Sensitive and Selective Detection of Tartrazine Based on TiO2-Electrochemically Reduced Graphene Oxide Composite-Modified Electrodes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
2.2. Synthesis of TiO2 Nanoparticles (NPs)
2.3. Synthesis of TiO2–GO Composite Nanomaterials
2.4. Fabrication of TiO2–ErGO-Modified GCE
2.5. Characterization
2.6. Electrochemical Experiments
2.7. Analysis of Real Samples
3. Result and Discussion
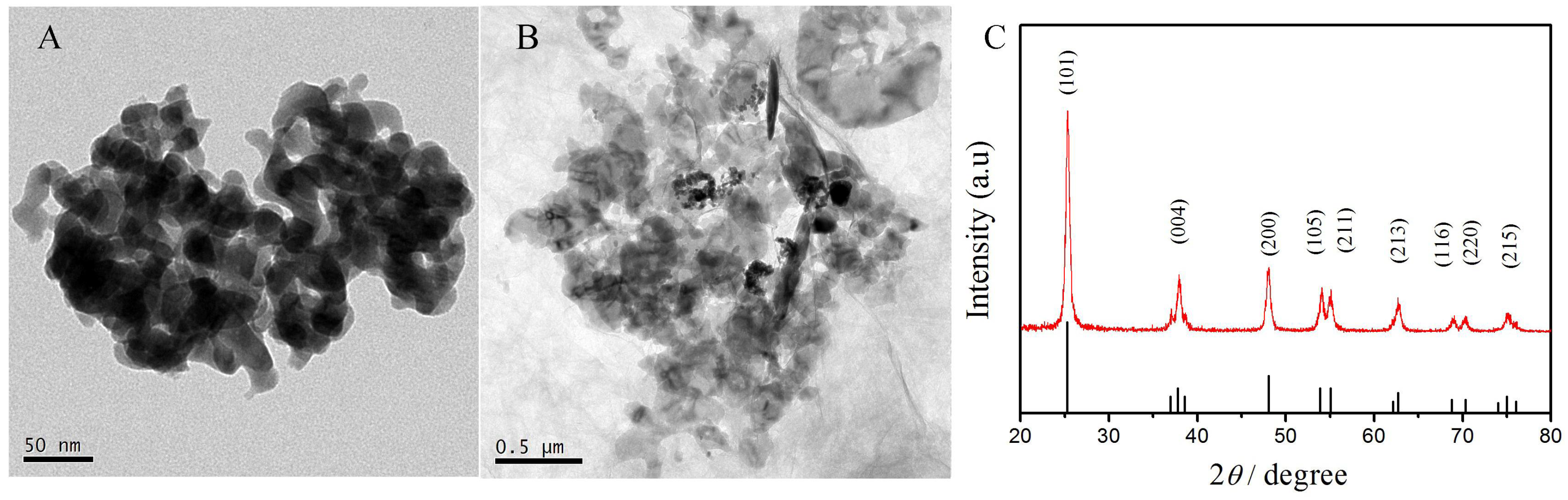
3.1. Morphologic and Structural Characterization of TiO2–GO Nanocomposites
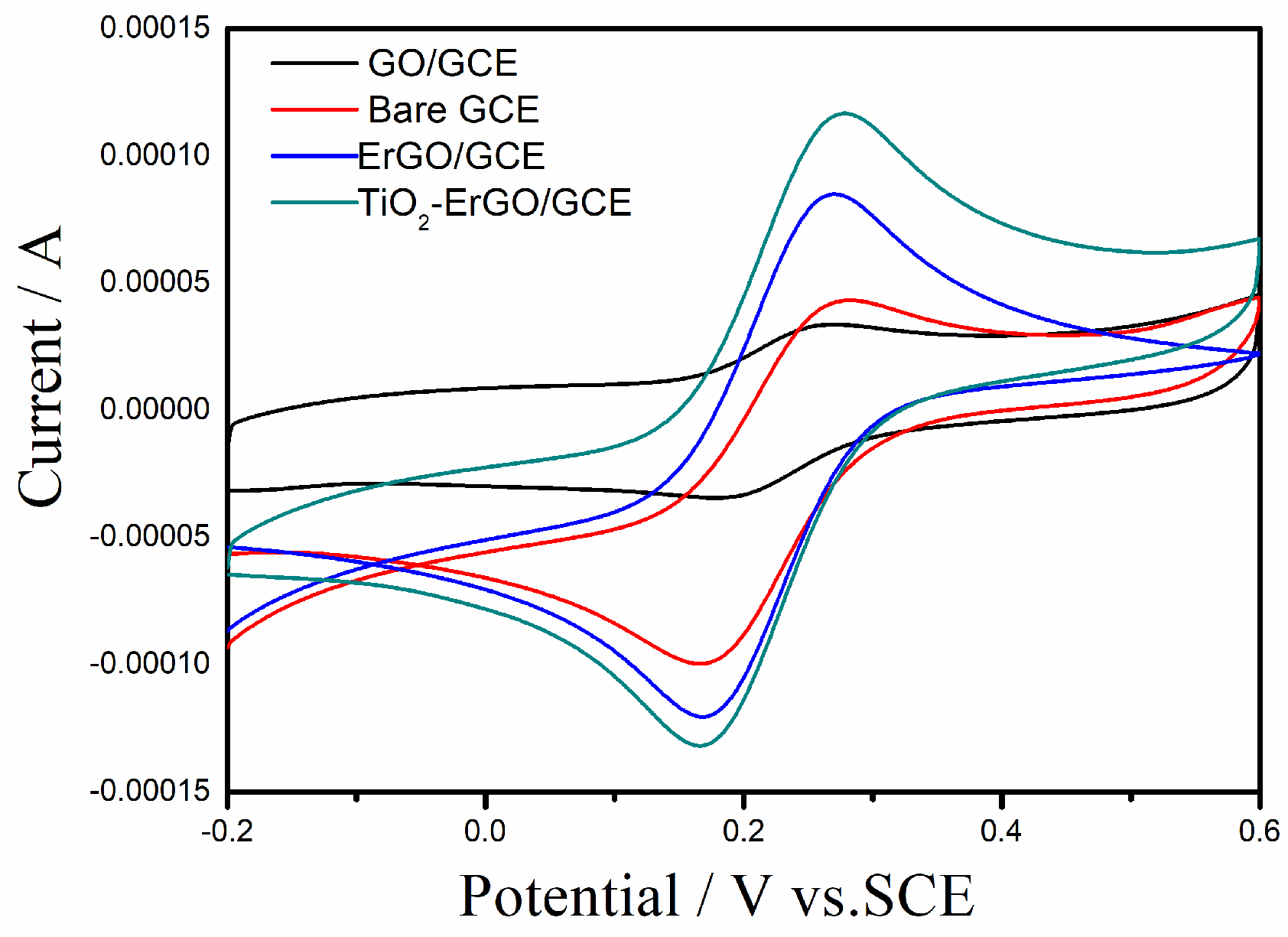
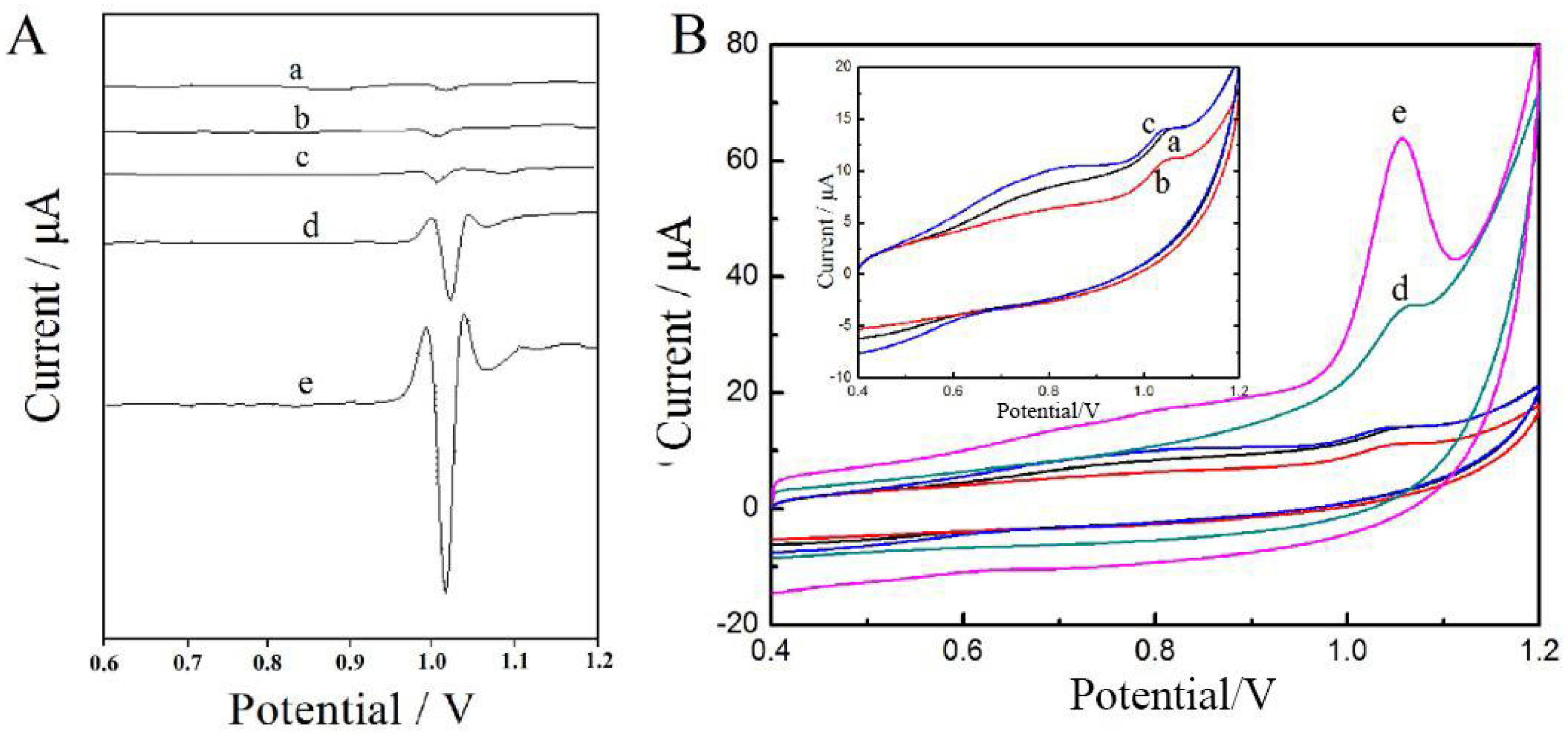
3.2. Electrochemical Behavior of Tartrazine on Different Electrodes
3.3. Optimization of the Detection Conditions of Tartrazine
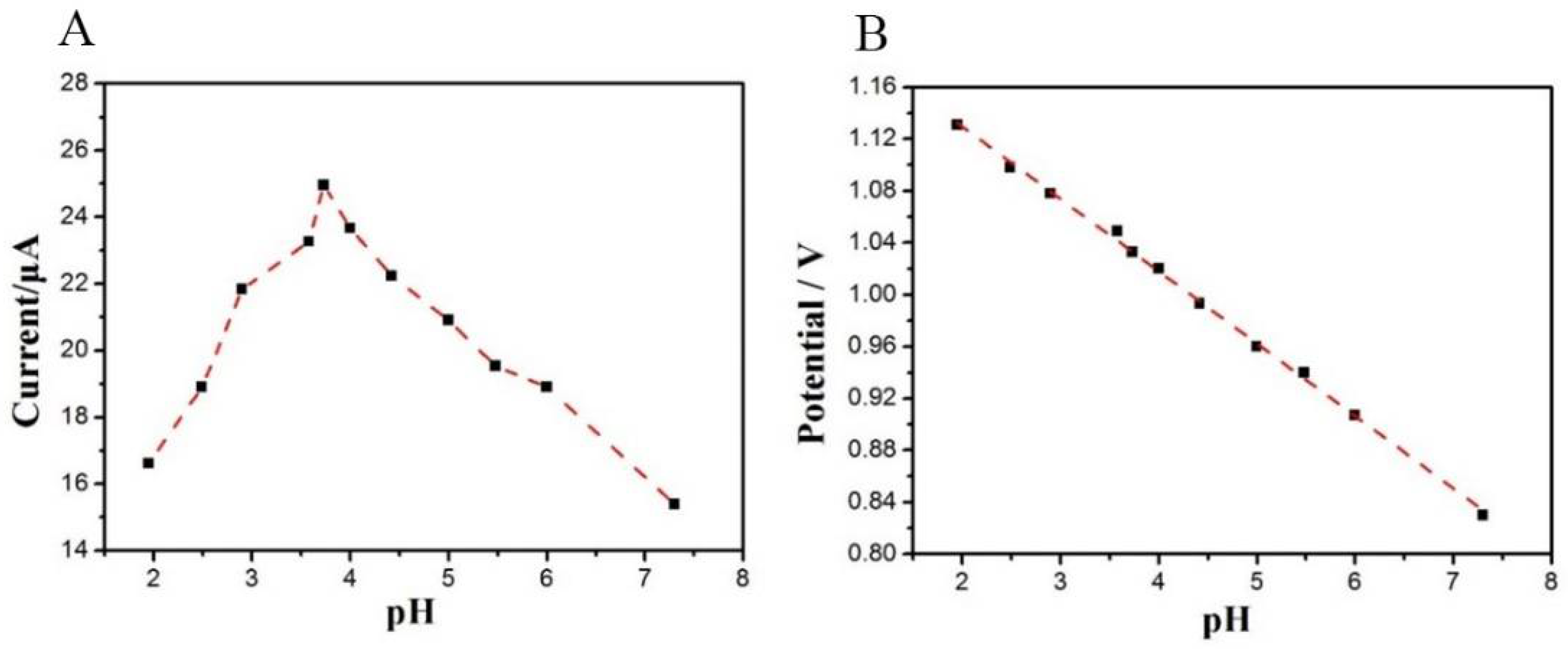
3.3.1. The Influence of pH
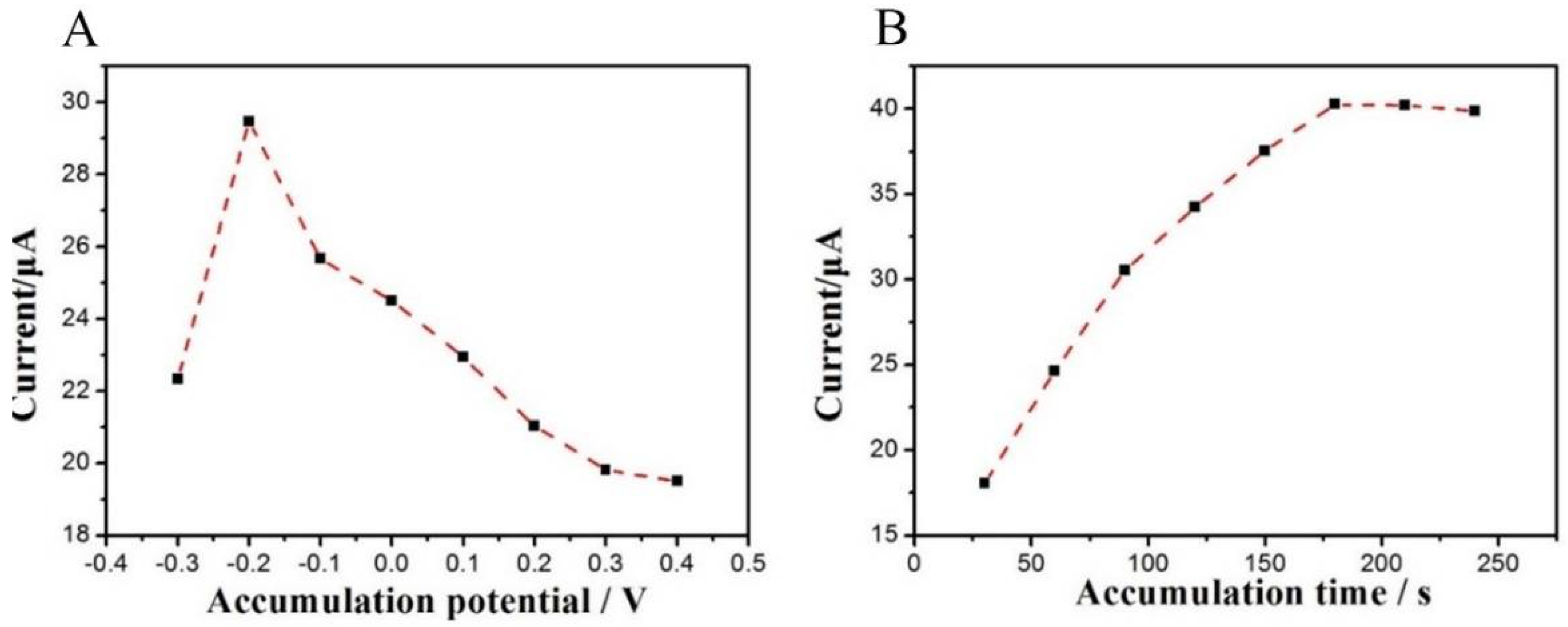
3.3.2. Effect of Accumulation Conditions
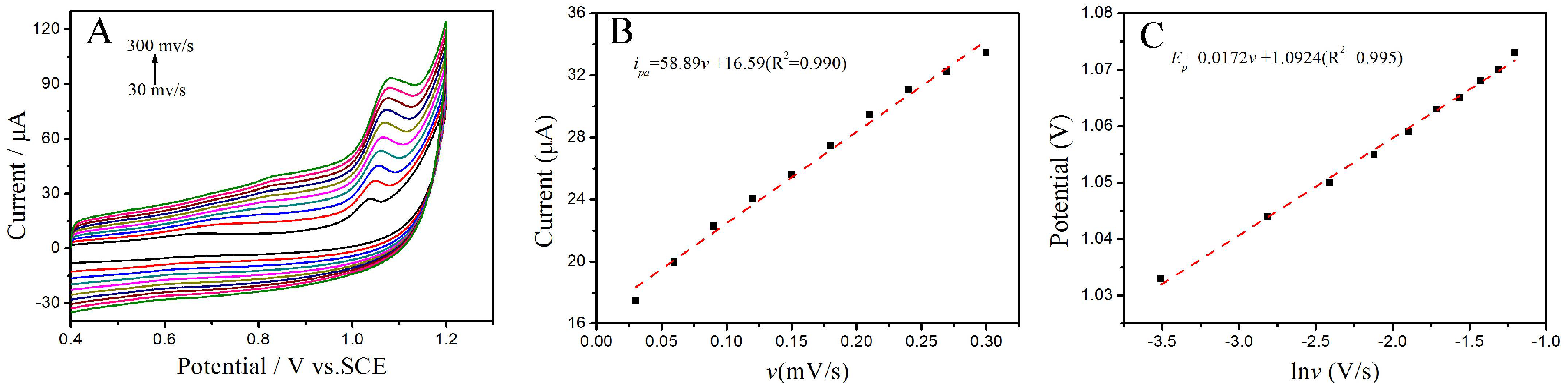
3.3.3. The Influence of the Scan Rate
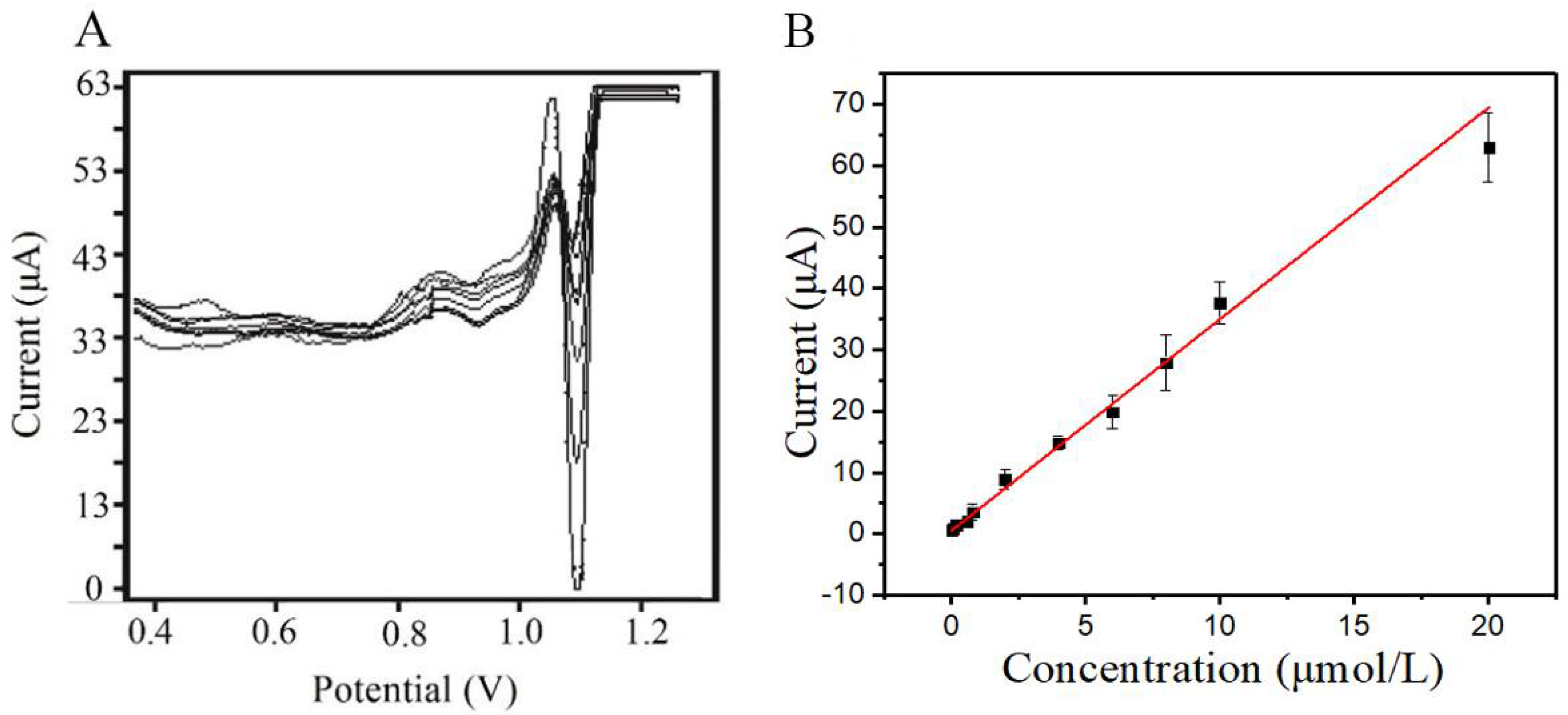
3.3.4. Calibration Curve and Detection Limit
3.4. Interference Studies
3.5. Reproducibility of the Detection
3.6. Real Sample Detection
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Llamas, N.E.; Garrido, M.; Nezio, M.S.D.; Band, B.S.F. Second order advantage in the determination of amaranth, sunset yellow FCF and tartrazine by UV–vis and multivariate curve resolution-alternating least squares. Anal. Chim. Acta 2009, 655, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Nevado, J.J.B.; Flores, J.R.; Llerena, M.J.V. Adsorptive stripping voltammetry of Tartrazine at the hanging mercury drop electrode in soft drinks. Fresenius J. Anal. Chem. 1997, 357, 989–994. [Google Scholar] [CrossRef]
- Vidotti, E.C.; Costa, W.F.; Oliveira, C.C. Development of a green chromatographic method for determination of colorants in food samples. Talanta 2006, 68, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Majidi, M.R.; Pournaghi-Azar, M.H.; Baj, R.F.B.; Naseri, A. Formation of graphene nanoplatelet-like structures on carbon–ceramic electrode surface: Application for simultaneous determination of sunset yellow and tartrazine in some food samples. Ionics 2015, 21, 863–875. [Google Scholar] [CrossRef]
- Ma, M.; Luo, X.; Chen, B.; Su, S.; Yao, S. Simultaneous determination of water-soluble and fat-soluble synthetic colorants in foodstuff by high-performance liquid chromatography-diode array detection-electrospray mass spectrometry. J. Chromatogr. A 2006, 1103, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Qin, P.; Liu, R.; Wang, J. Characterizing the Interaction between Tartrazine and Two Serum Albumins by a Hybrid Spectroscopic Approach. J. Agric. Food Chem. 2011, 59, 6650–6656. [Google Scholar] [CrossRef] [PubMed]
