Surface Plasmon Resonance and Bending Loss-Based U-Shaped Plastic Optical Fiber Biosensors
Abstract
1. Introduction
2. Working Principle
3. Materials and Methods
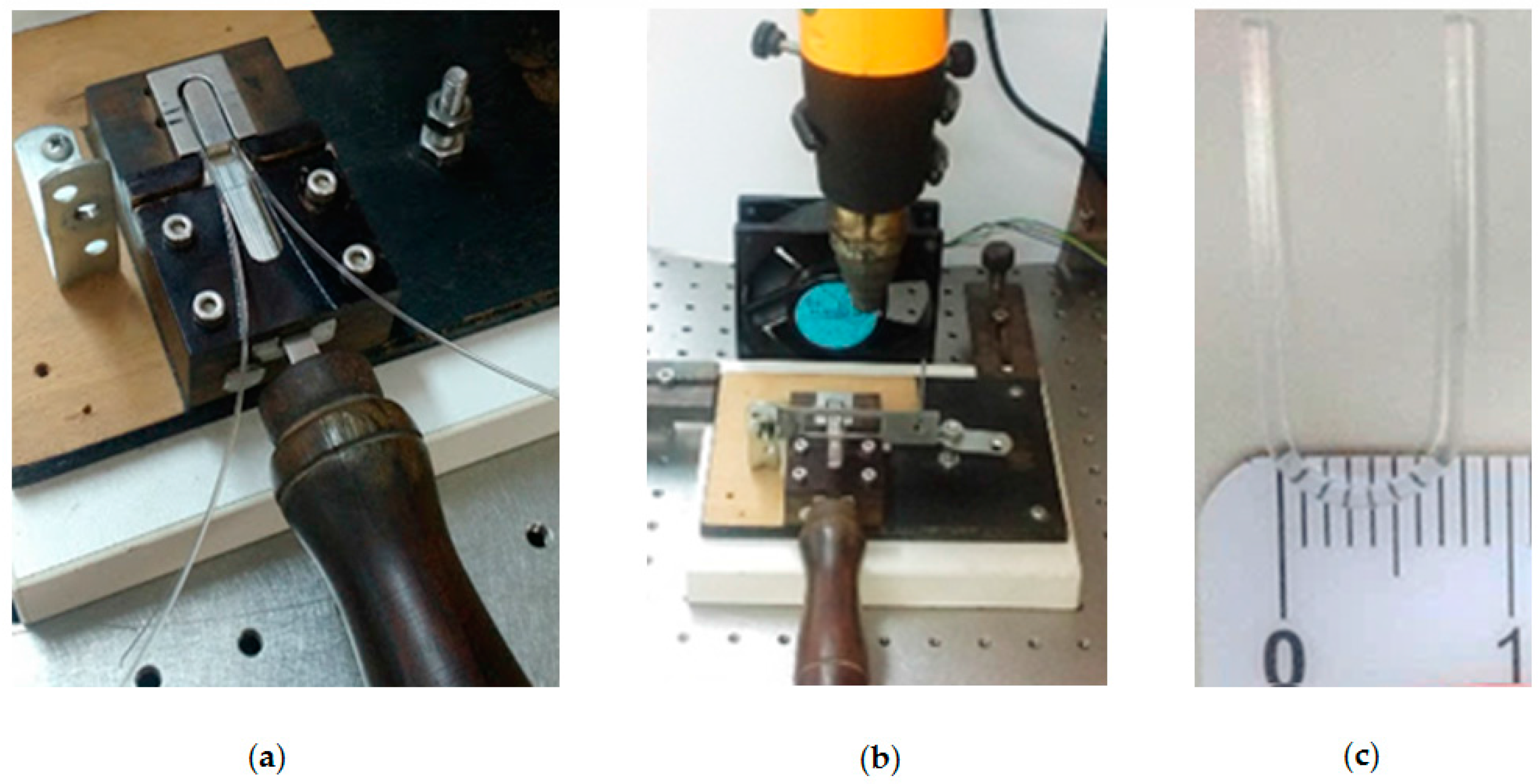
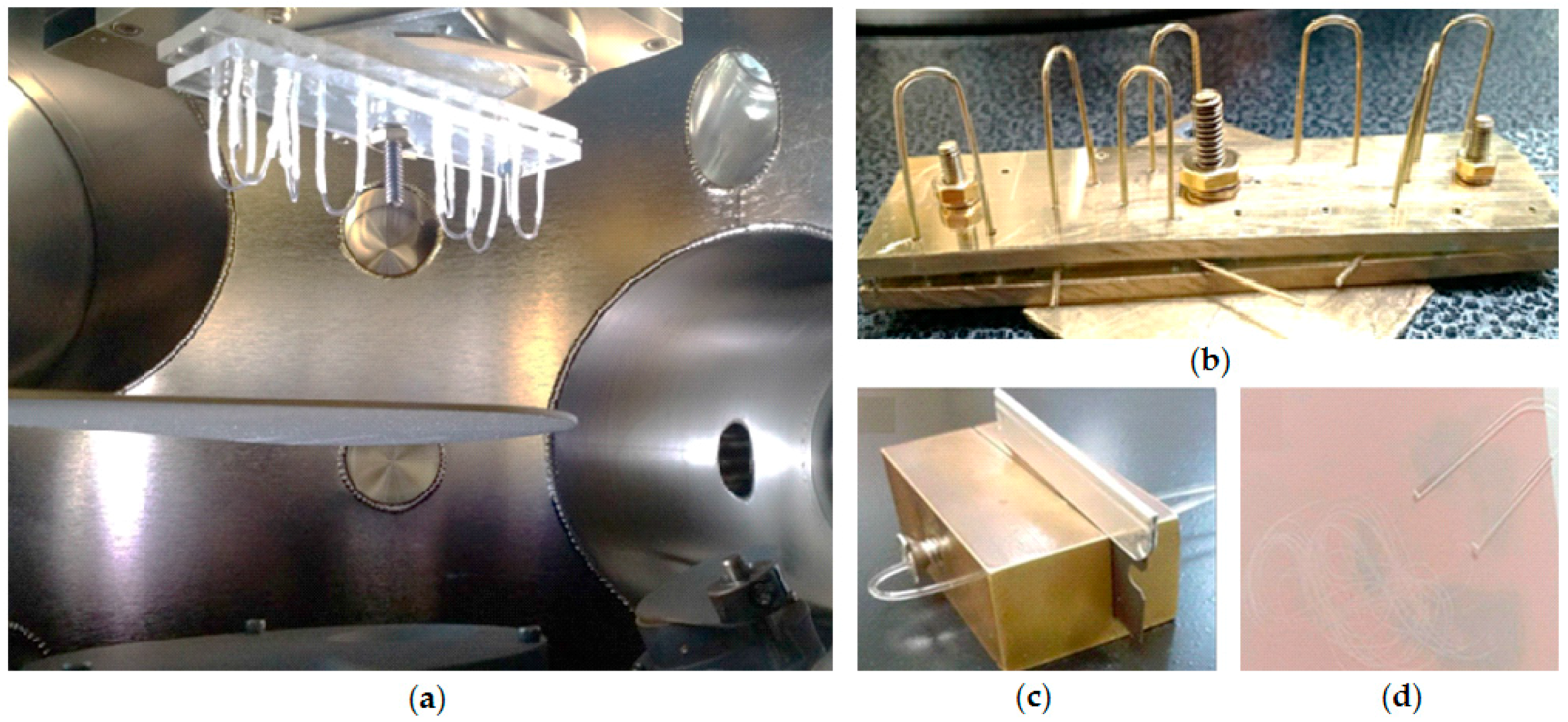
3.1. Probe Fabrication
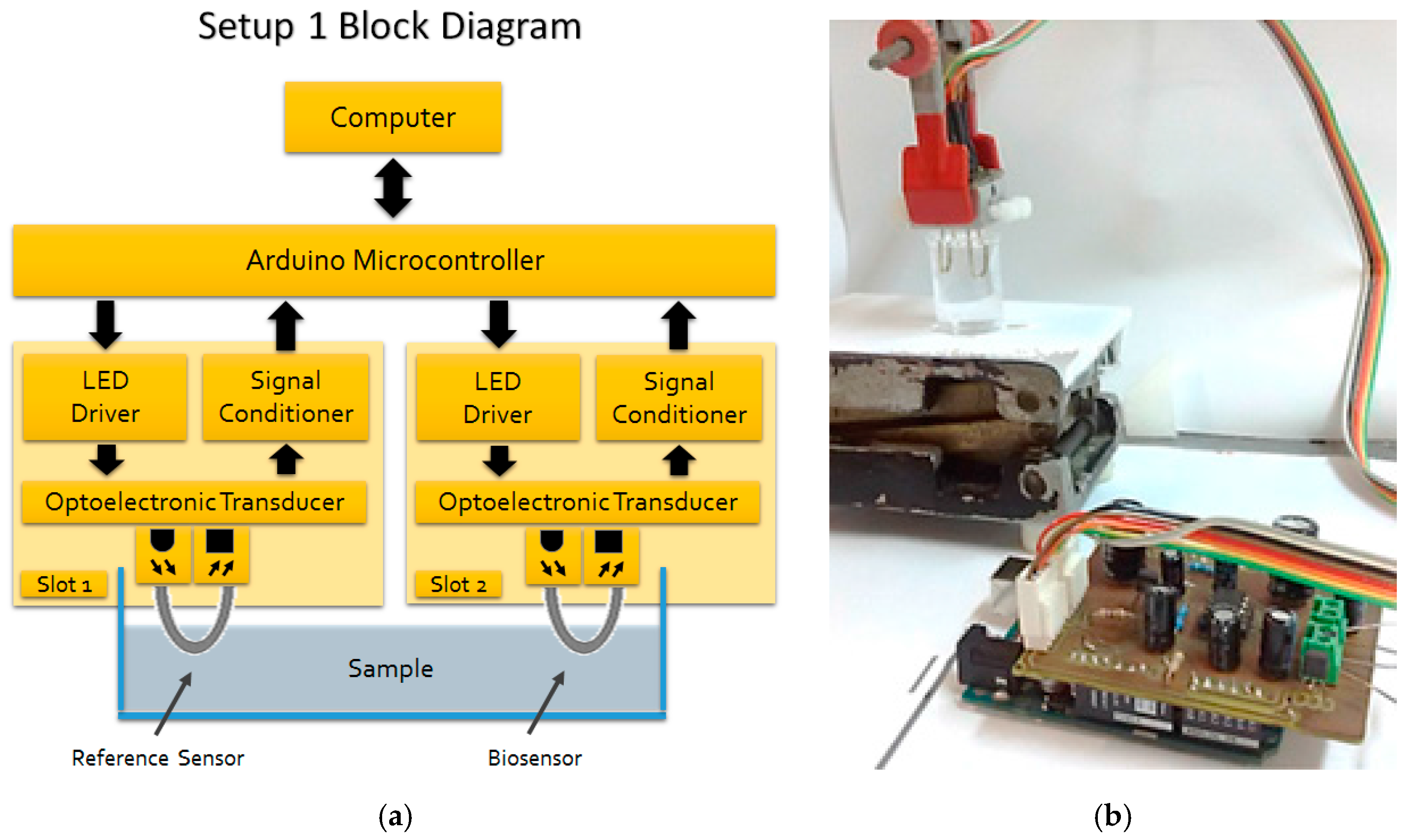
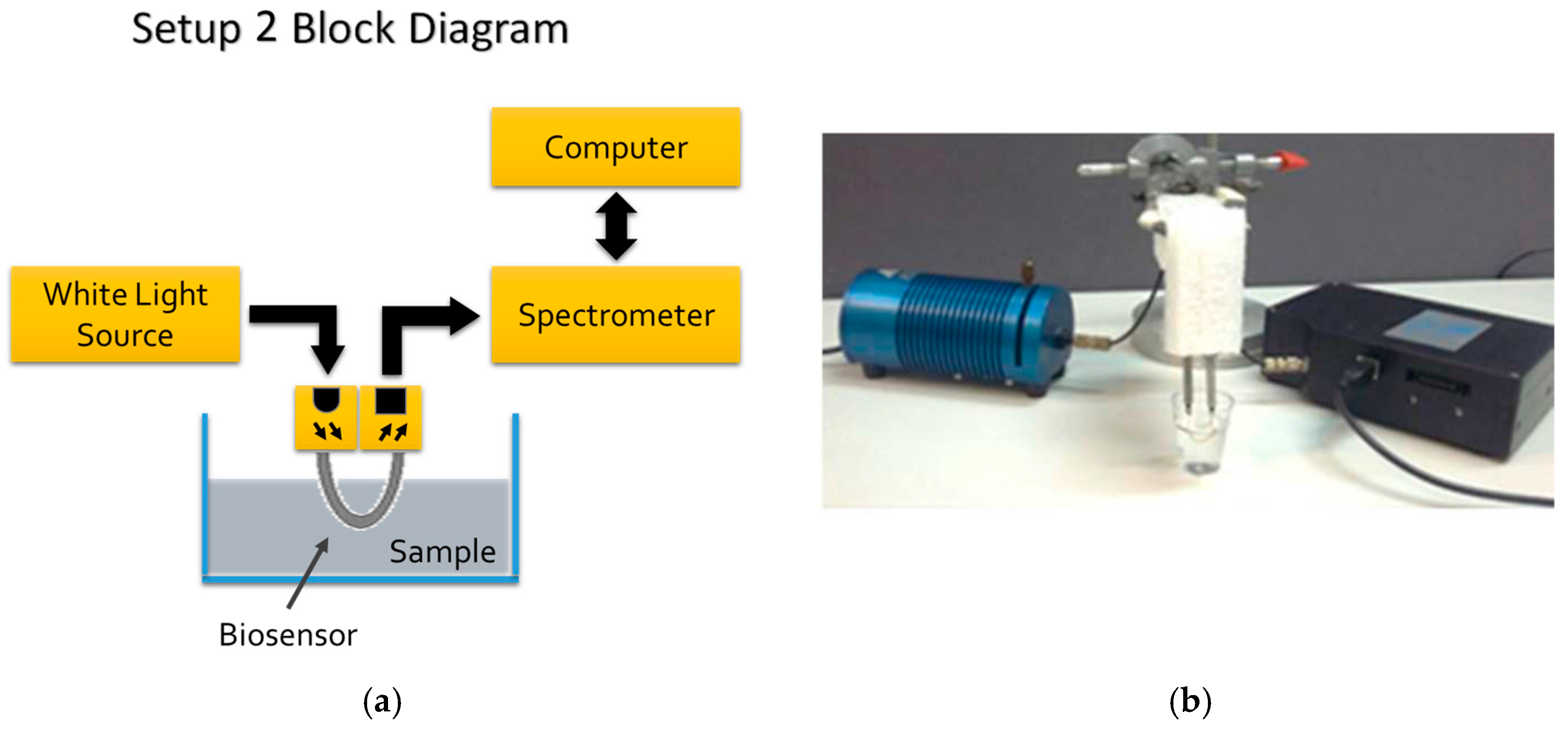
3.2. Optoelectronic Setups
3.3. Sucrose Solutions for RI Testing
