Comments to: A Novel Low-Cost Instrumentation System for Measuring the Water Content and Apparent Electrical Conductivity of Soils, Sensors, 15, 25546–25563
Abstract
:1. Introduction
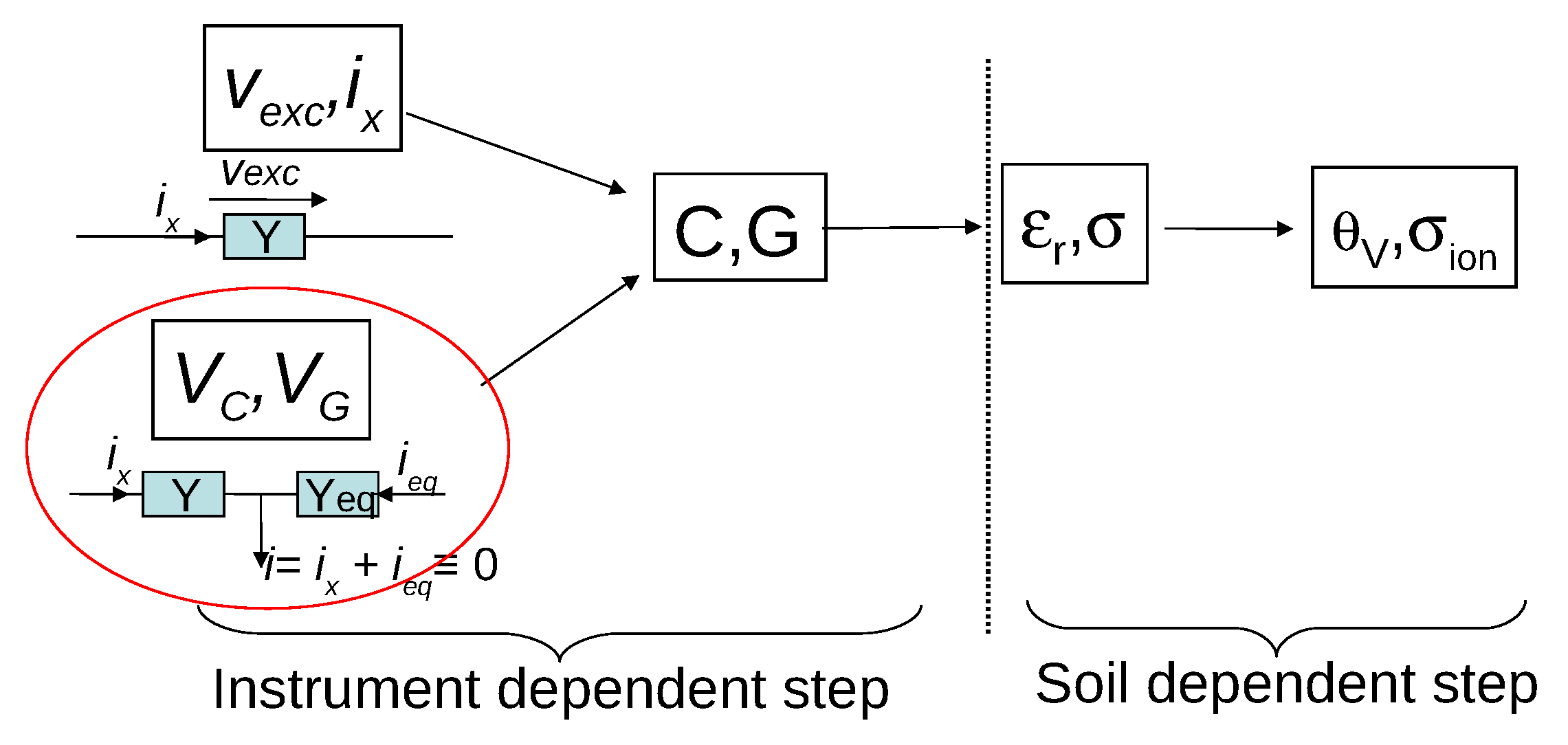
2. Distinction between the Various Measurement Techniques of Permittivity-Based Sensors
2.1. Overview
- Those using the phase velocity or travel time of an electromagnetic wave propagating along a guide in a soil. The velocity varies with the real part of the root square of (Equation (1)), approximated to at low conductivity. The most common sensors of this group are the Time Domain Reflectory probes (TDRs) such as CS659 used in the HydroSense system of Campbell Scientific Inc., Logan, UT, USA, or TRIME-TDR of IMKO (Ettlingen, Germany).