- Jager, A.V.; Tonin, F.G.; Tavares, M.F.M. Optimizing the separation of food dyes by capillary electrophoresis. J. Sep. Sci. 2005, 28, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Shi, Y.; Li, N.B.; Luo, H.Q. Sensitive turn-on fluorescent detection of tartrazine based on fluorescence resonance energy transfer. Chem. Commun. 2012, 48, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, X.; Li, G.; Zhao, C.; Liao, X. Green Synthesis of Fluorescent Carbon Dots for Selective Detection of Tartrazine in Food Samples. J. Agric. Food Chem. 2015, 63, 6707–6714. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, S.; Fang, L.; Hamid Akash, M.S.; Shi, W.; Sun, K.; Xu, Y.; Chen, S. A sensitive and specific enzyme immunoassay for detecting tartrazine in human urinary samples. Anal. Methods 2013, 5, 925–930. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, T.; Zheng, X.; Huang, W.; Wan, C. Surface-enhanced oxidation and detection of Sunset Yellow and Tartrazine using multi-walled carbon nanotubes film-modified electrode. Colloids Surf. B Biointerfaces 2009, 74, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, Y.; Sun, Q.; Zhao, J. Sensitively Simultaneous Determination of Sunset Yellow and Tartrazine in Foods Based on Polypyrrole Modified Oxidized Single-Walled Carbon Nanotubes. J. Electrochem. Soc. 2014, 161, B297–B304. [Google Scholar] [CrossRef]
- Sierra-Rosales, P.; Toledo-Neira, C.; Squella, J.A. Electrochemical determination of food colorants in soft drinks using MWCNT-modified GCEs. Sens. Actuators B Chem. 2017, 240, 1257–1264. [Google Scholar] [CrossRef]