3.4. Antibody Immobilization Protocol
3.5. Bacterial Suspensions and Testing
4. Results and Discussion
4.1. Gold Thin Film Transmittance
4.2. RI Measurements
4.2.1. Sucrose Solutions Testing in Setup 1
4.2.2. Sucrose Solutions Testing in Setup 2
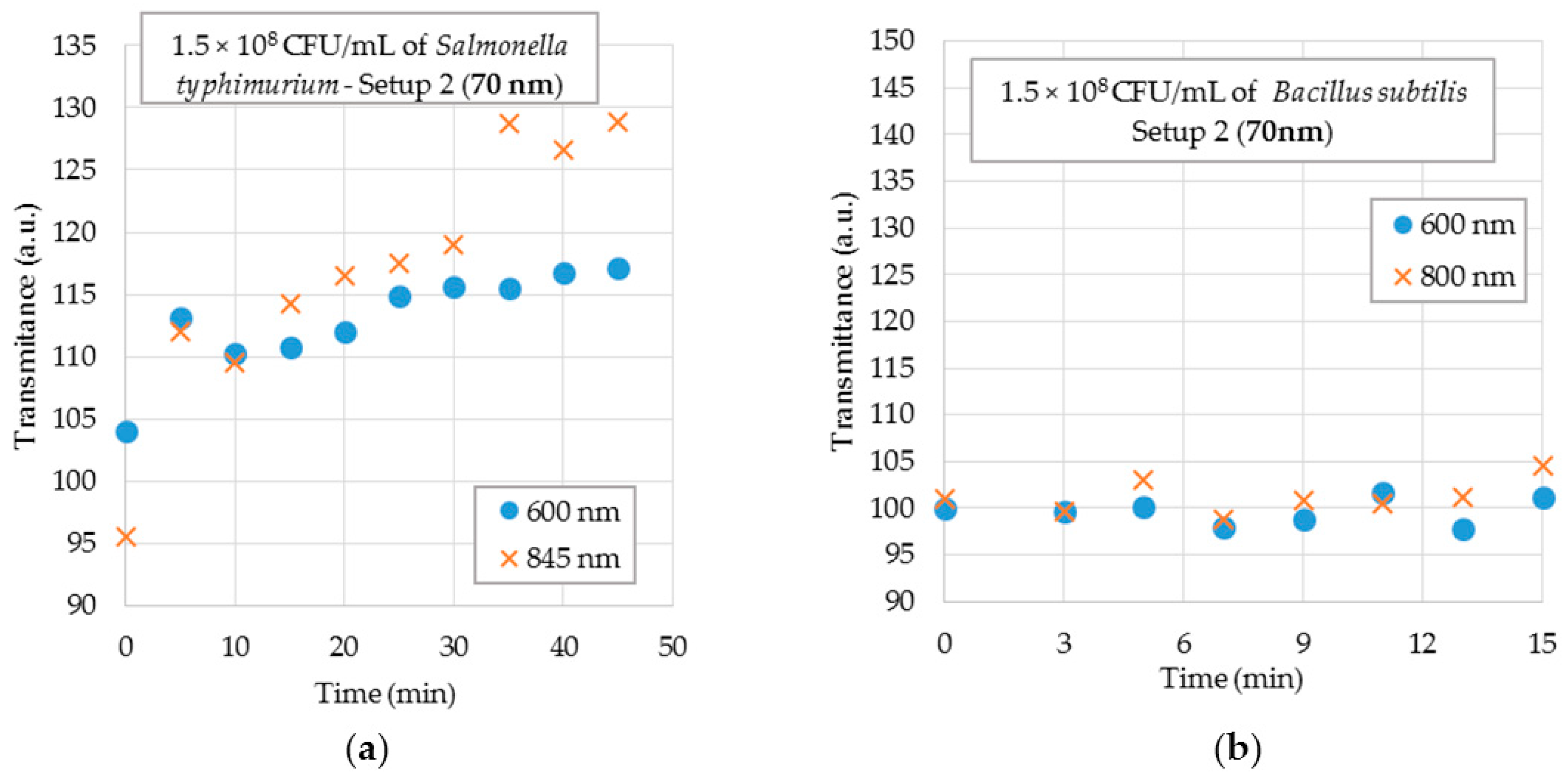
4.3. Bacterial Measurements
4.3.1. Bacterial Suspensions Testing in Setup 1
4.3.2. Bacterial Suspensions Testing in Setup 2
