Evaluation of Meropenem Pharmacokinetics in an Experimental Acute Respiratory Distress Syndrome (ARDS) Model during Extracorporeal Membrane Oxygenation (ECMO) by Using a PenP β-Lactamase Biosensor
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Animal Model
2.2.1. Ethics
2.2.2. Meropenem Infusion and Blood Sampling
2.2.3. Lung Injury and ECMO Support
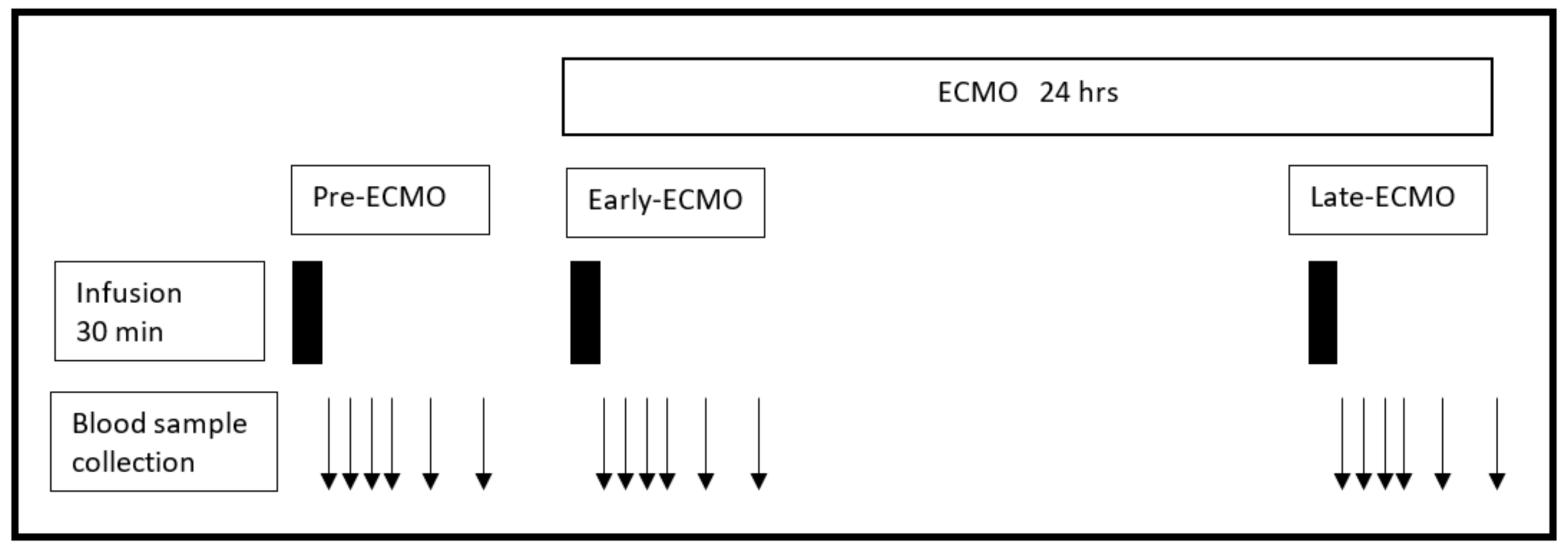
2.2.4. Timeframes of Meropenem Infusion during ECMO Support
2.3. Meropenem Measurements
Meropenem Measurement by the Biosensor PenP
2.4. Pharmacokinetic Analysis
2.5. Statistical Analysis
3. Results
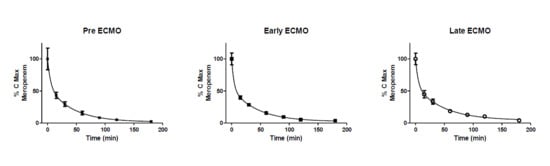
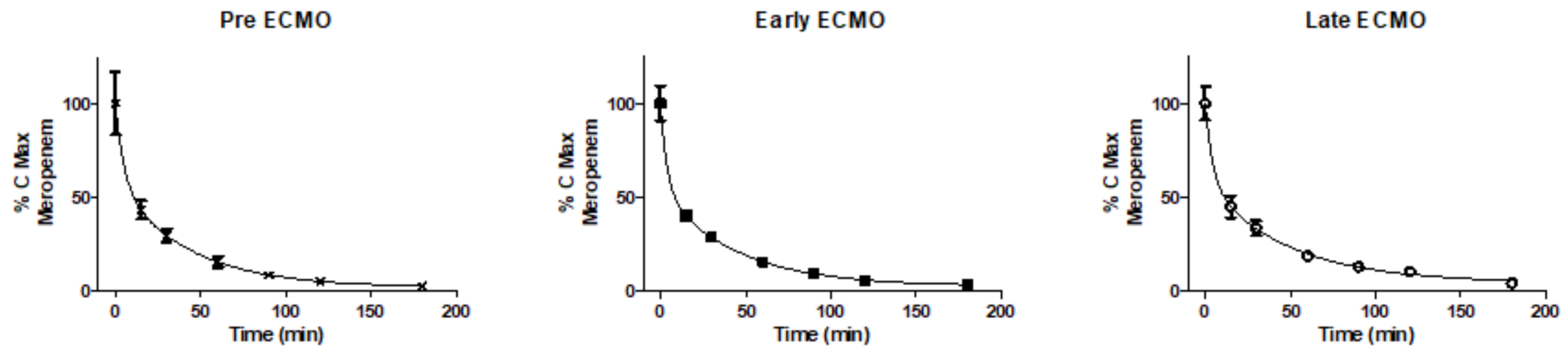
Pharmacokinetics of Meropenem during ECMO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Emmerson, M. Antibiotic usage and prescribing policies in the intensive care unit. Intensiv. Care Med. 2000, 26 (Suppl. 1), S26–S30. [Google Scholar] [CrossRef]
- Thomas, Z.; Bandali, F.; Sankaranarayanan, J.; Reardon, T.; Olsen, K.M. A Multicenter Evaluation of Prolonged Empiric Antibiotic Therapy in Adult ICUs in the United States. Crit. Care Med. 2015, 43, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- DeRyke, C.A.; Lee, S.Y.; Kuti, J.L.; Nicolau, D.P. Optimising dosing strategies of antibacterials utilising pharmacodynamic principles: Impact on the development of resistance. Drugs 2006, 66, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L. Prevention of resistance: A goal for dose selection for antimicrobial agents. Clin. Infect. Dis. 2003, 36, S42–S50. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.; Brodie, D. Emerging indications for extracorporeal membrane oxygenation in adults with respiratory failure. Ann. Am. Thorac. Soc. 2013, 10, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Hodgson, C.; Combes, A. Extracorporeal gas exchange for acute respiratory failure in adult patients: A systematic review. Crit. Care 2015, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- The Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009, 302, 1888–1895. [Google Scholar]
- Noah, M.A.; Peek, G.J.; Finney, S.J.; Griffiths, M.J.; Harrison, D.A.; Grieve, R.; Sadique, M.Z.; Sekhon, J.S.; McAuley, D.F.; Firmin, R.K.; et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A (H1N1). JAMA 2011, 306, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Combes, A.; Roze, H.; Chevret, S.; Mercat, A.; Roch, A.; Mourvillier, B.; Ara-Somohano, C.; Bastien, O.; Zogheib, E.; et al. Extracorporeal membrane oxygenation for pandemic influenza A (H1N1)-induced acute respiratory distress syndrome: A cohort study and propensity-matched analysis. Am. J. Respir. Crit. Care Med. 2013, 187, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Laterre, P.-F.; Dugernier, T.; Spapen, H.; Dalettre, I.; Wittebole, X.; De Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.L.; et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010, 14, R126. [Google Scholar] [CrossRef] [PubMed]