- Chang, M.; Hu, H.; Zhang, Y.; Chen, D.; Wu, L.; Li, X. Improving Visible Light-Absorptivity and Photoelectric Conversion Efficiency of a TiO2 Nanotube Anode Film by Sensitization with Bi2O3 Nanoparticles. Nanomaterials 2017, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Li, C.; Li, X.; Huang, H. Simultaneous detection of sunset yellow and tartrazine using the nanohybrid of gold nanorods decorated graphene oxide. J. Electroanal. Chem. 2016, 780, 296–302. [Google Scholar] [CrossRef]
- Jing, Z.; Xue, W.; Zhang, S.; Wang, W.; Hojo, M.; Chen, Z. An Electrochemical Sensor for Simultaneous Determination of Ponceau 4R and Tartrazine Based on an Ionic Liquid Modified Expanded Graphite Paste Electrode. J. Electrochem. Soc. 2014, 161, H453–H457. [Google Scholar]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2014, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Pattar, V.P.; Nandibewoor, S.T. Selective and Sensitive Electro Chemical Determination of D-Cycloserine Using Graphene Paste Sensor and its Application Studies. Anal. Chem. Lett. 2016, 6, 478–491. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-Reduced Graphene Nanocomposite Modified Electrodes towards Ultrasensitive Dopamine Detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. Fabrication of Amine-Modified Magnetite-Electrochemically Reduced Graphene Oxide Nanocomposite Modified Glassy Carbon Electrode for Sensitive Dopamine Determination. Nanomaterials 