4.4. Selectivity Testing
4.5. Bacterial Adhesion on Sensor Surface
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Lin, C.-T.J.; Jensen, K.L.; Yen, S. Determinants of Consumer Awareness of Foodborne Pathogens. In AAEA Annual Meeting; AAEA: Denver, CO, USA, 2004; pp. 1–4. [Google Scholar]
- Lu, L.; Jun, S. Evaluation of a microwire sensor functionalized to detect Escherichia coli bacterial cells. Biosens. Bioelectron. 2012, 36, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Havelaar, A.H.; Brul, S.; de Jong, A.; de Jonge, R.; Zwietering, M.H.; ter Kuile, B.H. Future challenges to microbial food safety. Int. J. Food Microbiol. 2010, 139, S79–S94. [Google Scholar] [CrossRef] [PubMed]
- Júnior, A.I.; Belotto, A.J.; Dhalia, C.B.C. Manual Integrado de Vigilância, Prevenção e Controle de Doenças Transmitidas por Alimentos; Ministério da Saúde: Brasília, Brazil, 2010. (In Portuguese)
- Ana Regina de Freitas, A. Doenças Alimentares de Origem Bacteriana; Universidade Fernando Pessoa: Porto, Portugal, 2012. (In Portuguese) [Google Scholar]
- General Information| E. coli | CDC. Available online: https://www.cdc.gov/ecoli/general/index.html (accessed on 15 September 2017).
