Towards an Electrochemical Immunosensor System with Temperature Control for Cytokine Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. The Sensor Principle in Detail
2.2. Chemicals
2.3. Electrochemical System
2.4. Performance of Reference ELISA
2.5. Immunosensor Preparation
2.6. Measurement and Characterization of Immunosensors
2.6.1. Measurements in Beaker
2.6.2. Measurements in Flow Cell
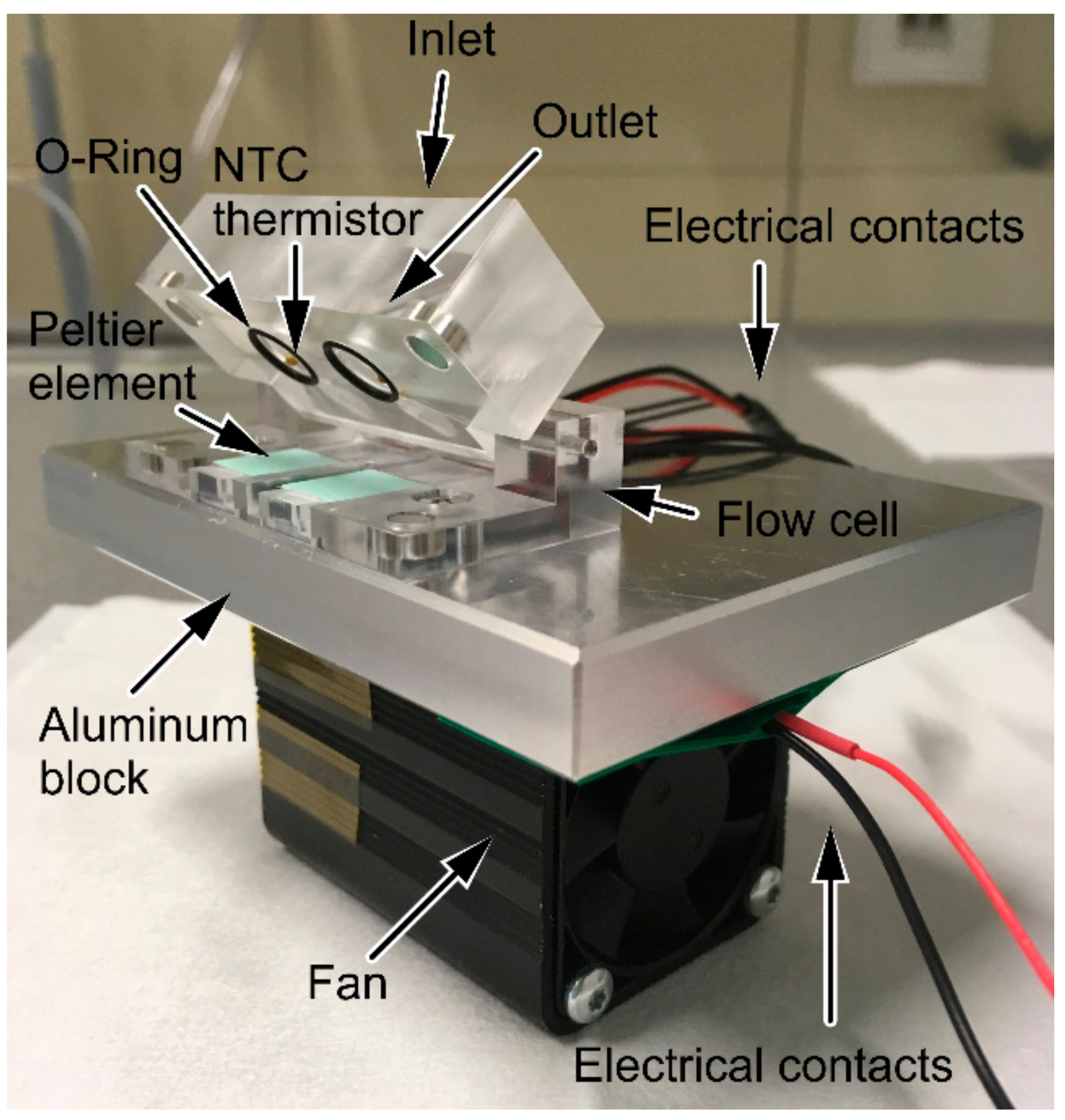
2.7. Design and Set-Up of Thermostat-Controlled Flow Cell
2.8. Selectivity of the IL-13 Immunosensor
3. Results and Discussion
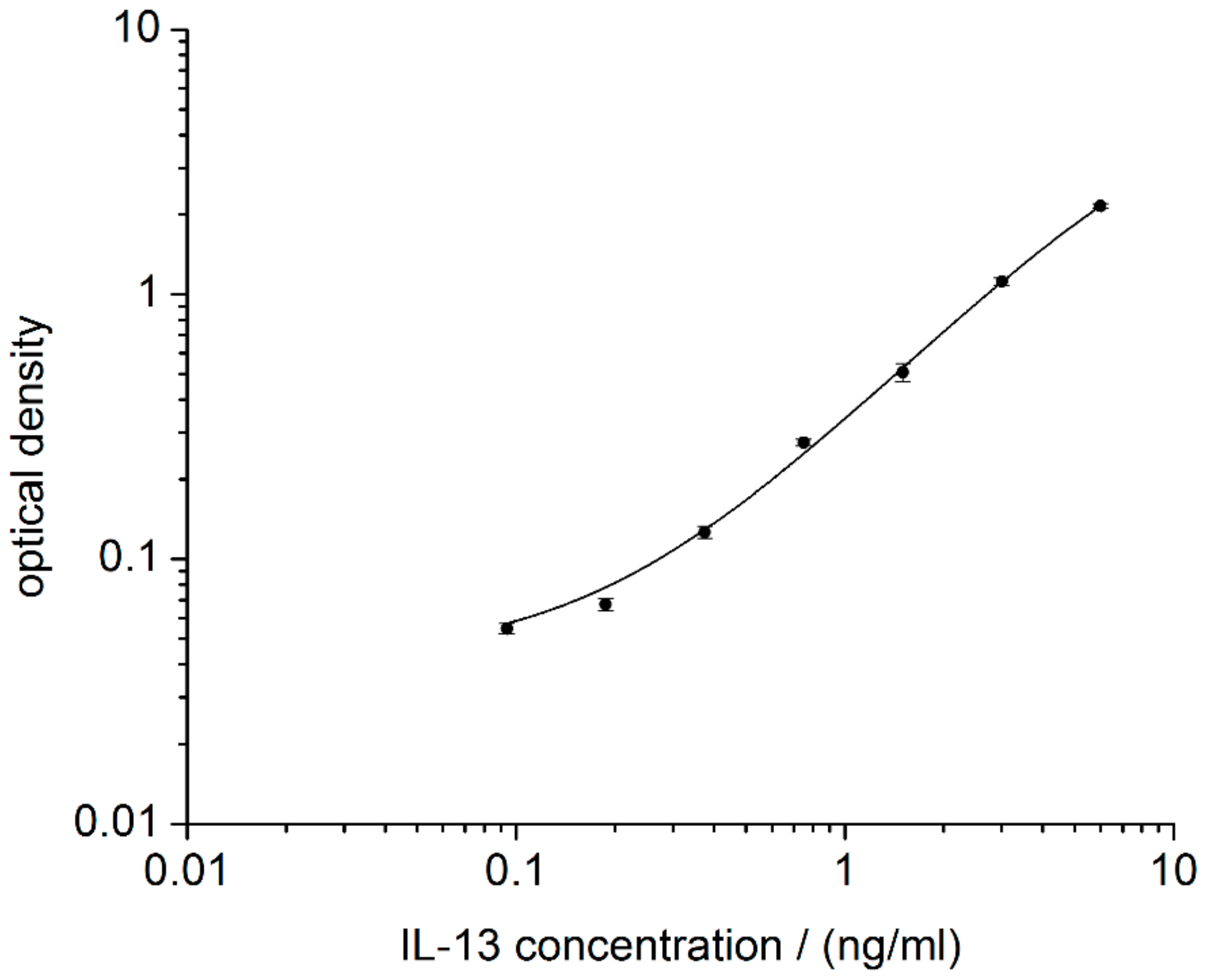
3.1. Performance of Reference ELISA
3.2. Sensor Characterization in Beaker
3.3. Development of a Fluidic System with Temperature Control
3.3.1. Determination of Flow Parameters
3.3.2. Application of the Fluidic System for Sensor Measurements
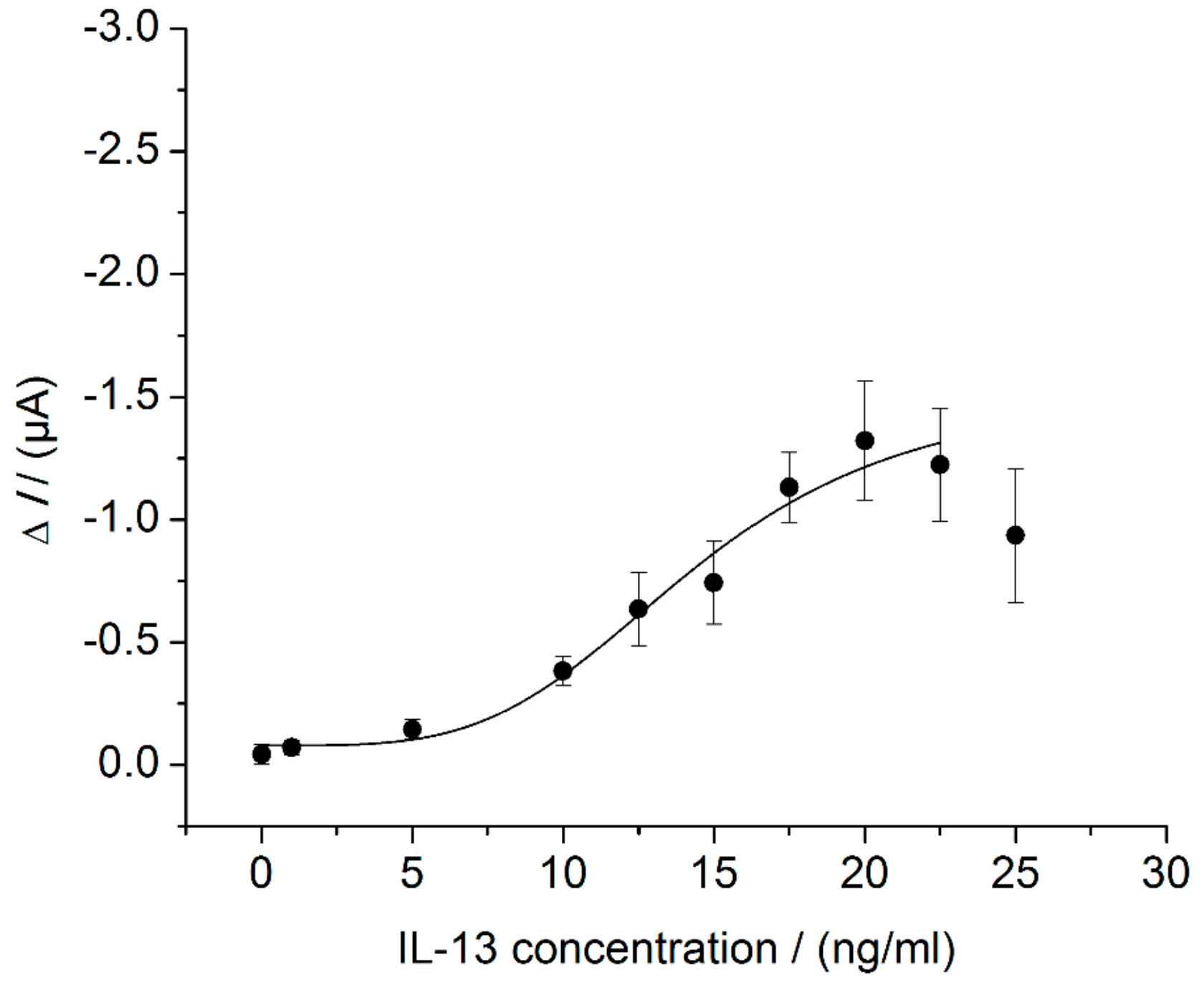
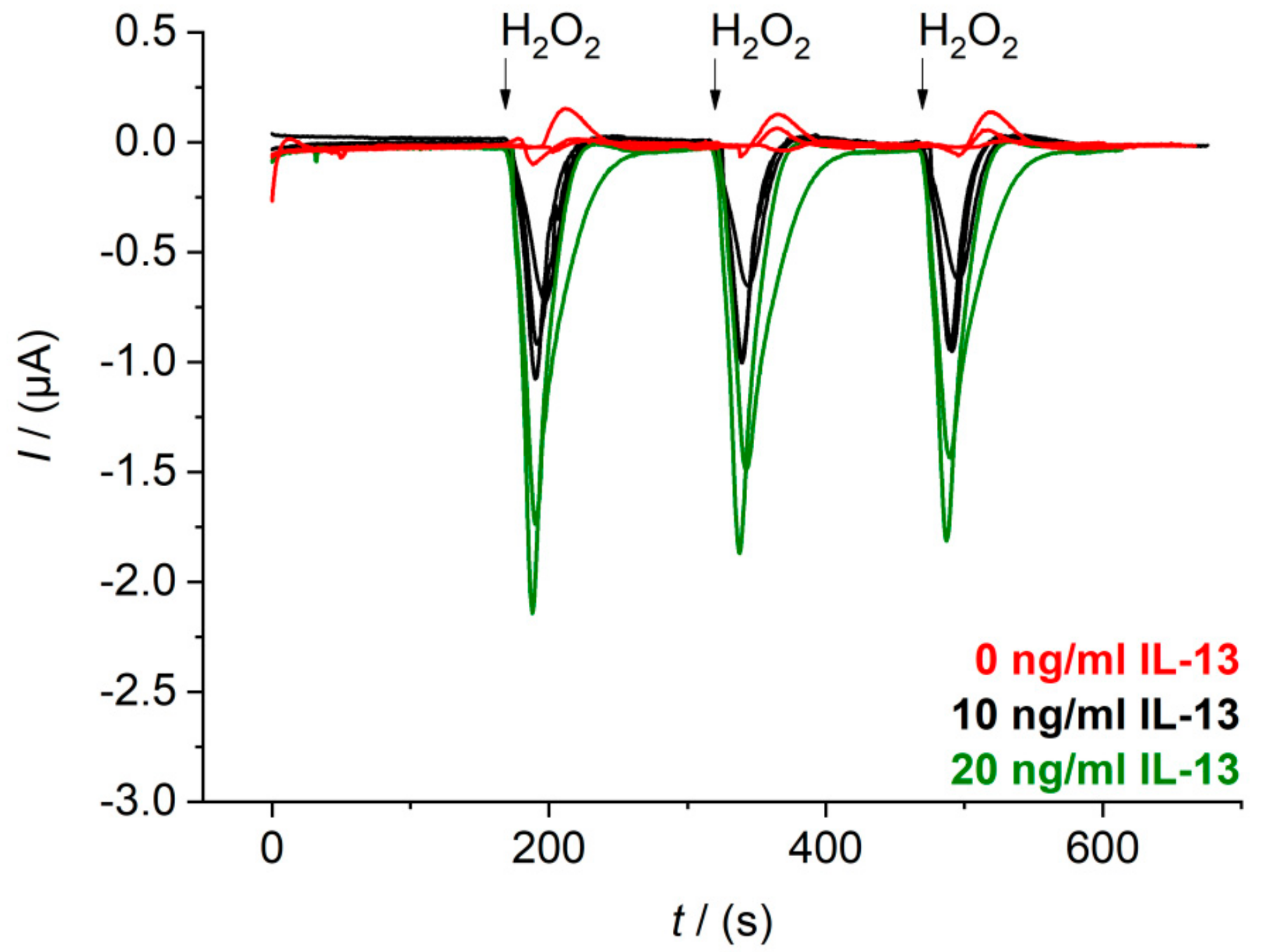
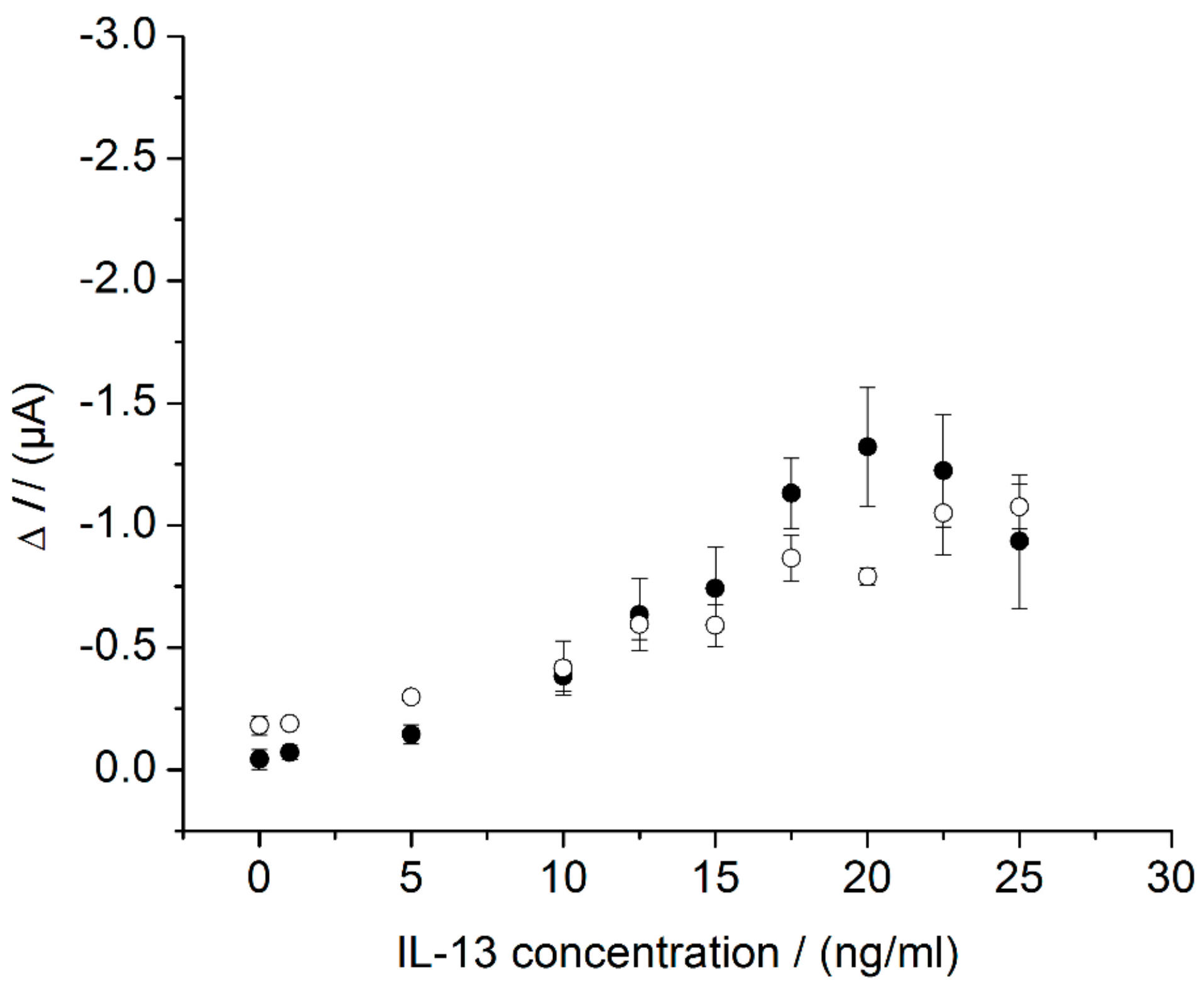
3.4. Sensor Characteristics in Thermostat-Controlled Flow Cell
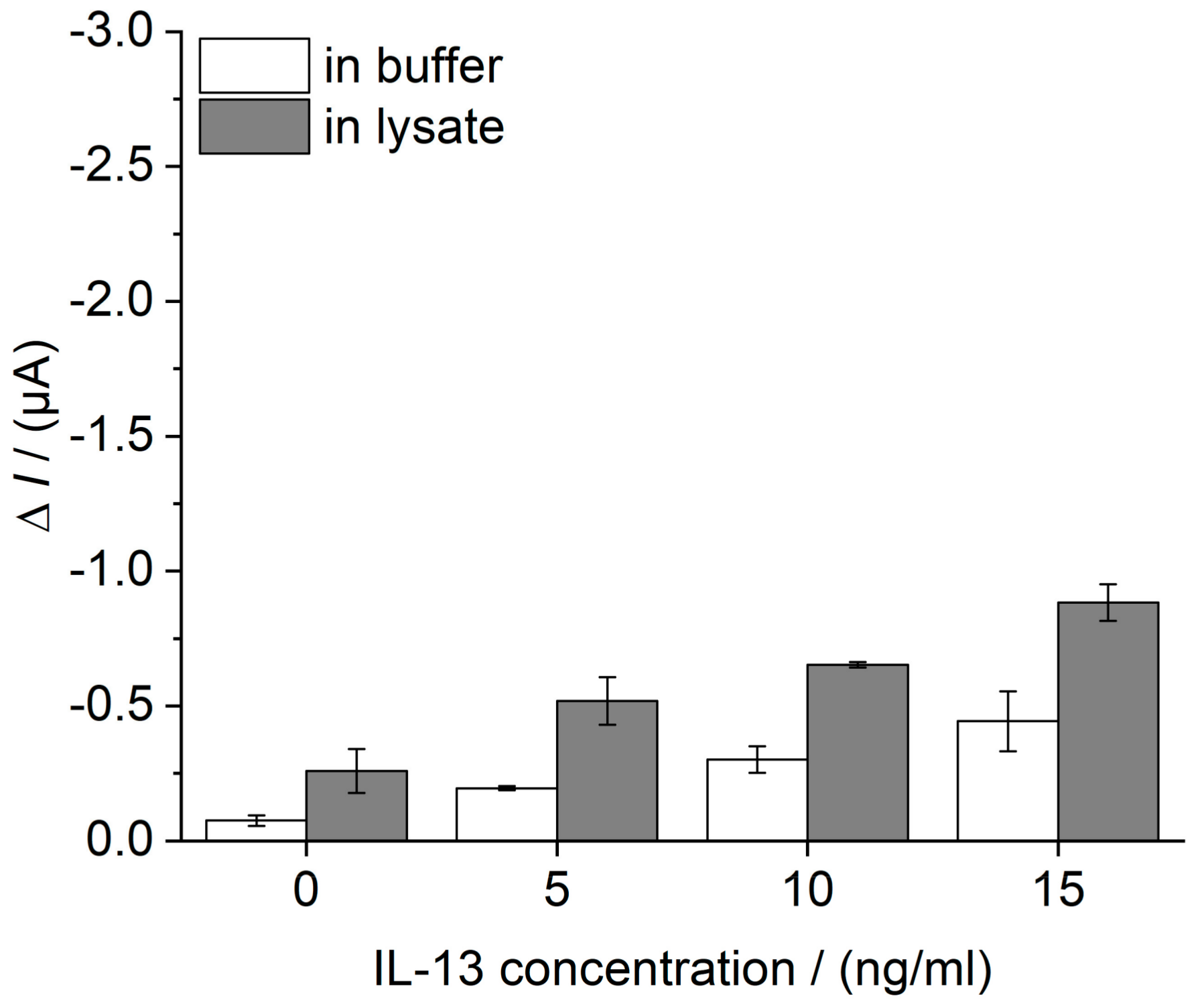
3.5. Selectivity of the IL-13 Immunosensor
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A. Determination of the Working Potential


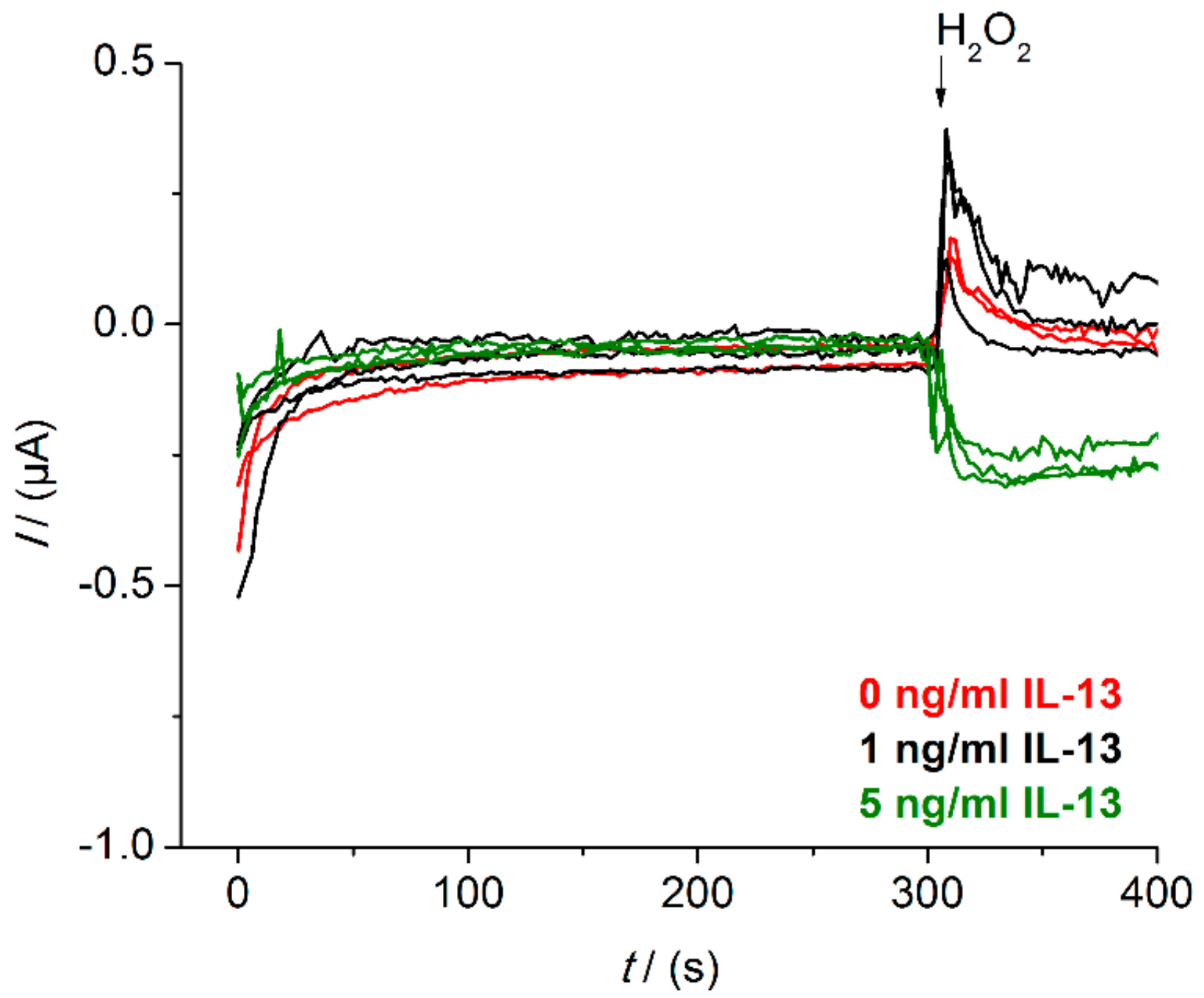
Appendix B. Raw Data Signals of Measurements in Beaker
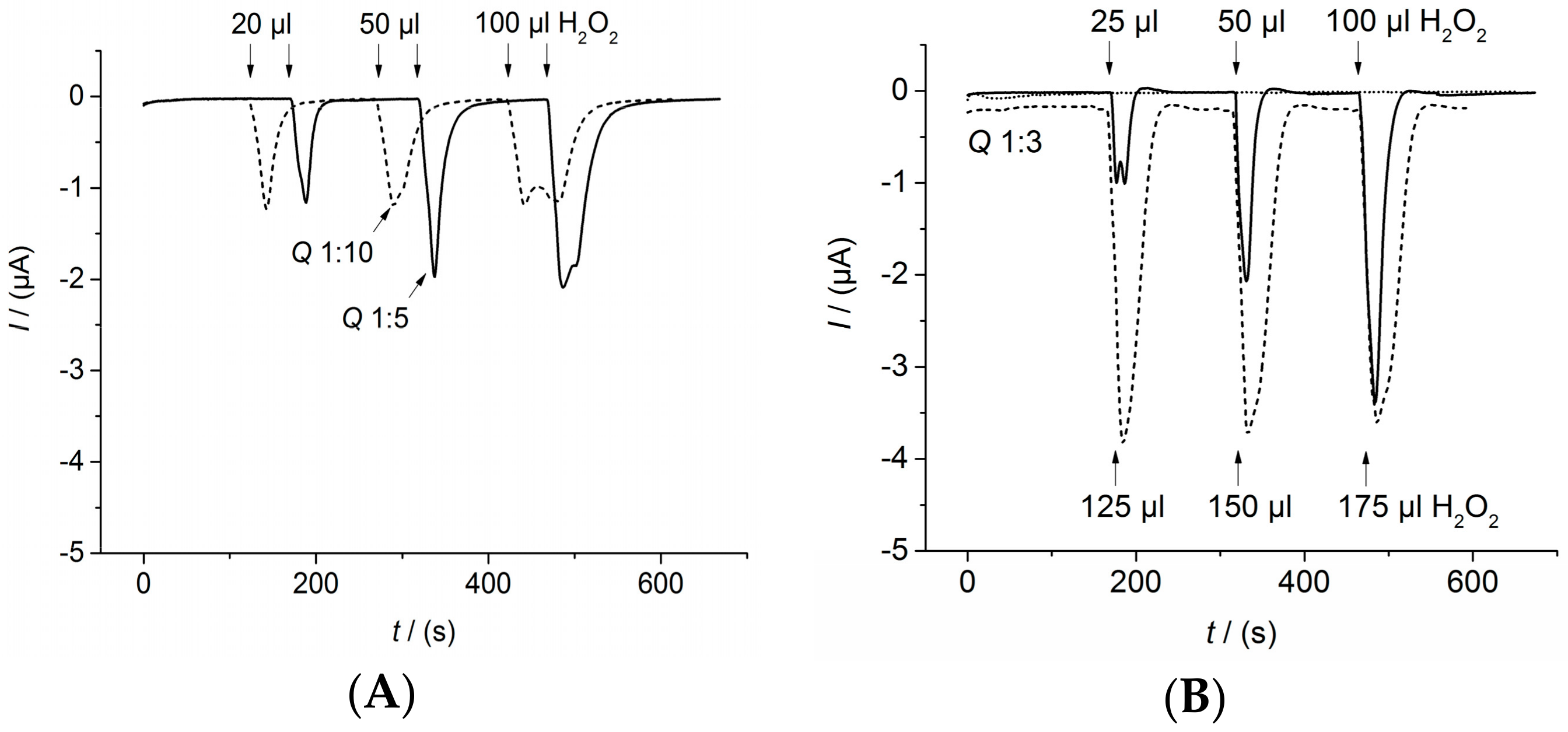
Appendix C. Determination of the Flow Parameters
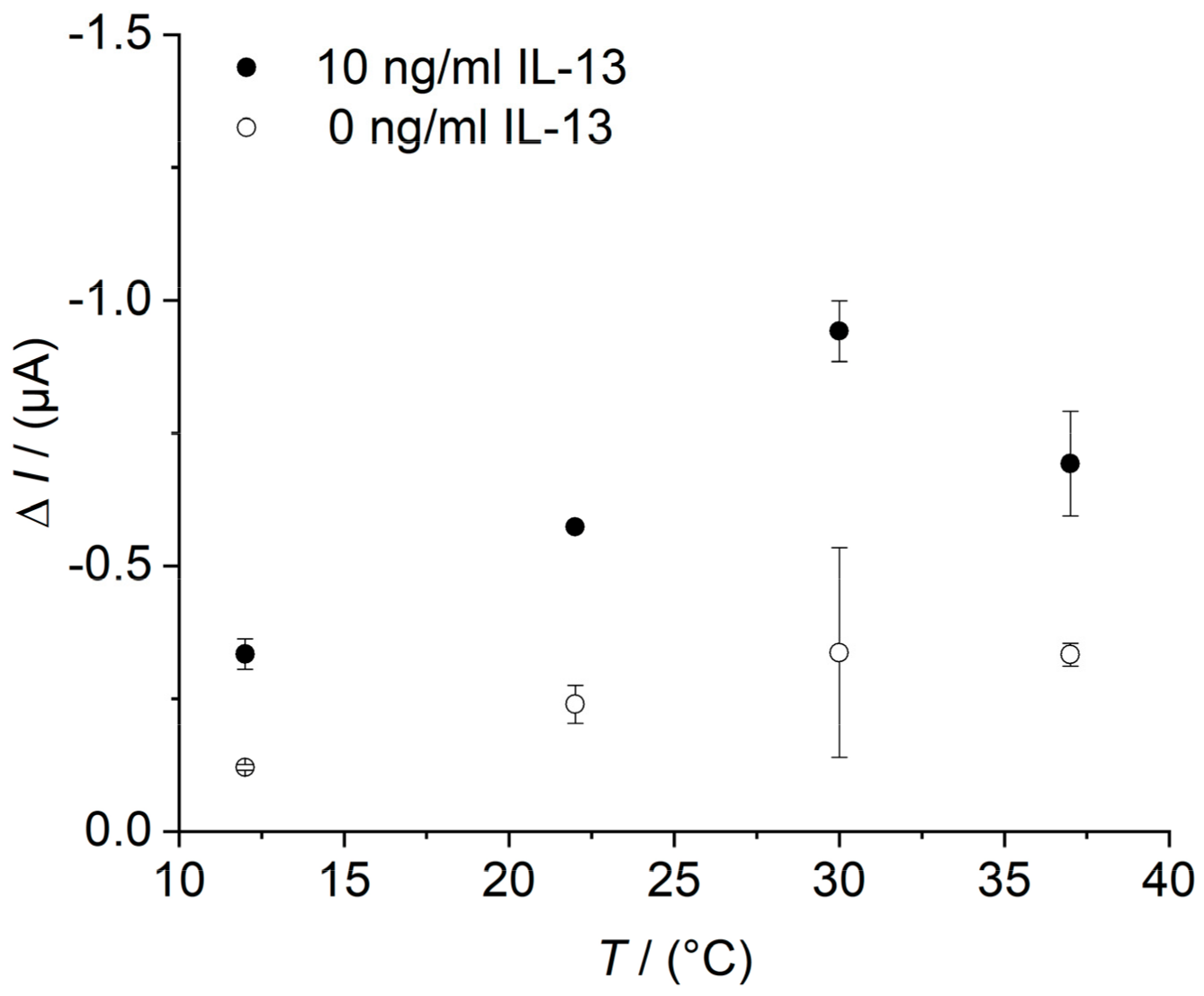
Appendix D. Determination of the Temperature Optimum of the IL-13 Immunosensor
References