2018, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xu, Z.; Zeng, R.; Ding, C. Electrochemical behavior and voltammetric determination of vanillin based on an acetylene black paste electrode modified with graphene–polyvinylpyrrolidone composite film. Food Chem. 2015, 180, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Zhou, W.Y.; Jiang, M.; Guo, Z.; Liu, J.H.; Zhang, L.; Huang, X.J. Surface Fe(II)/Fe(III) Cycle Promoted Ultra-Highly Sensitive Electrochemical Sensing of Arsenic(III) with Dumbbell-Like Au/Fe3O4 Nanoparticles. Anal. Chem. 2018, 90, 4569–4577. [Google Scholar] [CrossRef] [PubMed]
- Shetti, N.P.; Nayak, D.S.; Kuchinad, G.T.; Naik, R.R. Electrochemical behavior of thiosalicylic acid at γ-Fe2O3 nanoparticles and clay composite carbon electrode. Electrochim. Acta 2018, 269, 204–211. [Google Scholar] [CrossRef]
- Dong, S.; Xi, J.; Wu, Y.; Liu, H.; Fu, C.; Liu, H.; Xiao, F. High loading MnO2 nanowires on graphene paper: Facile electrochemical synthesis and use as flexible electrode for tracking hydrogen peroxide secretion in live cells. Anal. Chim. Acta 2015, 853, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Guo, H.; Wang, Z.; Long, Y.; Li, W.; Tu, Y. Au@Cu2O core-shell structure for high sensitive non-enzymatic glucose sensor. Sens. Actuators B Chem. 2018, 255, 2510–2519. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Wu, W.; Tian, Q.; Cui, S.; Dai, Z.; Ren, F.; Xiao, X.; Jiang, C. 3D Flowerlike α-Fe2O3@TiO2 Core–Shell Nanostructures: General Synthesis and Enhanced Photocatalytic Performance. ACS Sustain. Chem. Eng. 2015, 3, 2975–2984. [Google Scholar] [CrossRef]
