Analysis of Consistency of Transthoracic Bioimpedance Measurements Acquired with Dry Carbon Black PDMS Electrodes, Adhesive Electrodes, and Wet Textile Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Transthoracic Bioimpedance (TBI)
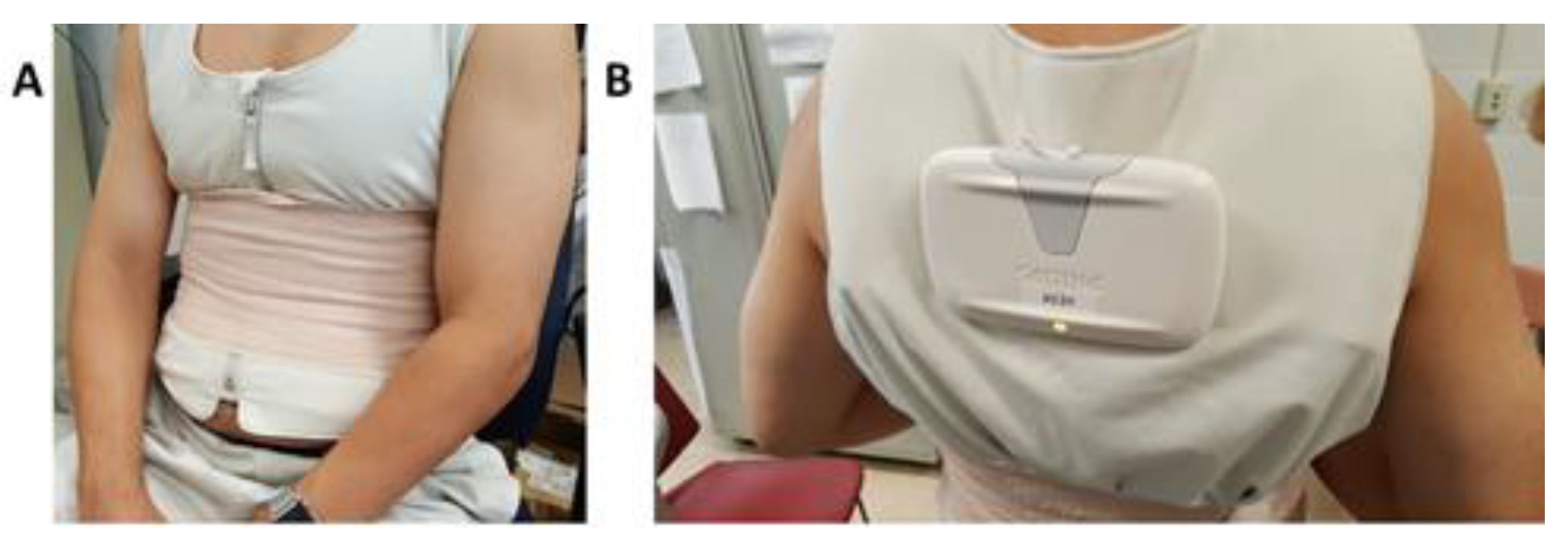
2.2. TBI and ECG Data Collection Protocol
- Copper mesh CB/PDMS (CM/CB/PDMS);
- Poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT) textile CB/PDMS (PT/CB/PDMS); and
- PEDOT salt textile, CB/PDMS (PST/CB/PDMS).
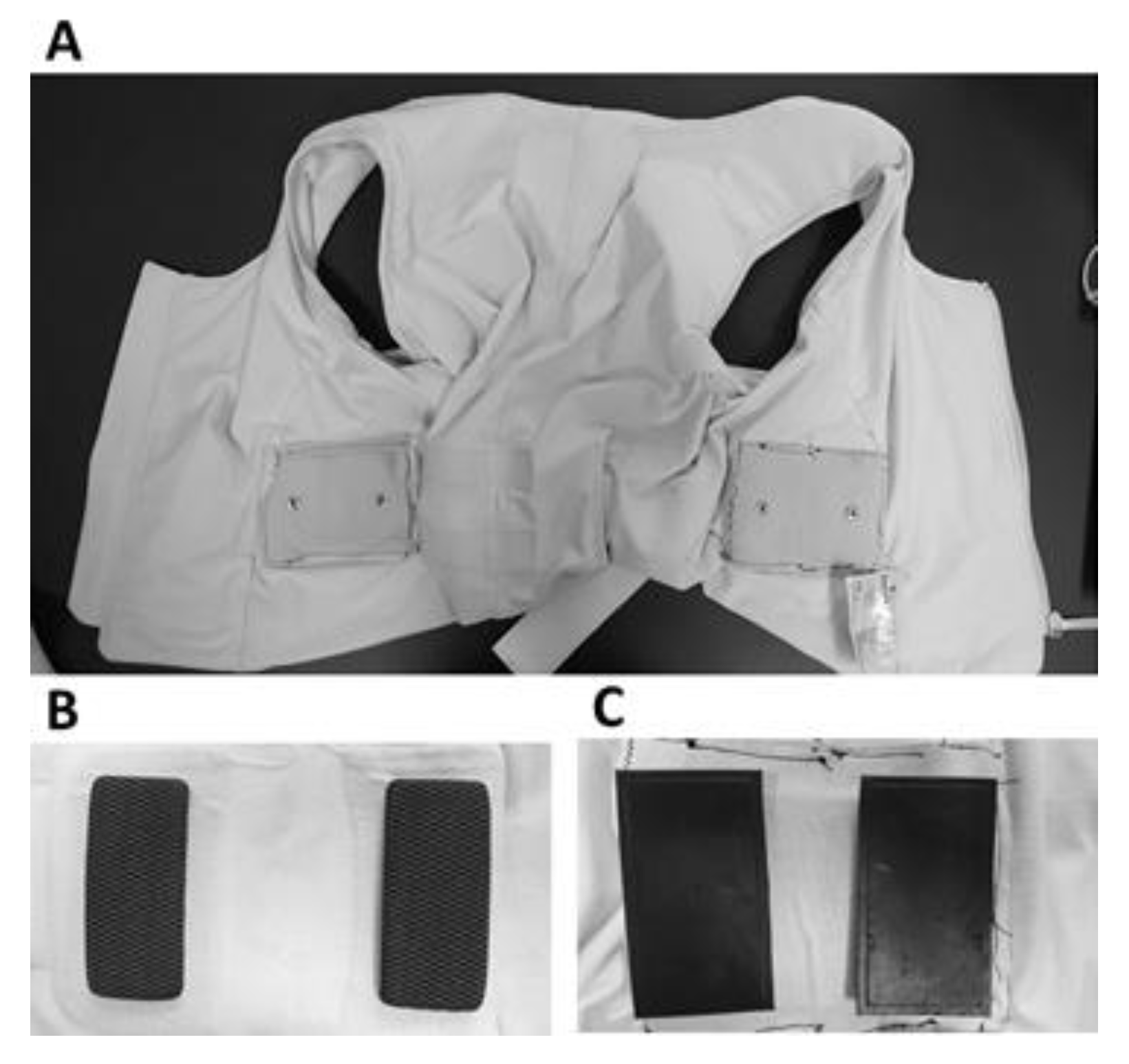
2.3. Fabrication of CB/PDMS Electrodes
- (A)
- The 3D printed Acrylonitrile Butadiene Styrene (ABS) cavity molds (Objet350 Connex, Stratasys, Eden Prairie, MN, USA) were filled with the conductive CB/PDMS composite and leveled so that no excess material remained.