- Stenken, J.A.; Poschenrieder, A.J. Bioanalytical chemistry of cytokines—A review. Anal. Chim. Acta 2015, 853, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Schuijs, M.J.; Willart, M.A.; Hammad, H.; Lambrecht, B.N. Cytokine targets in airway inflammation. Curr. Opin. Pharmacol. 2013, 13, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Mechanisms in COPD: Differences from asthma. Chest 2000, 117, 10S–14S. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.L.; Kraft, M. IL-13 in asthma and allergic disease: Asthma phenotypes and targeted therapies. J. Allergy Clin. Immunol. 2012, 130, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Adcock, I.M.; Caramori, G.; Chung, K.F. New targets for drug development in asthma. Lancet 2008, 372, 1073–1087. [Google Scholar] [CrossRef]
- Rackus, D.G.; Shamsi, M.H.; Wheeler, A.R. Electrochemistry, biosensors and microfluidics: A convergence of fields. Chem. Soc. Rev. 2015, 44, 5320–5340. [Google Scholar] [CrossRef] [PubMed]
- Hervás, M.; López, M.A.; Escarpa, A. Electrochemical immunosensing on board microfluidic chip platforms. TrAC Trends Anal. Chem. 2012, 31, 109–128. [Google Scholar] [CrossRef]
- Bange, A.; Halsall, H.B.; Heineman, W.R. Microfluidic immunosensor systems. Biosens. Bioelectron. 2005, 20, 2488–2503. [Google Scholar] [CrossRef] [PubMed]
- Heineman, W.R.; Halsall, H.B. Strategies for electrochemical immunoassay. Anal. Chem. 1985, 57, 1321A–1331A. [Google Scholar] [CrossRef] [PubMed]
- Holford, T.R.; Davis, F.; Higson, S.P. Recent trends in antibody based sensors. Biosens. Bioelectron. 2012, 34, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Sedeno, P.; Campuzano, S.; Pingarron, J.M. Multiplexed Electrochemical Immunosensors for Clinical Biomarkers. Sensors 2017, 17, 965. [Google Scholar] [CrossRef] [PubMed]