- Lee, A.; Libera, J.A.; Waldman, R.Z.; Ahmed, A.; Avila, J.R.; Elam, J.W.; Darling, S.B. Conformal Nitrogen-Doped TiO2 Photocatalytic Coatings for Sunlight-Activated Membranes. Adv. Sustain. Syst. 2017, 1, 1600041. [Google Scholar] [CrossRef]
- Zhou, D.; Dong, S.; Shi, J.; Cui, X.; Ki, D.; Torres, C.I.; Rittmann, B.E. Intimate coupling of an N-doped TiO2 photocatalyst and anode respiring bacteria for enhancing 4-chlorophenol degradation and current generation. Chem. Eng. J. 2017, 317, 882–889. [Google Scholar] [CrossRef]
- Arkan, E.; Paimard, G.; Moradi, K. A novel electrochemical sensor based on electrospun TiO2 nanoparticles/carbon nanofibers for determination of Idarubicin in biological samples. J. Electroanal. Chem. 2017, 801, 480–487. [Google Scholar] [CrossRef]
- Deng, P.; Fei, J.; Feng, Y. Sensitive voltammetric determination of tryptophan using an acetylene black paste electrode modified with a Schiff’s base derivative of chitosan. Analyst 2011, 136, 5211–5217. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, Y.; Deng, D.; Liu, X.; Xing, X.; Xiao, X.; Wang, Y. Acetone sensing performances based on nanoporous TiO2 synthesized by a facile hydrothermal method. Sens. Actuators B Chem. 2017, 238, 491–500. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Hu, H.; Chang, M.; Zhang, M.; Wang, X.; Chen, D. A new insight into PAM/graphene-based adsorption of water-soluble aromatic pollutants. J. Mater. Sci. 2017, 52, 8650–8664. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Wang, Z.; Zhang, J.; Duan, X.; Xu, J.; Wen, Y. Trace analysis of Ponceau 4R in soft drinks using differential pulse stripping voltammetry at SWCNTs composite electrodes based on PEDOT:PSS derivatives. Food Chem. 2015, 180, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zeng, B.; Zhao, F. Electrochemical determination of Tartrazine using a molecularly imprinted polymer–multiwalled carbon nanotubes-ionic liquid supported Pt nanoparticles composite film coated electrode. Electrochim. Acta 2014, 146, 611–617. [Google Scholar] [CrossRef]
- Song, X.; Shi, Z.; Tan, X.; Zhang, S.; Liu, G.; Wu, K. One-step solvent exfoliation of graphite to produce a highly-sensitive electrochemical sensor for tartrazine. Sens. Actuators B Chem. 2014, 197, 104–108. [Google Scholar] [CrossRef]
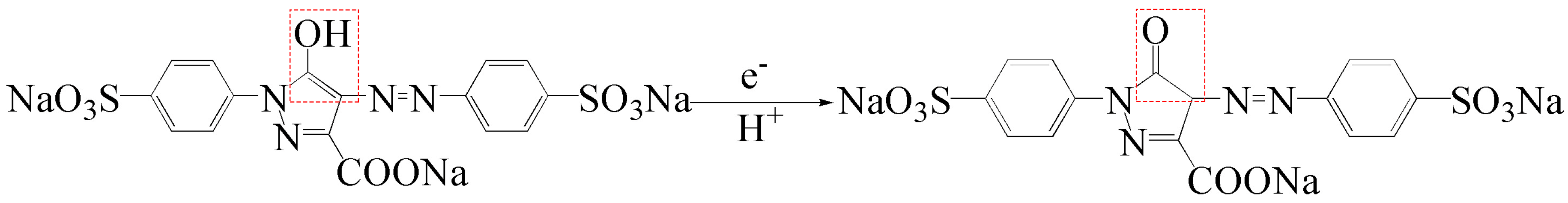
| Interferents | Peak Currents ipa (μA) |
|---|---|
| Without interferents (10 μmol/L of tartrazine) | 36.73 |
| Glucose (100-times concentration) | 36.80 |
| Benzoic acid (100-times concentration) | 36.30 |
| Citric acid (100-times concentration) | 36.27 |
| Na+ (100-times concentration) | 36.69 |
| K+ (100-times concentration) | 36.75 |
| Fe3+ (100-times concentration) | 36.71 |
| Amaranth (10-times concentration) | 36.54 |