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2008; Volume 1. [Google Scholar]
- Locking, M.E.; O’Brien, S.J.; Reilly, W.J.; Wright, E.M.; Campbell, D.M.; Coia, J.M.; Browning, L.M.; Ramsay, C.N. Risk Factors for Sporadic Cases of Escherichia coli O157 Infection: The Importance of Contact with Animal Excreta. Epidemiol. Infect. 2001, 127, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.d.; da Silva, J.J.; Bueno, P.R.; Watanabe, A.M. Determinação dos parâmetros cinéticos e termodinâmicos da adsorção de L-cisteína em ouro por meio da técnica de microbalança a cristal de quartzo. Quím. Nova 2012, 35, 1365–1368. (In Portuguese) [Google Scholar] [CrossRef]
- Lim, J.Y.; Yoon, J.W.; Hovde, C.J. A Brief Overview of Escherichia coli O157:H7 and Its Plasmid O157. J. Microbiol. Biotechnol. 2010, 20, 5–14. [Google Scholar] [PubMed]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiologia, 10th ed.; Grupo A: Artmed, Switzerland, 2000; ISBN 978-85-363-2698-6. [Google Scholar]
- Riley, L.W.; Semis, R.S.; Helgerson, S.D.; McGee, H.B. Hemorrhagic Colitis Associated with Rare Escherichia Coli Serotype. N. Engl. J. Med. 1983, 308, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Kretzer, J.W.; Biebl, M.; Miller, S. Sample Preparation–An Essential Prerequisite for High-Quality Bacteria Detection. In Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Springer: New York, NY, USA, 2008; pp. 15–30. [Google Scholar]
- Zourob, M.; Elwary, S.; Turner, A. Principles of Bacterial Detection: Biosensors, Recognition Receptors, and Microsystems; Springer: New York, NY, USA, 2008; ISBN 978-0-387-75112-2. [Google Scholar]
- Beres, C.; Nazaré, F.; Souza, N.; Miguel, M.; Werneck, M. Tapered plastic optical fiber-based biosensor—Tests and application. Biosens. Bioelectron. 2011, 30, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.; Uknalis, J.; Tu, S.-I.; Bhunia, A.K. Fiber-Optic Biosensor Employing Alexa-Fluor Conjugated Antibody for Detection of Escherichia coli O157H7 from Ground Beef for Four Hours. Sensors 2006, 6, 796–807. [Google Scholar] [CrossRef]
- Lazcka, O.; Campo, J.; Munoz, F. Pathogen detection a perspective of traditional methods and biosensors. Biosens. Bioelectron. 2006, 22, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Baccar, H.; Mejri, M.B.; Hafaiedh, I.; Ktari, T.; Aouni, M.; Abdelghani, A. Surface plasmon resonance immunosensor for bacteria detection. Talanta 2010, 82, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Wandermur, G.; Rodrigues, D.; Allil, R.; Queiroz, V.; Peixoto, R.; Werneck, M.; Miguel, M. Plastic optical fiber-based biosensor platform for rapid cell detection. Biosens. Bioelectron. 