- (B)
- A highly conductive layer was affixed on the CB/PDMS mixture to allow a signal acquisition via the monitoring device. An insulated and waterproofed wire was soldered to the material of the highly conductive layer and was used as a connector to an ECG monitoring device. Three different conductive materials were tested on the CB/PDMS electrodes for the TBI and ECG measurements with the FAV, as follows: (1) copper mesh; (2) poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT) screen printed synthetic leather; and (3) PEDOT-salt screen printed synthetic leather.
- (C)
- PDMS and curing agent mixture was then used to encapsulate the exposed surface with either an embedded copper mesh, PEDOT only leather, or PEDOT-salt leather.
- (D)
- All of the components were degassed for 15 min in a vacuum chamber.
- (E)
- The fasteners were soldered to the exposed end of the wire that was extended from the electrode.
- (F)
- The completed electrode assembly was then placed in a curing oven at 75 °C for 3 h.
- (G)
- After 3 h, the molds were removed from the curing oven and subsequently the electrodes were also removed from the cavity molds.
2.4. TBI and ECG Measurements
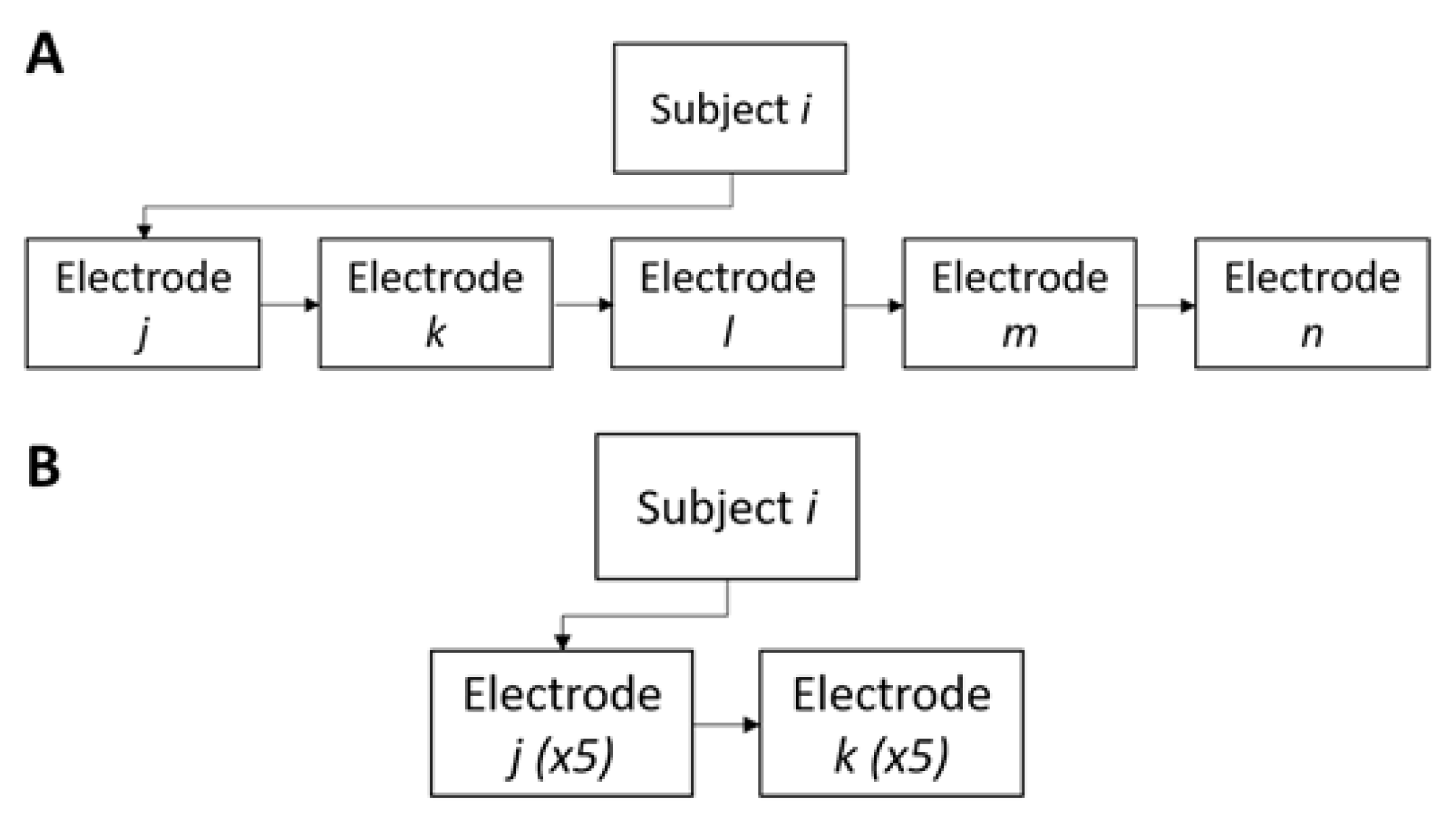
2.4.1. Inter-Subject TBI and ECG Tests
2.4.2. Intra-Subject Consistency Test
3. Results
3.1. Inter-Subject TBI and ECG Tests
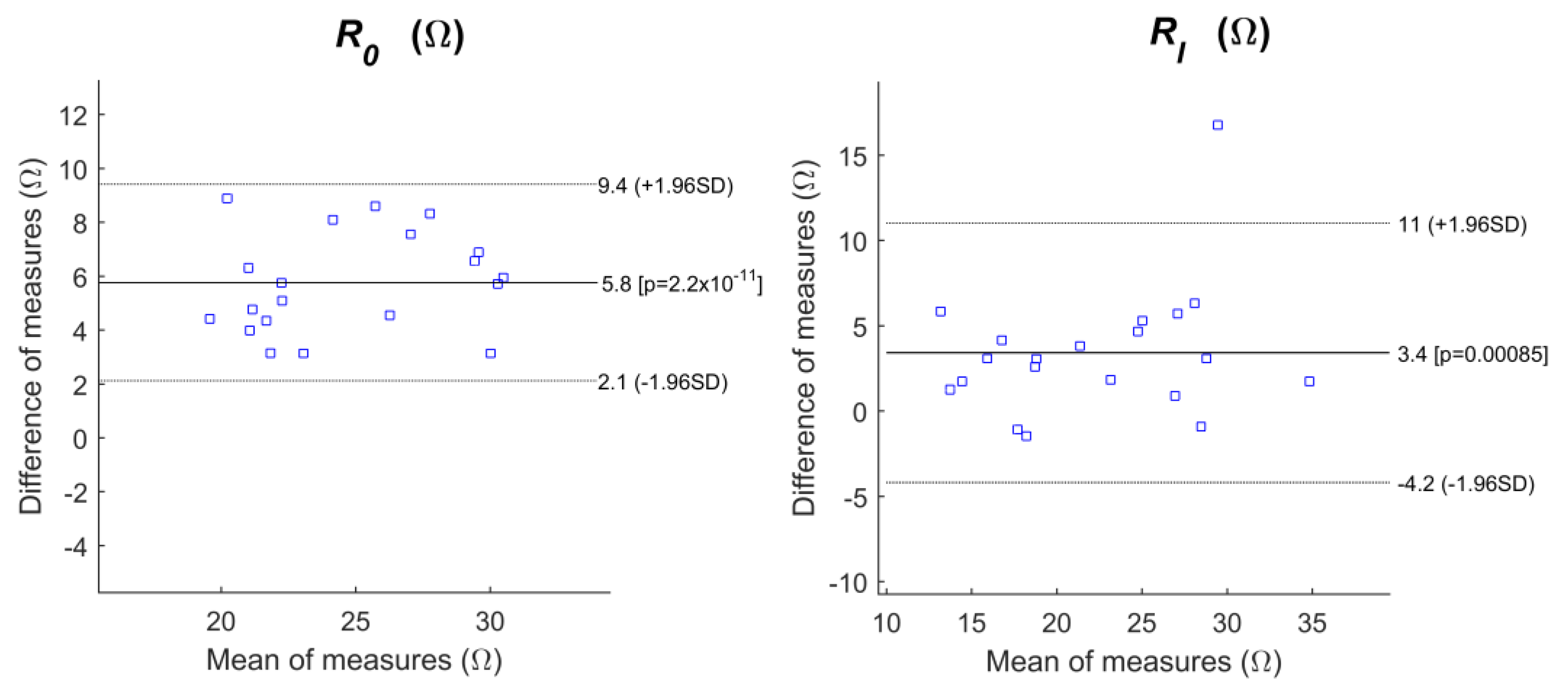
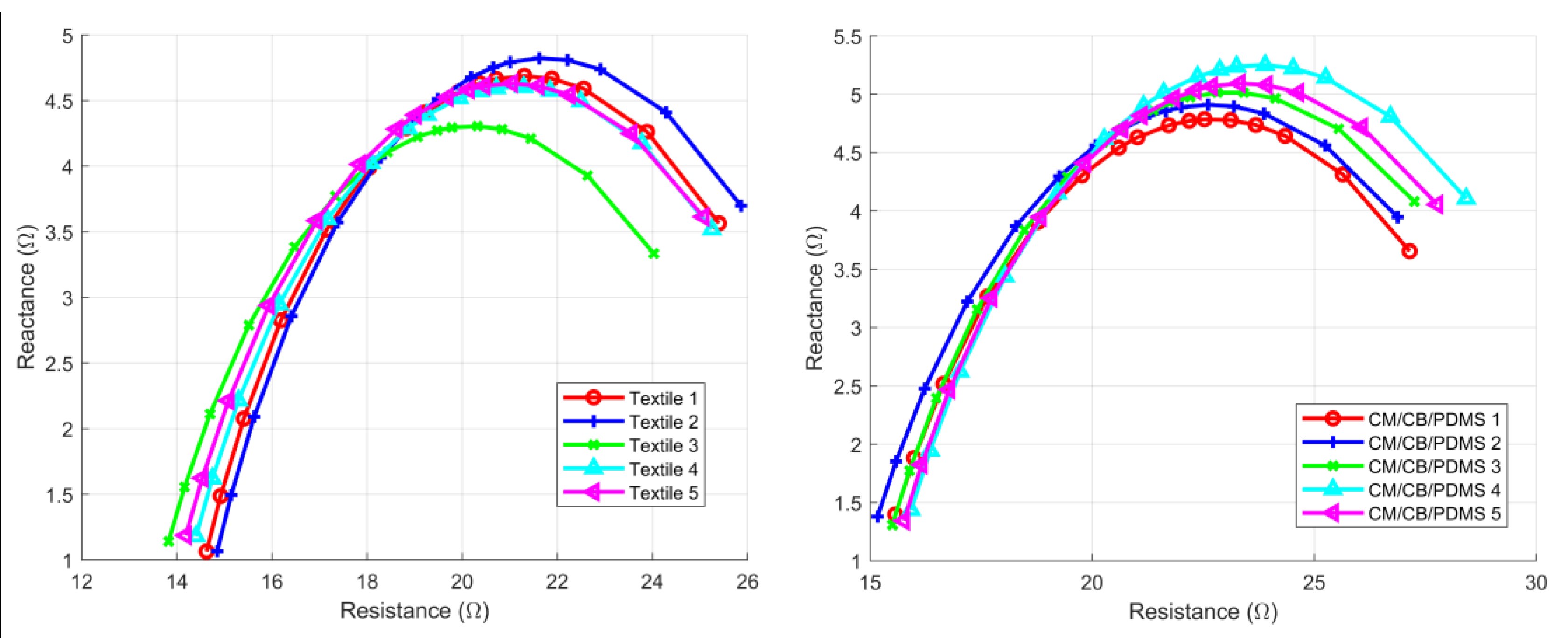
3.2. Intra-Subject Consistency Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baumgartner, R.N.; Chumlea, W.C.; Roche, A.F. Estimation of body composition from bioelectric impedance of body segments. Am. J. Clin. Nutr. 1989, 50, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Seppä, V.-P.; Viik, J.; Hyttinen, J. Assessment of pulmonary flow using impedance pneumography. IEEE Trans. Biomed. Eng. 2010, 57, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Grimnes, S.; Martinsen, Ø.G. Sources of error in tetrapolar impedance measurements on biomaterials and other ionic conductors. J. Phys. Appl. Phys. 2007, 40, 9. [Google Scholar] [CrossRef]