- Others using, instead, the amplitude of sensor electromagnetic fields to determine the capacitance (or admittance if they are able to measure simultaneously the conductance part) of electrodes embedded in a soil.
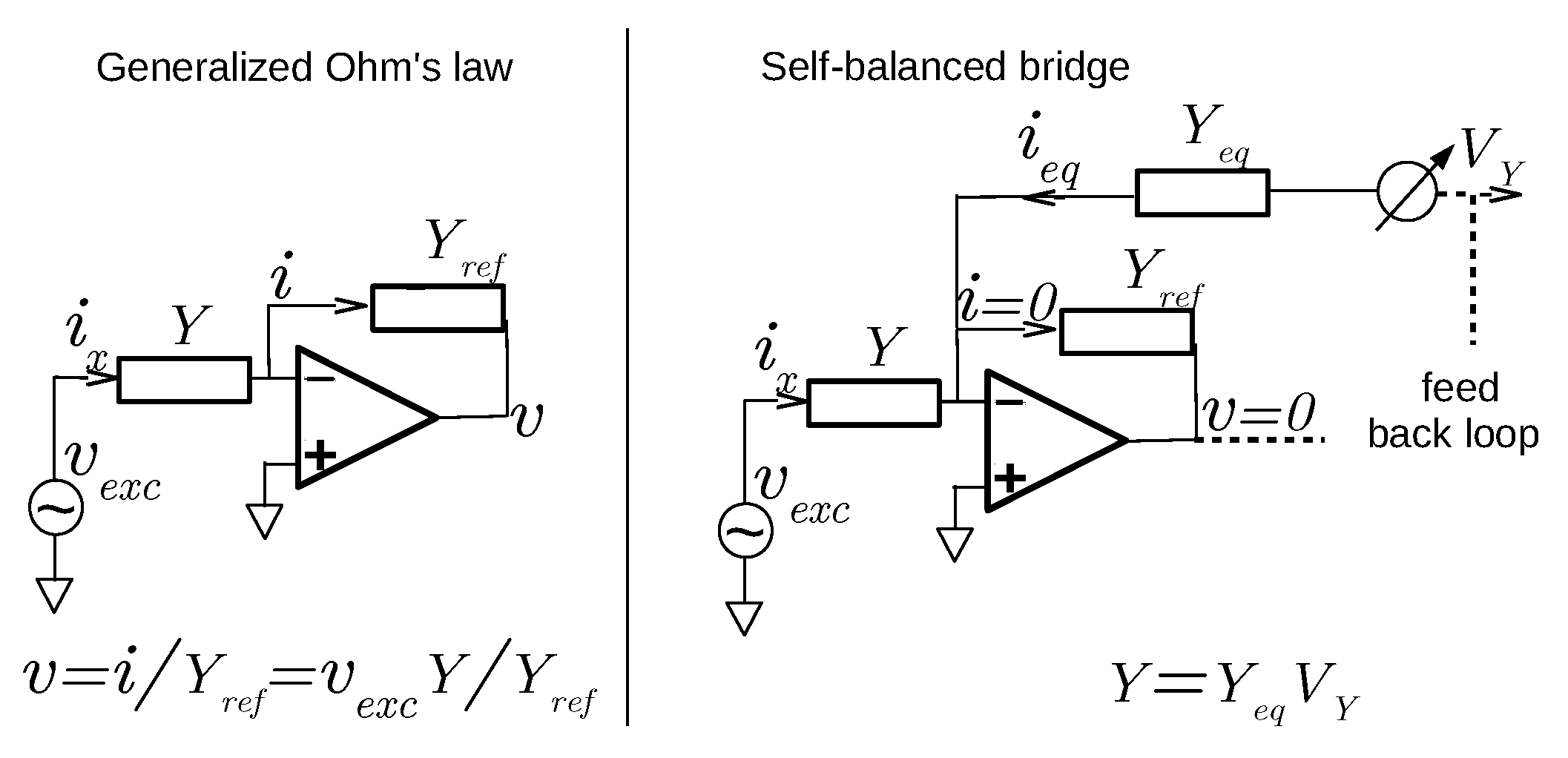
2.2. Ohm’s Law versus Self-Balanced Bridge
- The use of the simple or generalized Ohm’s law, which requires the measurement of voltage and current across the impedance to be determined.