2014, 54, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Halkare, P.; Punjabi, N.; Wangchuk, J.; Kondabagil, K.; Mukherji, S. LSPR based fiber optic sensor for detection of E.coli using bacteriophage T4. In Proceedings of the Workshop on Recent Advances in Photonics (WRAP), Bangalore, India, 16–17 December 2015. [Google Scholar]
- Balaev, A.E.; Dvoretski, K.N.; Doubrovski, V.A. Refractive index of Escherichia coli cells. In Proceedings of SPIE; SPIE: Bellingham, WA, USA, 2002; Volume 4707. [Google Scholar]
- Bryant, F.D.; Seiber, B.A.; Latimer, P. Absolute optical cross sections of cells and chloroplasts. Arch. Biochem. Biophys. 1969, 135, 97–108. [Google Scholar] [CrossRef]
- Werneck, M.; Lopes, R.; Costa, G.; Rodrigues, D.; Arcas, A.; Dutra, F.; Queiroz, V.; Allil, R. POF Biosensors Based on Refractive Index and Immunocapture Effect. In Fiber Optic Sensors; Matias, I.R., Ikezawa, S., Corres, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 21, pp. 69–93. ISBN 978-3-319-42624-2. [Google Scholar]
- Baccar, H.; Mejri, M.B.; Adams, C.P.; Aouini, M.; Obare, S.O.; Abdelghani, A. Functionalized Gold Nanoparticles for Biosensors Application. Sens. Lett. 2011, 9, 2336–2338. [Google Scholar] [CrossRef]
- De Carvalho, R.M.; Rath, S.; Kubota, L.T. SPR-Uma nova ferramenta para biossensores. Quím. Nova 2003, 26, 97–104. [Google Scholar] [CrossRef]
- Navarrete, M.-C.; Díaz-Herrera, N.; González-Cano, A.; Esteban, Ó. A Polarization-Independent SPR Fiber Sensor. Plasmonics 2010, 5, 7–12. [Google Scholar] [CrossRef]
- Christopher, C.; Subrahmanyam, A.; Sai, V.V.R. Gold Sputtered U-Bent Plastic Optical Fiber Probes as SPR- and LSPR-Based Compact Plasmonic Sensors. Plasmonics 2017. [Google Scholar] [CrossRef]
- Mitsushio, M.; Miyashita, K.; Higo, M. Sensor properties and surface characterization of the metal-deposited SPR optical fiber sensors with Au, Ag, Cu, and Al. Sens. Actuators Phys. 2006, 125, 296–303. [Google Scholar] [CrossRef]
- Cennamo, N.; Massarotti, D.; Conte, L.; Zeni, L. Low Cost Sensors Based on SPR in a Plastic Optical Fiber for Biosensor Implementation. Sensors 2011, 11, 11752–11760. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, C.A.J.; Baptista, J.M.; Jorge, P.A.S. Refractometric Optical Fiber Platforms for Label Free Sensing. In Current Developments in Optical Fiber Technology; Harun, S.W., Ed.; InTech: Rijeka, Croatia, 2013; ISBN 978-953-51-1148-1. [Google Scholar]