- Piro, B.; Reisberg, S. Recent Advances in Electrochemical Immunosensors. Sensors 2017, 17, 794. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAC Trends Anal. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Munge, B.S.; Stracensky, T.; Gamez, K.; DiBiase, D.; Rusling, J.F. Multiplex Immunosensor Arrays for Electrochemical Detection of Cancer Biomarker Proteins. Electroanalysis 2016, 28, 2644–2658. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.; Sartorius, D.; Ehrentreich-Förster, E.; Bier, F.F. Miniaturization for Point-of-Care Analysis: Platform Technology for Almost Every Biomedical Assay. Elec. J. Int. Fed. Clin. Chem. 2012, 23, 70–75. [Google Scholar]
- Krause, C.E.; Otieno, B.A.; Bishop, G.W.; Phadke, G.; Choquette, L.; Lalla, R.V.; Peterson, D.E.; Rusling, J.F. Ultrasensitive microfluidic array for serum pro-inflammatory cytokines and C-reactive protein to assess oral mucositis risk in cancer patients. Anal. Bioanal. Chem. 2015, 407, 7239–7243. [Google Scholar] [CrossRef] [PubMed]
- Kling, A.; Chatelle, C.; Armbrecht, L.; Qelibari, E.; Kieninger, J.; Dincer, C.; Weber, W.; Urban, G. Multianalyte Antibiotic Detection on An Electrochemical Microfluidic Platform. Anal. Chem. 2016, 88, 10036–10043. [Google Scholar] [CrossRef] [PubMed]
- Riahi, R.; Shaegh, S.A.M.; Ghaderi, M.; Zhang, Y.S.; Shin, S.R.; Aleman, J.; Massa, S.; Kim, D.; Dokmeci, M.R.; Khademhosseini, A. Automated microfluidic platform of bead-based electrochemical immunosensor integrated with bioreactor for continual monitoring of cell secreted biomarkers. Sci. Rep. 2016, 6, 24598. [Google Scholar] [CrossRef] [PubMed]