- The Wheatstone, or balanced, bridge, which is a zero method where a reference is adjusted to match the impedance.
2.2.1. Ohm’s Law-Based Techniques
2.2.2. Self-Balanced Bridge
2.2.3. Use of High-Gain-Bandwidth-Product Amplifiers in Both Techniques
3. Instrument Bias and Soil-Specific Effects
3.1. Frequency Role
3.1.1. Instrument Bias
3.1.2. Soil-Specific Effects
3.2. Temperature Influence
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rêgo Segundo, A.K.; Martins, J.H.; de Barros Monteiro, P.M.; de Oliveira, R.A.; Freitas, G.M. A novel low-cost instrumentation system for measuring the water content and apparent electrical conductivity of soils. Sensors 2015, 15, 25546–25563. [Google Scholar] [CrossRef] [PubMed]
- Bogena, H.; Huisman, J.; Oberdörster, C.; Vereecken, H. Evaluation of a low-cost soil water content sensor for wireless network applications. J. Hydrol. 2007, 344, 32–42. [Google Scholar] [CrossRef]
- Chavanne, X.; Frangi, J.P.; de Rosny, G. A New Device for In Situ Measurement of an Impedance Profile at 1–20 MHz. IEEE Trans. Instrum. Meas. 2010, 59, 1850–1859. [Google Scholar] [CrossRef]
- Chavanne, X.; Frangi, J.P. Presentation of a Complex Permittivity-Meter with Applications for Sensing the Moisture and Salinity of a Porous Media. Sensors 2014, 14, 15815–15835. [Google Scholar] [CrossRef] [PubMed]
- Chavanne, X.; Frangi, J.P. Autonomous Sensors for Measuring Continuously the Moisture and Salinity of a Porous Medium. Sensors 2017, 17, 1094. [Google Scholar] [CrossRef] [PubMed]
- Hilhorst, M. Dielectric Characterisation of Soil. Ph.D. Thesis, Agricultural University of Wageningen, Wageningen, The Netherlands, 1998. [Google Scholar]
- Chang, Z.Y.; Iliev, B.P.; Meijer, G.C. A Sensor Interface System for Measuring the Impedance (Cx, Rx) of Soil at a Signal Frequency of 20 MHz. In Proceedings of the 2007 IEEE Sensors, Atlanta, GA, USA, 28–31 October 2007; pp. 268–271. [Google Scholar]
- Agilent Technologies. Agilent Technologies Impedance Measurement Handbook, 4th ed.; Agilent Technologies: Santa Clara, CA, USA, 2003. [Google Scholar]
- Keysight Technologies. Keysight Technologies Impedance Measurement Handbook, 6th ed.; Keysight Technologies: Santa Rosa, CA, USA, 2016. [Google Scholar]
- Giffard, R.P. A simple low power self-balancing resistance bridge. J. Phys. E Sci. Instrum. 1973, 6, 719–723. [Google Scholar] [CrossRef]
- Aalto, M.I.; Ehnholm, G.J. A self-balancing resistance bridge. J. Phys. E Sci. Instrum. 1973, 6, 614–618. [Google Scholar] [CrossRef]
- Jones, S.B.; Blonquist, J.; Robinson, D.; Rasmussen, V.P.; Or, D. Standardizing characterization of electromagnetic water content sensors. Vadose Zone J. 2005, 4, 1048–1058. [Google Scholar] [CrossRef]
- Kelleners, T.; Soppe, R.; Robinson, D.; Schaap, M.; Ayars, J.; Skaggs, T. Calibration of capacitance probe sensors using electric circuit theory. Soil Sci. Soc. Am. J. 2004, 68, 770–778. [Google Scholar] [CrossRef]
- Campbell, J.E. Dielectric properties and influence of conductivity in soils at one to fifty megahertz. Soil Sci. Soc. Am. J. 1990, 54, 332–341. [Google Scholar] [CrossRef]
- Gaskin, G.; Miller, J. Measurement of soil water content using a simplified impedance measuring technique. J. Agric. Eng. Res. 1996, 63, 153–159. [Google Scholar] [CrossRef]
- Rosenbaum, U.; Huisman, J.; Vrba, J.; Vereecken, H.; Bogena, H. Correction of Temperature and Electrical Conductivity Effects on Dielectric Permittivity Measurements with ECH2O Sensors. Vadose Zone J. 2011, 10, 582–593. [Google Scholar] [CrossRef]
- Kuphaldt, T.R. Lessons in Electric Circuits (Volume II Chapter 12 of AC METERING CIRCUITS), 5th ed. Open Book Project. 2006. Available online: https://www.ibiblio.org/kuphaldt/electricCircuits/ (accessed on 25 May 2018).