- Hernández-Balaguera, E.; López-Dolado, E.; Polo, J.L. Obtaining electrical equivalent circuits of biological tissues using the current interruption method, circuit theory and fractional calculus. RSC Adv. 2016, 6, 22312–22319. [Google Scholar] [CrossRef]
- Khan, M.I.H.; Karim, M.A. Cellular water distribution, transport, and its investigation methods for plant-based food material. Food Res. Int. 2017, 99, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Darling, C.E.; Dovancescu, S.; Saczynski, J.S.; Riistama, J.; Kuniyoshi, F.S.; Rock, J.; Meyer, T.E.; McManus, D.D. Bioimpedance-Based Heart Failure Deterioration Prediction Using a Prototype Fluid Accumulation Vest-Mobile Phone Dyad: An Observational Study. JMIR Cardio 2017, 1, e1. [Google Scholar] [CrossRef]
- Marquez, J.C.; Seoane, F.; Lindecrantz, K. Skin-electrode contact area in electrical bioimpedance spectroscopy. Influence in total body composition assessment. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 1867–1870. [Google Scholar] [CrossRef]
- Márquez, J.C.; Seoane, F.; Lindecrantz, K. Textrode functional straps for bioimpedance measurements—Experimental results for body composition analysis. Eur. J. Clin. Nutr. 2013, 67 (Suppl. 1), S22–S27. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, L.; Neuhaus, C.; Medrano, G.; Jungbecker, N.; Walter, M.; Gries, T.; Leonhardt, S. Characterization of textile electrodes and conductors using standardized measurement setups. Physiol. Meas. 2010, 31, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.; Bales, J.R.; Reyes, B.A.; Molignano, J.; Clement, A.L.; Pins, G.D.; Florian, J.P.; Chon, K.H. Novel Conductive Carbon Black and Polydimethlysiloxane ECG Electrode: A Comparison with Commercial Electrodes in Fresh, Chlorinated, and Salt Water. Ann. Biomed. Eng. 2016, 44. [Google Scholar] [CrossRef] [PubMed]
- Reyes, B.A.; Posada-Quintero, H.F.; Bales, J.R.; Clement, A.L.; Pins, G.D.; Swiston, A.; Riistama, J.; Florian, J.P.; Shykoff, B.; Qin, M.; et al. Novel Electrodes for Underwater ECG Monitoring. IEEE Trans. Biomed. Eng. 2014, 61, 1863–1876. [Google Scholar] [CrossRef] [PubMed]