| Sunset yellow (10-times concentration) | 36.60 |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| ipa/μA | 36.82 | 37.52 | 36.99 | 37.20 | 37.00 | 36.95 | 36.98 |
| Average value/μA | 37.06 | ||||||
| RSD/% | 0.45 | ||||||
| No. | Added/(μmol/L) | Total Found/(μmol/L) | Recovery (%) | RSD/(%) |
|---|---|---|---|---|
| 1 | — | 0.20 | — | 2.32 |
| 2 | 2 | 2.24 | 112.0 | 1.05 |
| 3 | 4 | 4.26 | 106.5 | 1.50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J.; Chen, D. Sensitive and Selective Detection of Tartrazine Based on TiO2-Electrochemically Reduced Graphene Oxide Composite-Modified Electrodes. Sensors 2018, 18, 1911. https://doi.org/10.3390/s18061911
He Q, Liu J, Liu X, Li G, Deng P, Liang J, Chen D. Sensitive and Selective Detection of Tartrazine Based on TiO2-Electrochemically Reduced Graphene Oxide Composite-Modified Electrodes. Sensors. 2018; 18(6):1911. https://doi.org/10.3390/s18061911
Chicago/Turabian StyleHe, Quanguo, Jun Liu, Xiaopeng Liu, Guangli Li, Peihong Deng, Jing Liang, and Dongchu Chen. 2018. "Sensitive and Selective Detection of Tartrazine Based on TiO2-Electrochemically Reduced Graphene Oxide Composite-Modified Electrodes" Sensors 18, no. 6: 1911. https://doi.org/10.3390/s18061911
APA StyleHe, Q., Liu, J., Liu, X., Li, G., Deng, P., Liang, J., & Chen, D. (2018). Sensitive and Selective Detection of Tartrazine Based on TiO2-Electrochemically Reduced Graphene Oxide Composite-Modified Electrodes. Sensors, 18(6), 1911. https://doi.org/10.3390/s18061911






