95, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Soltani, K.; Ming, M.; He, Y.-Y. Deregulation of XPC and CypA by cyclosporin A: An immunosuppression-independent mechanism of skin carcinogenesis. Cancer Prev. Res. Phila. Pa 2012, 5, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Muellenhoff, M.W.; Koo, J.Y. Cyclosporine and skin cancer: An international dermatologic perspective over 25 years of experience. A comprehensive review and pursuit to define safe use of cyclosporine in dermatology. J. Dermatol. Treat. 2012, 23, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Roekevisch, E.; Spuls, P.I.; Kuester, D.; Limpens, J.; Schmitt, J. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: A systematic review. J. Allergy Clin. Immunol. 2014, 133, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Van der Schaft, J.; Politiek, K.; van den Reek, J.M.P.A.; Christoffers, W.A.; Kievit, W.; de Jong, E.M.G.J.; Bruijnzeel-Koomen, C.A.F.M.; Schuttelaar, M.L.A.; de Bruin-Weller, M.S. Drug survival for ciclosporin A in a long-term daily practice cohort of adult patients with atopic dermatitis. Br. J. Dermatol. 2015, 172, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Commission Decision of 20 November 2008 on Guidelines for the Purpose of the Risk-Based Animal Health Surveillance Schemes Provided for in Council Directive 2006/88/EC (Notified under Document Number C(2008) 6787) (Text with EEA relevance) (2008/896/EC). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32008D0896 (accessed on 6 June 2018).
- Council Directive 2006/88/EC of 24 October 2006 on Animal Health Requirements for Aquaculture Animals and Products Thereof, and on the Prevention and Control of Certain Diseases in Aquatic Animals. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32006L0088 (accessed on 6 June 2018).
- Legrand, J.-J.; Bouchez, C.; Mimouni, C.; N’Guyen, A.; Bouchard, J.; Ameller, T.; Descotes, J. Immunotoxic effects of cyclophosphamide and cyclosporine in the dog. J. Immunotoxicol. 2013, 10, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Elshama, S.S.; EL-Kenawy, A.E.-M.; Osman, H.-E.H. Histopathological Study of Cyclosporine Pulmonary Toxicity in Rats. Available online: https://www.hindawi.com/journals/jt/2016/2973274/ (accessed on 15 May 2018).
- Kovalik, M.; Thoday, K.L.; Handel, I.G.; Bronsvoort, B.M.C.; Evans, H.; van den Broek, A.H.M.; Mellanby, R.J. Ciclosporin A therapy is associated with disturbances in glucose metabolism in dogs with atopic dermatitis. Vet. Dermatol. 2011, 22, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Last, R.D.; Suzuki, Y.; Manning, T.; Lindsay, D.; Galipeau, L.; Whitbread, T.J. A case of fatal systemic toxoplasmosis in a cat being treated with cyclosporin A for feline atopy. Vet. Dermatol. 2004, 15, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Bechstein, W.O. Neurotoxicity of calcineurin inhibitors: Impact and clinical management. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2000, 13, 313–326. [Google Scholar] [CrossRef]
- Gauglitz, G.; Vo-Dinh, T. Handbook of Spectroscopy; Wiley-VCH: Weinheim, Germany, 2003; ISBN 3-527-29782-0. [Google Scholar]
- Benavides, A.L.; Aragones, J.L.; Vega, C. Consensus on the solubility of NaCl in water from computer simulations using the chemical potential route. J. Chem. Phys. 2016, 144, 124504. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.O.G. Carbohydrates. In Encyclopedia of Inland Waters; Academic Press: Oxford, UK, 2009; pp. 727–742. ISBN 978-0-12-370626-3. [Google Scholar]
- Rai, H. Glucose in Freshwater of Central Amazon Lakes: Natural Substrate Concentrations Determined by Dilution Bioassay. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1979, 64, 141–146. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).