- Joukhadar, C.; Frossard, M.; Mayer, B.; Brunner, M.; Klein, N.; Siostrzonek, P.; Eichler, H.G.; Müller, M. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit. Care Med. 2001, 29, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Donadello, K.; Antonucci, E.; Cristallini, S.; Roberts, J.A.; Beumier, M.; Scolletta, S.; Jacobs, F.; Rondelet, B.; de Backer, D.; Vincent, J.-L.; et al. β-Lactam pharmacokinetics during extracorporeal membrane oxygenation therapy: A case-control study. Int. J. Antimicrob. Agents 2015, 45, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; Fraser, J.F.; Taccone, F.S.; Welch, S.; Wallis, S.C.; Mullany, D.V.; Lipman, J.; Roberts, J.A. The combined effects of extracorporeal membrane oxygenation and renal replacement therapy on meropenem pharmacokinetics: A matched cohort study. Crit. Care 2014, 18, 565. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, M.; Cairoli, S.; Goffredo, B.M.; Stoppa, F.; D’Argenio, P.; Corsetti, T.; Ranieri, V.M. Therapeutic drug monitoring for meropenem after the extracorporeal membrane oxygenation circuit change in children: Is it necessary? Minerva Anestesiol. 2016, 82, 1018–1019. [Google Scholar] [PubMed]
- Cies, J.J.; Moore, W.S., II; Conley, S.B.; Dickerman, M.J.; Small, C.; Carella, B.; Shea, P.; Parker, J.; Chopra, A. Pharmacokinetics of continuous infusion meropenem with concurrent extracorporeal life support and continuous renal replacement therapy: A case report. J. Pediatr. Pharmacol. Ther. 2016, 21, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; Roberts, J.A.; Barnett, A.G.; Diab, S.; Wallis, S.C.; Fung, Y.L.; Fraser, J.F. Can physicochemical properties of antimicrobials be used to predict their pharmacokinetics during extracorporeal membrane oxygenation? Illustrative data from ovine models. Crit. Care 2015, 19, 437. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.M.; Jacobs, R.; Hendrickx, I.; De Waele, E.; Van Gorp, V.; Spapen, H.D. Meropenem therapy in extracorporeal membrane oxygenation patients: An ongoing pharmacokinetic challenge. Crit. Care 2015, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Cies, J.J.; Moore, W.S., II; Dickerman, M.J.; Small, C.; Carella, D.; Chopra, A.; Parker, J. Pharmacokinetics of continuous-infusion meropenem in a pediatric patient receiving extracorporeal life support. Pharmacotherapy 2014, 34, e175–e179. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; Roberts, J.A.; Welch, S.; Buscher, H.; Rudham, S.; Burrows, F.; Ghassabian, S.; Wallis, S.C.; Levkovich, B.; Pellegrino, V.; et al. ASAP ECMO: Antibiotic, Sedative and Analgesic Pharmacokinetics during Extracorporeal Membrane Oxygenation: A multi-centre study to optimise drug therapy during ECMO. BMC Anesthesiol. 2012, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Levcovich, B.; Mojtahedzadeh, M. A systematic review on pharmacokinetic changes in critically ill patients: Role of extracorporeal membrane oxygenation. Daru 2011, 19, 312–321. [Google Scholar] [PubMed]