- Anter, E.; Jessup, M.; Callans, D.J. Atrial fibrillation and heart failure: Treatment considerations for a dual epidemic. Circulation 2009, 119, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Parise, H.; D’Agostino, R.B.; Lloyd-Jones, D.M.; Vasan, R.S.; Wang, T.J.; Levy, D.; Wolf, P.A.; Benjamin, E.J. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA 2004, 291, 2851–2855. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Larson, M.G.; Leip, E.P.; Beiser, A.; D’Agostino, R.B.; Kannel, W.B.; Murabito, J.M.; Vasan, R.S.; Benjamin, E.J.; Levy, D. Framingham Heart Study Lifetime risk for developing congestive heart failure: The Framingham Heart Study. Circulation 2002, 106, 3068–3072. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Wang, T.J.; Leip, E.P.; Larson, M.G.; Levy, D.; Vasan, R.S.; D’Agostino, R.B.; Massaro, J.M.; Beiser, A.; Wolf, P.A.; et al. Lifetime risk for development of atrial fibrillation: The Framingham Heart Study. Circulation 2004, 110, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Executive summary: Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010, 121, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.G.; Stevenson, L.W.; Middlekauff, H.R.; Fonarow, G.C.; Hamilton, M.A.; Woo, M.A.; Saxon, L.A.; Natterson, P.D.; Steimle, A.; Walden, J.A.; et al. Improving survival for patients with atrial fibrillation and advanced heart failure. J. Am. Coll. Cardiol. 1996, 28, 1458–1463. [Google Scholar] [CrossRef]
- Vasan, R.S.; Levy, D. Defining diastolic heart failure: A call for standardized diagnostic criteria. Circulation 2000, 101, 2118–2121. [Google Scholar] [CrossRef] [PubMed]
- Gummeson, J.; Clark, S.S.; Fu, K.; Ganesan, D. On the Limits of Effective Hybrid Micro-energy Harvesting on Mobile CRFID Sensors. In Proceedings of the 8th International Conference on Mobile Systems, Applications, and Services, San Francisco, CA, USA, 15–18 June 2010; ACM: New York, NY, USA, 2010; pp. 195–208. [Google Scholar]
- Reiter, H.; Muehlsteff, J.; Sipilä, A. Medical application and clinical validation for reliable and trustworthy physiological monitoring using functional textiles: Experience from the HeartCycle and MyHeart project. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 3270–3273. [Google Scholar] [CrossRef]