- Uthuppu, B.; Heiskanen, A.; Kofoed, D.; Aamand, J.; Jørgensen, C.; Dufva, M.; Jakobsen, M.H. Micro-flow-injection analysis (muFIA) immunoassay of herbicide residue 2,6-dichlorobenzamide—Towards automated at-line monitoring using modular microfluidics. Analyst 2015, 140, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.; Faulkner, L. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; ISBN 978-0471043720. [Google Scholar]
- Lau, C.; Reiter, S.; Schuhmann, W.; Gründler, P. Application of heated electrodes operating in a non-isothermal mode for interference elimination with amperometric biosensors. Anal. Bioanal. Chem. 2004, 379, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Garcinuno, B.; Ojeda, I.; Moreno-Guzmán, M.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Amperometric immunosensor for the determination of ceruloplasmin in human serum and urine based on covalent binding to carbon nanotubes-modified screen-printed electrodes. Talanta 2014, 118, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; He, T.; Jiang, F.; Shi, J.J.; Zhu, J.J. A competitive electrochemical immunosensor for the detection of human interleukin-6 based on the electrically heated carbon electrode and silver nanoparticles functionalized labels. Talanta 2014, 122, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Borgmann, S.; Maciejewska, M.; Ngounou, B.; Gründler, P.; Schuhmann, W. Improved specificity of reagentless amperometric PQQ-sGDH glucose biosensors by using indirectly heated electrodes. Biosens. Bioelectron. 2007, 22, 3014–3020. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jasinski, M.; Flechsig, G.U.; Grundler, P.; Tian, B. Hot-wire amperometric monitoring of flowing streams. Talanta 2000, 50, 1205–1210. [Google Scholar] [CrossRef]