- Shekar, K.; Roberts, J.A.; Mcdonald, C.I.; Fisquet, S.; Barnett, A.G.; Mullany, D.V.; Ghassabian, S.; Wallis, S.C.; Fung, Y.L.; Smith, M.T.; et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit. Care 2012, 16, R194. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; Silva, C.; Andresen-Vasquez, M.; Soto, N.; Wong, K.-Y.; Andresen, M. Monitorización terapéutica de antibióticos: Nuevas metodologías: Biosensores. Rev. Med. Chile 2015, 143, 1050–1057. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andresen, M.; Wong, K.Y.; Leung, Y.C.; Wong, W.T.; Chan, P.H.; Andresen-Vasquez, M.; Alegria, L.; Silva, C.; Tapia, P.; Downey, P.; et al. Method Based on the β-Lactamase PenPC Fluorescent Labeled for β-Lactam Antibiotic Quantification in Human Plasma. Biomed. Res. Int. 2016, 2016, 4307987. [Google Scholar] [CrossRef] [PubMed]
- Araos, J.; Alegria, L.; Garcia, P.; Damiani, F.; Tapia, P.; Soto, D.; Salomon, T.; Rodriguez, F.; Amthauer, M.; Erranz, B.; et al. Extracorporeal membrane oxygenation improves survival in a novel 24-hour pig model of severe acute respiratory distress syndrome. Am. J. Transl. Res. 2016, 8, 2826–2837. [Google Scholar] [PubMed]
| Pre-ECMO (n = 3) | Early ECMO (n = 5) | Late ECMO (n = 5) | |
|---|---|---|---|
| Cmax (umol/Kg) | 2.196 ± 0.877 | 2.507 ± 0.677 | 2.311 ± 0.567 |
| Cl (L/min/Kg) | 11.92 ± 3.93 | 8.96 ± 1.84 | 9.26 ± 1.69 |
| Ke (min−1) | 0.024 ± 0.007 | 0.020 ± 0.003 | 0.019 ± 0.002 |
| Vd (L/Kg) | 497 ± 134 | 448 ± 92 | 490 ± 89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andresen, M.; Araos, J.; Wong, K.-Y.; Leung, Y.-C.; So, L.-Y.; Wong, W.-T.; Cabrera, S.; Silva, C.; Alegria, L.; Bruhn, A.; et al. Evaluation of Meropenem Pharmacokinetics in an Experimental Acute Respiratory Distress Syndrome (ARDS) Model during Extracorporeal Membrane Oxygenation (ECMO) by Using a PenP β-Lactamase Biosensor. Sensors 2018, 18, 1424. https://doi.org/10.3390/s18051424
Andresen M, Araos J, Wong K-Y, Leung Y-C, So L-Y, Wong W-T, Cabrera S, Silva C, Alegria L, Bruhn A, et al. Evaluation of Meropenem Pharmacokinetics in an Experimental Acute Respiratory Distress Syndrome (ARDS) Model during Extracorporeal Membrane Oxygenation (ECMO) by Using a PenP β-Lactamase Biosensor. Sensors. 2018; 18(5):1424. https://doi.org/10.3390/s18051424
Chicago/Turabian StyleAndresen, Max, Joaquin Araos, Kwok-Yin Wong, Yun-Chung Leung, Lok-Yan So, Wai-Ting Wong, Salvador Cabrera, Camila Silva, Leyla Alegria, Alejandro Bruhn, and et al. 2018. "Evaluation of Meropenem Pharmacokinetics in an Experimental Acute Respiratory Distress Syndrome (ARDS) Model during Extracorporeal Membrane Oxygenation (ECMO) by Using a PenP β-Lactamase Biosensor" Sensors 18, no. 5: 1424. https://doi.org/10.3390/s18051424
APA StyleAndresen, M., Araos, J., Wong, K.-Y., Leung, Y.-C., So, L.-Y., Wong, W.-T., Cabrera, S., Silva, C., Alegria, L., Bruhn, A., & Soto, D. (2018). Evaluation of Meropenem Pharmacokinetics in an Experimental Acute Respiratory Distress Syndrome (ARDS) Model during Extracorporeal Membrane Oxygenation (ECMO) by Using a PenP β-Lactamase Biosensor. Sensors, 18(5), 1424. https://doi.org/10.3390/s18051424