- Fang, J.; Mensah, G.A.; Croft, J.B.; Keenan, N.L. Heart failure-related hospitalization in the U.S., 1979 to 2004. J. Am. Coll. Cardiol. 2008, 52, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.H.; Rogers, J.G.; Hasselblad, V.; Tasissa, G.; Binanay, C.; Califf, R.M.; O’Connor, C.M. Association of Weight Change with Subsequent Outcomes in Patients Hospitalized with Acute Decompensated Heart Failure. Am. J. Cardiol. 2009, 103, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Brisco, M.A.; Kociol, R.D.; Jacoby, D.; Bellumkonda, L.; Parikh, C.R.; Coca, S.G.; Wilson Tang, W.H. Substantial discrepancy between fluid and weight loss during acute decompensated heart failure treatment: Important lessons for research and clinical care. Am. J. Med. 2015, 128, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.J.; Goldberg, J.H.; Pruell, S.; Yarzebski, J.; Lessard, D.; Spencer, F.A.; Gore, J.M. Delays in seeking medical care in hospitalized patients with decompensated heart failure. Am. J. Med. 2008, 121, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.; Morris, M. Improving heart failure in home care with chronic disease management and telemonitoring. Home Healthc. Nurse 2010, 28, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Riistama, J.; Muehlsteff, J.; Reiter, H.; Sartor, F.; Sipila, A. Minimally obtrusive measurement devices for monitoring of cardiovascular parameters—An overview. In Proceedings of the 2014 8th Conference of the European Study Group on Cardiovascular Oscillations (ESGCO), Trento, Italy, 25–28 May 2014; pp. 137–138. [Google Scholar]
- Reljin, N.; Posada-Quintero, H.; Noh, Y.; Robb, C.E.; Dimitrov, T.; Murphy, L.; Riistama, J.; Chon, K.H. Preliminary results on transthoracic bioimpedance measurements with a variety of electrode materials. In Proceedings of the 2018 IEEE EMBS International Conference on Biomedical Health Informatics (BHI), Las Vegas, NV, USA, 4–7 March 2018; pp. 62–65. [Google Scholar]
- Luepker, R.V.; Michael, J.R.; Warbasse, J.R. Transthoracic electrical impedance; quantitative evaluation of a non-invasive measure of thoracic fluid volume. Am. Heart J. 1973, 85, 83–93. [Google Scholar] [CrossRef]