- Bruere, A.; Galaud, C. Capacitive Dimensional Measurement Chain with Linear Output. U.S. Patent 5,065,105, 12 November 1991. [Google Scholar]
- Bruere, A.; Galaud, C. Dispositif de Mesure D’impédance. French Patent FR2693555 (A1), 14 January 1994. [Google Scholar]
- Rêgo Segundo, A.K.; Pinto, E.S.; de Barros Monteiro, P.M.; Martins, J.H. Sensor for measuring electrical parameters of soil based on auto-balancing bridge circuit. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; Volume 15, pp. 1–3. [Google Scholar]
- Friedman, S.P. Soil properties influencing apparent electrical conductivity: A review. Comput. Electron. Agric. 2005, 46, 45–70. [Google Scholar] [CrossRef]
- Chelidze, T.L.; Gueguen, Y.; Ruffet, C. Electrical spectroscopy of porous rocks: A review II. Geophys. J. Int. 1999, 137, 16–34. [Google Scholar] [CrossRef]
- Revil, A. Effective conductivity and permittivity of unsaturated porous materials in the frequency range 1 mHz–1 GHz. Water Resour. Res. 2013, 49, 306–327. [Google Scholar] [CrossRef] [PubMed]
- Hilhorst, M. A pore water conductivity sensor. Soil Sci. Soc. Am. J. 2000, 64, 1922–1925. [Google Scholar] [CrossRef]
- Metrohm AG. Conductometry. In Metrohm Application Bulletin No 102/2 e; Metrohm: Herisau, Switzerland, 1996; pp. 1–9. [Google Scholar]
- Rhoades, J.D.; Chanduvi, F.; Lesch, S.M. Soil Salinity Assessment: Methods and Interpretation of Electrical Conductivity Measurements; Food & Agriculture Organization of the United Nations: Rome, Italy, 1999. [Google Scholar]
- Kaatze, U. Complex permittivity of water as a function of frequency and temperature. J. Chem. Eng. Data 1989, 34, 371–374. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavanne, X.; Bruère, A.; Frangi, J.-P. Comments to: A Novel Low-Cost Instrumentation System for Measuring the Water Content and Apparent Electrical Conductivity of Soils, Sensors, 15, 25546–25563. Sensors 2018, 18, 1730. https://doi.org/10.3390/s18061730
Chavanne X, Bruère A, Frangi J-P. Comments to: A Novel Low-Cost Instrumentation System for Measuring the Water Content and Apparent Electrical Conductivity of Soils, Sensors, 15, 25546–25563. Sensors. 2018; 18(6):1730. https://doi.org/10.3390/s18061730
Chicago/Turabian StyleChavanne, Xavier, Alain Bruère, and Jean-Pierre Frangi. 2018. "Comments to: A Novel Low-Cost Instrumentation System for Measuring the Water Content and Apparent Electrical Conductivity of Soils, Sensors, 15, 25546–25563" Sensors 18, no. 6: 1730. https://doi.org/10.3390/s18061730
APA StyleChavanne, X., Bruère, A., & Frangi, J.-P. (2018). Comments to: A Novel Low-Cost Instrumentation System for Measuring the Water Content and Apparent Electrical Conductivity of Soils, Sensors, 15, 25546–25563. Sensors, 18(6), 1730. https://doi.org/10.3390/s18061730