- dos Santos, V.B.; Fava, E.L.; de Miranda Curi, N.S.; Faria, R.C.; Fatibello-Filho, O. A thermostated electrochemical flow cell with a coupled bismuth film electrode for square-wave anodic stripping voltammetric determination of cadmium(II) and lead(II) in natural, wastewater and tap water samples. Talanta 2014, 126, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Eletxigerra, U.; Martinez-Perdiguero, J.; Merino, S.; Barderas, R.; Ruiz-Valdepeñas Montiel, V.; Villalonga, R.; Pingarrón, J.M.; Campuzano, S. Electrochemical Magnetoimmunosensor for Progesterone Receptor Determination. Application to the Simultaneous Detection of Estrogen and Progesterone Breast-cancer Related Receptors in Raw Cell Lysates. Electroanalysis 2016, 28, 1787–1794. [Google Scholar] [CrossRef]
- Ojeda, I.; Moreno-Guzmán, M.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical magnetoimmunosensor for the ultrasensitive determination of interleukin-6 in saliva and urine using poly-HRP streptavidin conjugates as labels for signal amplification. Anal. Bioanal. Chem. 2014, 406, 6363–6371. [Google Scholar] [CrossRef] [PubMed]
- Gottheil, R.; Baur, N.; Becker, H.; Link, G.; Maier, D.; Schneiderhan-Marra, N.; Stelzle, M. Moving the solid phase: A platform technology for cartridge based sandwich immunoassays. Biomed. Microdevices 2014, 16, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.W.; Dillard, R.F. Appropriate calibration curve fitting in ligand binding assays. AAPS J. 2007, 9, E260–E267. [Google Scholar] [CrossRef] [PubMed]
- Banica, F.-G. Chemical Sensors and Biosensors: Fundamentals and Applications, 1st ed.; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2012; ISBN 978-1-118-35423-0. [Google Scholar]