- Yancy, C.; Abraham, W.T. Noninvasive hemodynamic monitoring in heart failure: Utilization of impedance cardiography. Congest. Heart Fail. (Greenwich Conn.) 2003, 9, 241–250. [Google Scholar] [CrossRef]
- Patterson, R.; Kubicek, W.G.; Kinnen, E.; Witsoe, D.; Noren, G. Development of an electrical impedance plethysmographic system to monitor cardiac output. In Proceedings of the 1st Annual Rocky Mountain Bioengineering Symposium, Colorado Springs, CO, USA, 4–5 May 1964; p. 56. [Google Scholar]
- Kubicek, W.G.; Patterson, R.P.; Witsoe, D.A. Impedance cardiography as a noninvasive method of monitoring cardiac function and other parameters of the cardiovascular system*. Ann. N. Y. Acad. Sci. 1970, 170, 724–732. [Google Scholar] [CrossRef]
- Kubicek, W.G.; Kottke, J.; Ramos, M.U.; Patterson, R.P.; Witsoe, D.A.; Labree, J.W.; Remole, W.; Layman, T.E.; Schoening, H.; Garamela, J.T. The Minnesota impedance cardiograph—Theory and applications. Biomed. Eng. 1974, 9, 410–416. [Google Scholar] [PubMed]
- Kauppinen, P.K.; Hyttinen, J.A.; Malmivuo, J.A. Sensitivity distributions of impedance cardiography using band and spot electrodes analyzed by a three-dimensional computer model. Ann. Biomed. Eng. 1998, 26, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Belalcazar, A.; Patterson, R.P. Improved lung edema monitoring with coronary vein pacing leads: A simulation study. Physiol. Meas. 2004, 25, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Brodin, L.A.; Jogestrand, T.; Larsen, F.F.; Tedner, B.; Walldius, G. Effects of furosemide and slow-release furosemide on thoracic fluid volumes. Clin. Cardiol. 1986, 9, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Ayllón, D.; Gil-Pita, R.; Seoane, F. Detection and classification of measurement errors in bioimpedance spectroscopy. PLoS ONE 2016, 11, e0156522. [Google Scholar] [CrossRef] [PubMed]
- Cornish, B.H.; Thomas, B.J.; Ward, L.C. Improved prediction of extracellular and total body water using impedance loci generated by multiple frequency bioelectrical impedance analysis. Phys. Med. Biol. 1993, 38, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.S. Membranes, Ions and Impulses: A Chapter of Classical Biophysics, 1st ed.; University of California Press: Berkeley, CA, USA, 1968; ISBN 978-0-520-00251-7. [Google Scholar]
- Taubin, G. Estimation of planar curves, surfaces, and nonplanar space curves defined by implicit equations with applications to edge and range image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 1991, 13, 1115–1138. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A Simplex Method for Function Minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Martinsen, O.G.; Grimnes, S. Bioimpedance and Bioelectricity Basics; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Weyer, S.; Zink, M.D.; Wartzek, T.; Leicht, L.; Mischke, K.; Vollmer, T. Steffen Leonhardt Bioelectrical impedance spectroscopy as a fluid management system in heart failure. Physiol. Meas. 2014, 35, 917. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Chon, K.H.; Lu, S.; Raeder, E.A. Automatic real time detection of atrial fibrillation. Ann. Biomed. Eng. 2009, 37, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Reyes, B.A.; McManus, D.; Maitas, O.; Chon, K.H. Atrial fibrillation detection using an iPhone 4s. IEEE Trans. Biomed. Eng. 2013, 60, 203–206. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.D.; Lee, J.; Maitas, O.; Esa, N.; Pidikiti, R.; Carlucci, A.; Harrington, J.; Mick, E.; Chon, K.H. A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm 2013, 10, 315–319. [Google Scholar] [CrossRef] [PubMed]
- McGraw, K.O.; Wong, S.P. Forming inferences about some intraclass correlation coefficients. Psychol. Methods 1996, 1, 30. [Google Scholar] [CrossRef]
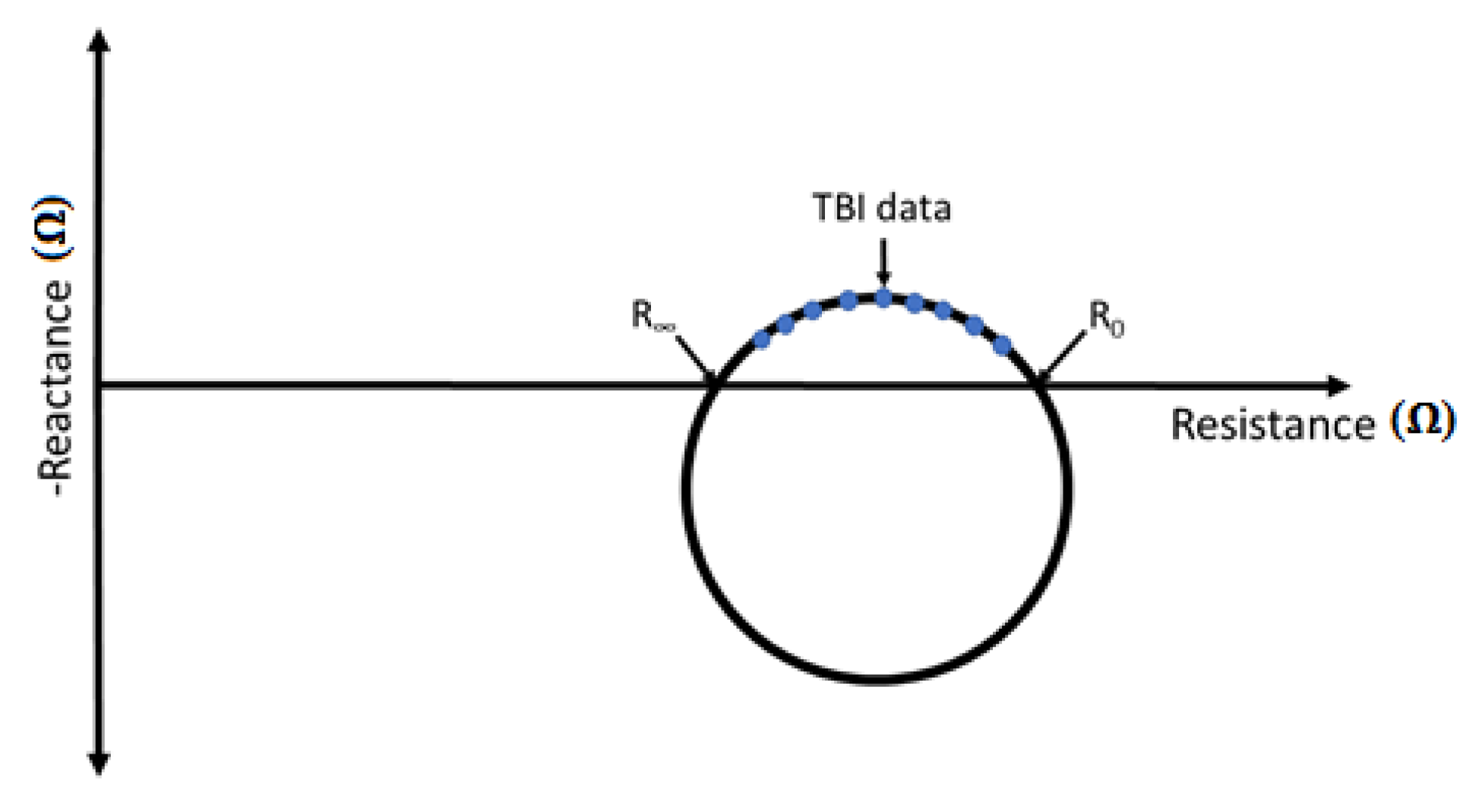
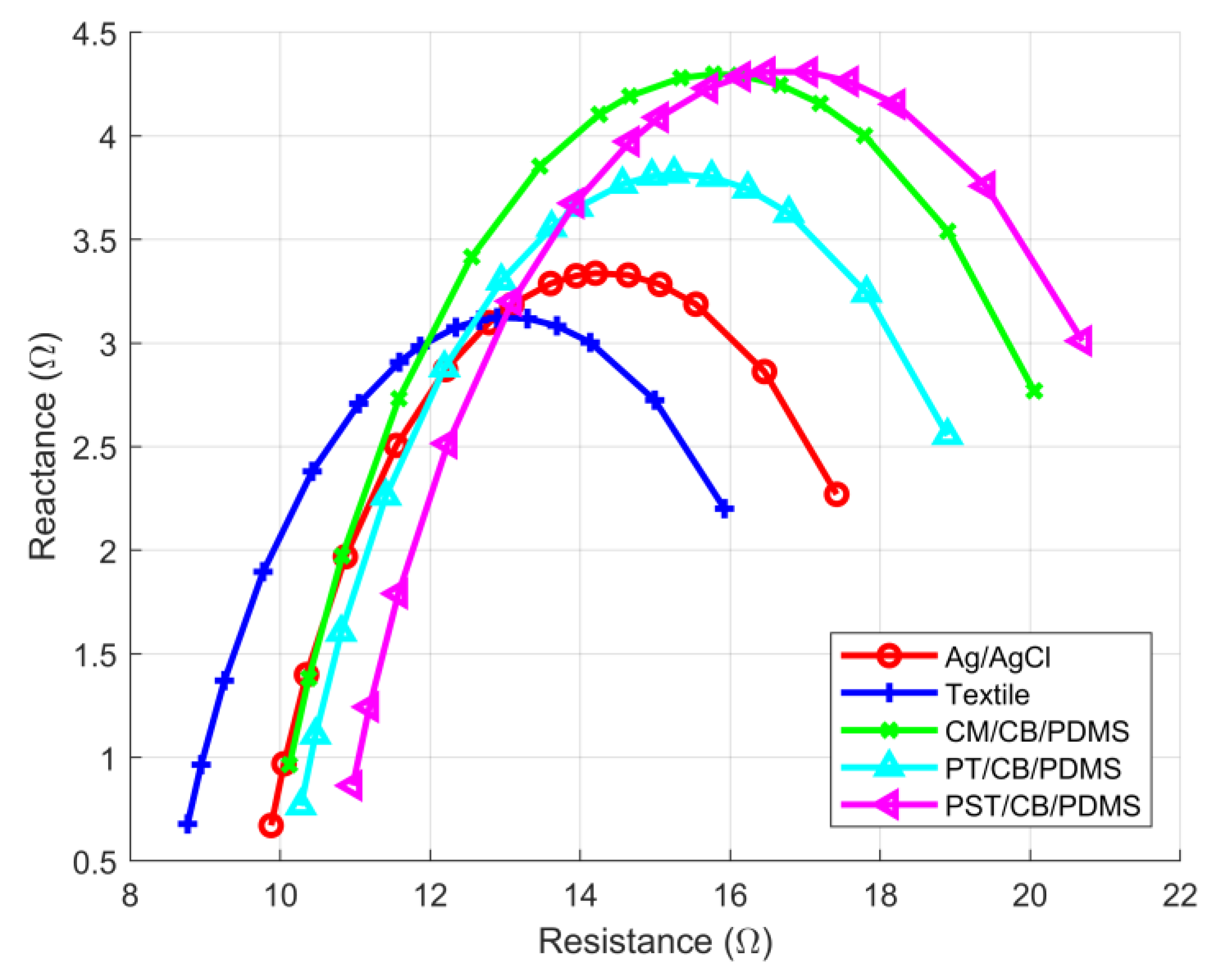
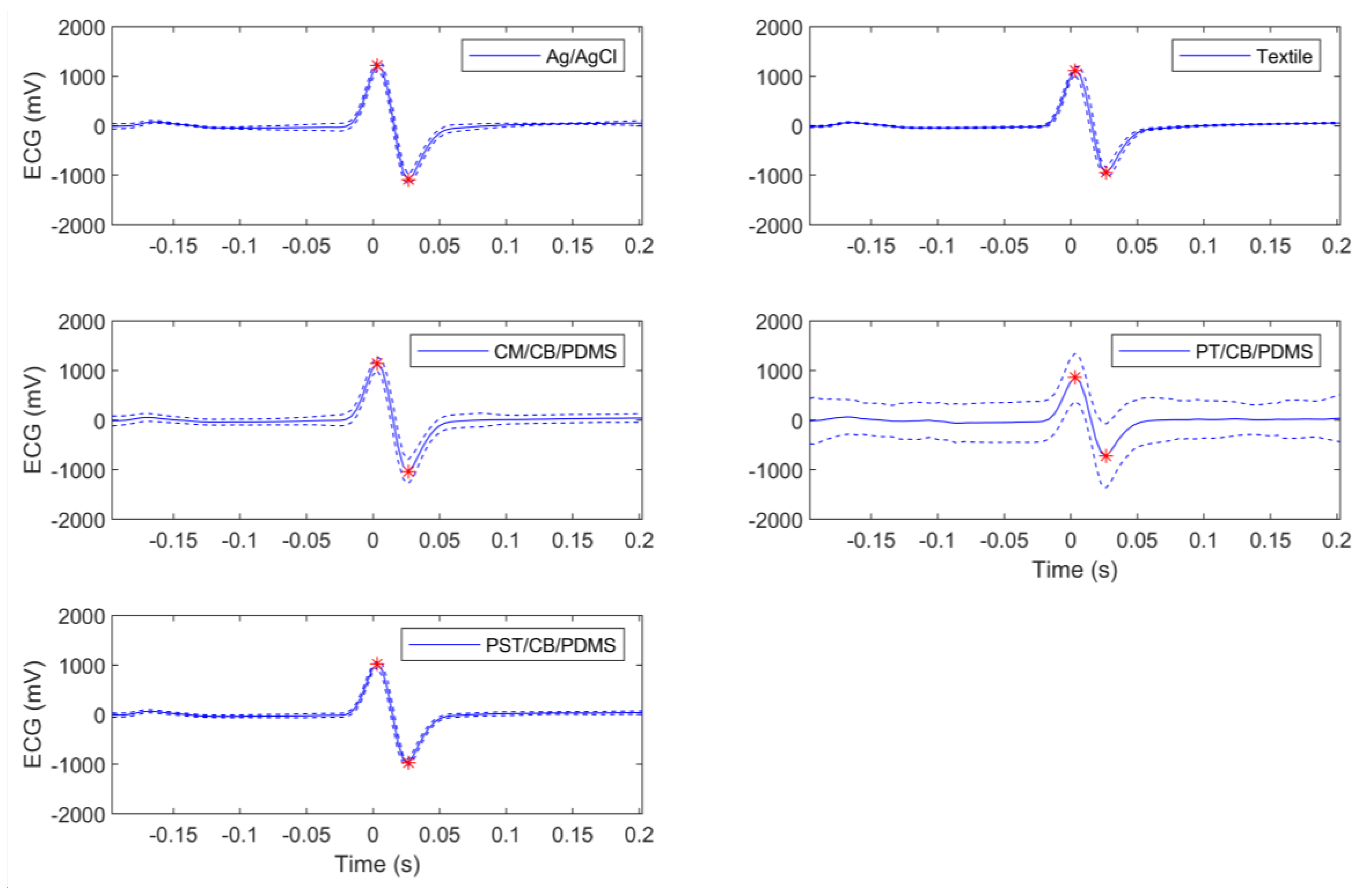

| Electrode Type | R0 (Ω) | RI (Ω) | Peak-To-Peak ECG Amplitude (V) |
|---|---|---|---|
| Ag/AgCl | 24 ± 3.7 * | 24 ± 5.2 * | 1.53 ± 0.69 |
| Textile | 21 ± 3.1 | 19 ± 8.2 | 1.55 ± 0.63 |
| CM/CB/PDMS | 26 ± 4.5 * | 24 ± 5.6 * | 1.51 ± 0.74 |
| PT/CB/PDMS | 26 ± 6.6 * | 25 ± 11 * | 1.44 ± 0.82 |
| PST/CB/PDMS | 26 ± 5.4 * | 27 ± 13 * | 1.5 ± 0.69 |
| Electrode Type | R0 | RI |
|---|---|---|
| Ag/AgCl | 0.15 (0.14 0.16) | 0.22 (0.2 0.24) |
| Textile | 0.15 (0.14 0.16) | 0.42 (0.39 0.46) |
| CM/CB/PDMS | 0.17 (0.16 0.18) | 0.24 (0.22 0.26) |
| PT/CB/PDMS | 0.25 (0.23 0.27) | 0.42 (0.39 0.46) |