- Luckert, K.; Götschel, F.; Sorger, P.K.; Hecht, A.; Joos, T.O.; Pötz, O. Snapshots of protein dynamics and post-translational modifications in one experiment—β-catenin and its functions. Mol. Cell. Proteom. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Ho, C.Y.; Ko, F.W.S.; Chan, C.H.S.; Ho, A.S.S.; Hui, D.S.C.; Lam, C.W.K. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin. Exp. Immunol. 2001, 125, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Cruse, G.; Mckenna, S.; Monteiro, W.; Mistry, V.; Wardlaw, A.; Brightling, C. IL-13 expression by blood T cells and not eosinophils is increased in asthma compared to non-asthmatic eosinophilic bronchitis. BMC Pulm. Med. 2009, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Wild, D. The Immunoassay Handbook, 3rd ed.; Elsevier: New York City, NY, USA, 2005; ISBN 978-0080445267. [Google Scholar]
- Rodbard, D.; Feldman, Y.; Jaffe, M.L.; Miles, L.E.M. Kinetics of two-site immunoradiometric (‘sandwich’) assays-II. Studies on the nature of the ‘high-dose hook effect’. Immunochemistry 1978, 15, 77–82. [Google Scholar] [CrossRef]
- Ricci, F.; Adornetto, G.; Palleschi, G. A review of experimental aspects of electrochemical immunosensors. Electrochim. Acta 2012, 84, 74–83. [Google Scholar] [CrossRef]
- Sanchez-Tirado, E.; Salvo, C.; González-Cortés, A.; Yáñez-Sedeño, P.; Langa, F.; Pingarrón, J.M. Electrochemical immunosensor for simultaneous determination of interleukin-1 beta and tumor necrosis factor alpha in serum and saliva using dual screen printed electrodes modified with functionalized double-walled carbon