| PST/CB/PDMS | 0.2 (0.19 0.22) | 0.50 (0.46 0.55) |
| r2 | Bias (CI) (Ω) | CR (Ω) | sd (Ω) | |
|---|---|---|---|---|
| R0 | ||||
| Ag/AgCl | 0.6940 | 2.3 (−1.9 6.5) | 4.2 | 2.1 |
| CM/CB/PDMS | 0.7937 | 5.8 (2.123 9.4) | 3.6 | 1.9 |
| PT/CB/PDMS | 0.5310 | 4.5 (−4.6 13.6) | 9.1 | 4.6 |
| PST/CB/PDMS | 0.6707 | 4.3 (−1.9 10.5) | 6.2 | 3.1 |
| RI | ||||
| Ag/AgCl | 0.7867 | 3.2 (−2.3 8.6) | 5.4 | 2.8 |
| CM/CB/PDMS | 0.6915 | 3.4 (−4.2 11.0) | 7.6 | 3.9 |
| PT/CB/PDMS | 0.1456 | 4.9 (−14.9 24.8) | 19.8 | 10.1 |
| PST/CB/PDMS | 0.5865 | 6.2 (−12.8 25.2) | 18.9 | 9.7 |
| CV (CI) | ICC (CI) | |||
|---|---|---|---|---|
| Electrode | R0 | RI | R0 | RI |
| Textile | 0.034 (0.029 0.04) | 0.058 (0.048 0.067) | 0.99 (0.98 1) | 0.99 (0.98 0.99) |
| CM/CB/PDMS | 0.041 (0.035 0.048) | 0.079 (0.066 0.091) | 0.98 (0.97 0.99) | 0.96 (0.92 0.98) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posada-Quintero, H.F.; Reljin, N.; Eaton-Robb, C.; Noh, Y.; Riistama, J.; Chon, K.H. Analysis of Consistency of Transthoracic Bioimpedance Measurements Acquired with Dry Carbon Black PDMS Electrodes, Adhesive Electrodes, and Wet Textile Electrodes. Sensors 2018, 18, 1719. https://doi.org/10.3390/s18061719
Posada-Quintero HF, Reljin N, Eaton-Robb C, Noh Y, Riistama J, Chon KH. Analysis of Consistency of Transthoracic Bioimpedance Measurements Acquired with Dry Carbon Black PDMS Electrodes, Adhesive Electrodes, and Wet Textile Electrodes. Sensors. 2018; 18(6):1719. https://doi.org/10.3390/s18061719
Chicago/Turabian StylePosada-Quintero, Hugo F., Natasa Reljin, Caitlin Eaton-Robb, Yeonsik Noh, Jarno Riistama, and Ki H. Chon. 2018. "Analysis of Consistency of Transthoracic Bioimpedance Measurements Acquired with Dry Carbon Black PDMS Electrodes, Adhesive Electrodes, and Wet Textile Electrodes" Sensors 18, no. 6: 1719. https://doi.org/10.3390/s18061719
APA StylePosada-Quintero, H. F., Reljin, N., Eaton-Robb, C., Noh, Y., Riistama, J., & Chon, K. H. (2018). Analysis of Consistency of Transthoracic Bioimpedance Measurements Acquired with Dry Carbon Black PDMS Electrodes, Adhesive Electrodes, and Wet Textile Electrodes. Sensors, 18(6), 1719. https://doi.org/10.3390/s18061719