nanotubes. Anal. Chim. Acta 2017, 959, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Sharafeldin, M.; Bishop, G.W.; Bhakta, S.; El-Sawy, A.; Suib, S.L.; Rusling, J.F. Fe3O4 nanoparticles on graphene oxide sheets for isolation and ultrasensitive amperometric detection of cancer biomarker proteins. Biosens. Bioelectron. 2017, 91, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, B.; Ayala, M.; Vazquez-Duhalt, R. Suicide inactivation of peroxidases and the challenge of engineering more robust enzymes. Chem. Biol. 2002, 9, 555–565. [Google Scholar] [CrossRef]
- Banks, C.E.; Foster, C.W.; Kadara, R.O. Screen-Printing Electrochemical Architectures, 1st ed.; Springer: Berlin, Germany, 2016; ISBN 978-3319251936. [Google Scholar]
- Kukobat, R.; Hayashi, T.; Matsuda, T.; Sunaga, M.; Futamura, R.; Sakai, T.; Kaneko, K. Essential Role of Viscosity of SWCNT Inks in Homogeneous Conducting Film Formation. Langmuir 2016, 32, 6909–6916. [Google Scholar] [CrossRef] [PubMed]
- Dixit, C.K.; Kadimisetty, K.; Otieno, B.A.; Tang, C.; Malla, S.; Krause, C.E.; Rusling, J.F. Electrochemistry-based approaches to low cost, high sensitivity, automated, multiplexed protein immunoassays for cancer diagnostics. Analyst 2016, 141, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Kusnezow, W.; Syagailo, Y.V.; Goychuk, I.; Hoheisel, J.D.; Wild, D.G. Antibody microarrays: The crucial impact of mass transport on assay kinetics and sensitivity. Expert Rev. Mol. Diagn. 2006, 6, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Squires, T.M.; Messinger, R.J.; Manalis, S.R. Making it stick: Convection, reaction and diffusion in surface-based biosensors. Nat. Biotechnol. 2008, 26, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Lavery, C.B.; MacInnis, M.C.; MacDonald, M.J.; Williams, J.B.; Spencer, C.A.; Burke, A.A.; Irwin, D.J.; D’Cunha, G.B. Purification of peroxidase from Horseradish (Armoracia rusticana) roots. J. Agric. Food Chem. 2010, 58, 8471–8476. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Aly, A.S.; Mohamed, T.M.; Salah, H.A. Immobilization of horseradish peroxidase on nonwoven polyester fabric coated with chitosan. Appl. Biochem. Biotechnol. 2008, 144, 169–179. [Google Scholar]
- Chattopadhyay, K.; Mazumdar, S. Structural and conformational stability of horseradish peroxidase: Effect of temperature and pH. Biochemistry 2000, 39, 263–270. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metzner, J.; Luckert, K.; Lemuth, K.; Hämmerle, M.; Moos, R. Towards an Electrochemical Immunosensor System with Temperature Control for Cytokine Detection. Sensors 2018, 18, 1309. https://doi.org/10.3390/s18051309
Metzner J, Luckert K, Lemuth K, Hämmerle M, Moos R. Towards an Electrochemical Immunosensor System with Temperature Control for Cytokine Detection. Sensors. 2018; 18(5):1309. https://doi.org/10.3390/s18051309
Chicago/Turabian StyleMetzner, Julia, Katrin Luckert, Karin Lemuth, Martin Hämmerle, and Ralf Moos. 2018. "Towards an Electrochemical Immunosensor System with Temperature Control for Cytokine Detection" Sensors 18, no. 5: 1309. https://doi.org/10.3390/s18051309
APA StyleMetzner, J., Luckert, K., Lemuth, K., Hämmerle, M., & Moos, R. (2018). Towards an Electrochemical Immunosensor System with Temperature Control for Cytokine Detection. Sensors, 18(5), 1309. https://doi.org/10.3390/s18051309