- Chen, C.-H.; Tsao, T.-C.; Tang, J.-L.; Wu, W.-T. A Multi-D-Shaped Optical Fiber for Refractive Index Sensing. Sensors 2010, 10, 4794–4804. [Google Scholar] [CrossRef] [PubMed]
- Arrue, J.; Zubia, J. Analysis of the decrease in attenuation achieved by properly bending plastic optical fibres. IEE Proc. Optoelectron. 1996, 143, 135–138. [Google Scholar] [CrossRef]
- Durana, G.; Zubia, J.; Arrue, J.; Aldabaldetreku, G.; Mateo, J. Dependence of bending losses on cladding thickness in plastic optical fibers. Appl. Opt. 2003, 42, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Gloge, D. Bending loss in multimode fibers with graded and ungraded core index. Appl. Opt. 1972, 11, 2506–2513. [Google Scholar] [CrossRef] [PubMed]
- Celikkol-Aydin, S.; Suo, Z.; Yang, X.; Ince, B.; Avci, R. Sharp Transition in the Immunoimmobilization of E. coli O157:H7. Langmuir 2014, 30, 7755–7761. [Google Scholar] [CrossRef] [PubMed]
- BD McFarland Turbidity Standard No 5; Becton, Dickinson and Company: Sparks, NV, USA, 2010.
- Zibaii, M.I.; Latifi, H.; Saeedian, Z.; Chenari, Z. Nonadiabatic tapered optical fiber sensor for measurement of antimicrobial activity of silver nanoparticles against Escherichia coli. J. Photochem. Photobiol. B 2014, 135, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Axelevitch, A.; Apter, B.; Golan, G. Simulation and experimental investigation of optical transparency in gold island films. Opt. Express 2013, 21, 4126–4138. [Google Scholar] [CrossRef] [PubMed]
- Ronot-Trioli, C.; Trouillet, A.; Veillas, C.; Gagnaire, H. Monochromatic excitation of surface plasmon resonance in an opticalfibre refractive-index sensor. Sens. Actuators 1996, 54, 589–593. [Google Scholar] [CrossRef]
- Trouillet, A.; Ronot-Trioli, C.; Veillas, C.; Gagnaire, H. Chemical sensing by surface plasmon resonance in a multimode optical fibre. Pure Appl. Opt. J. Eur. Opt. Soc. Part A 1996, 5, 227. [Google Scholar] [CrossRef]
- Kurihara, K.; Nakamura, K.; Suzuki, K. Asymmetric SPR sensor response curve-fitting equation for the accurate determination of SPR resonance angle. Sens. Actuators B Chem. 2002, 86, 49–57. [Google Scholar] [CrossRef]
- Rofrigues, D.M.C.; Lopes, R.N.; Franco, M.A.R.; Werneck, M.M.; Allil, R.C.S.B. Sensitivity Analysis of Different Shapes of a Plastic Optical Fiber-Based Immunosensor for Escherichia coli: Simulation and Experimental Results. Sensors 2017, 17, 2944. [Google Scholar] [CrossRef] [PubMed]

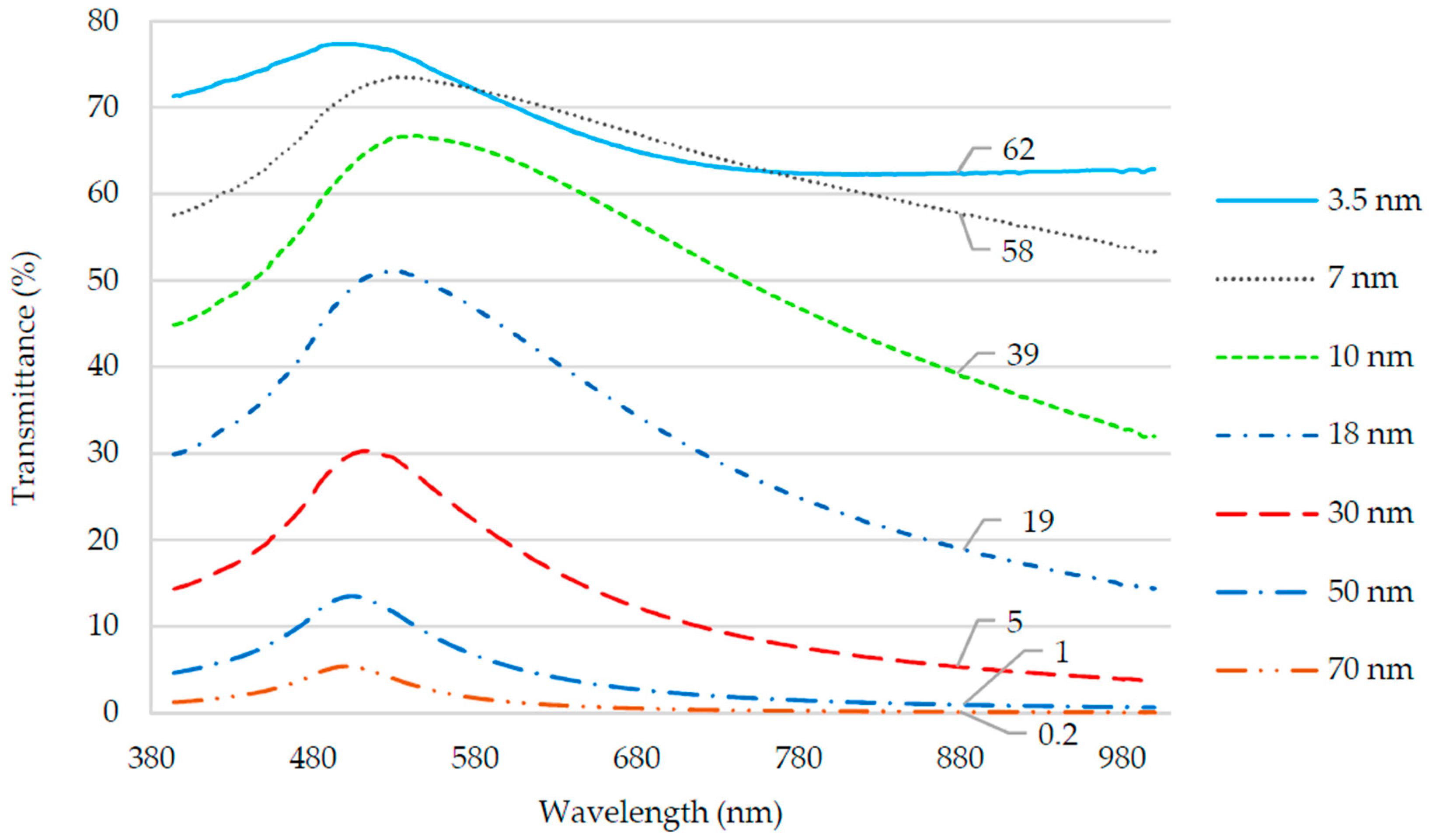

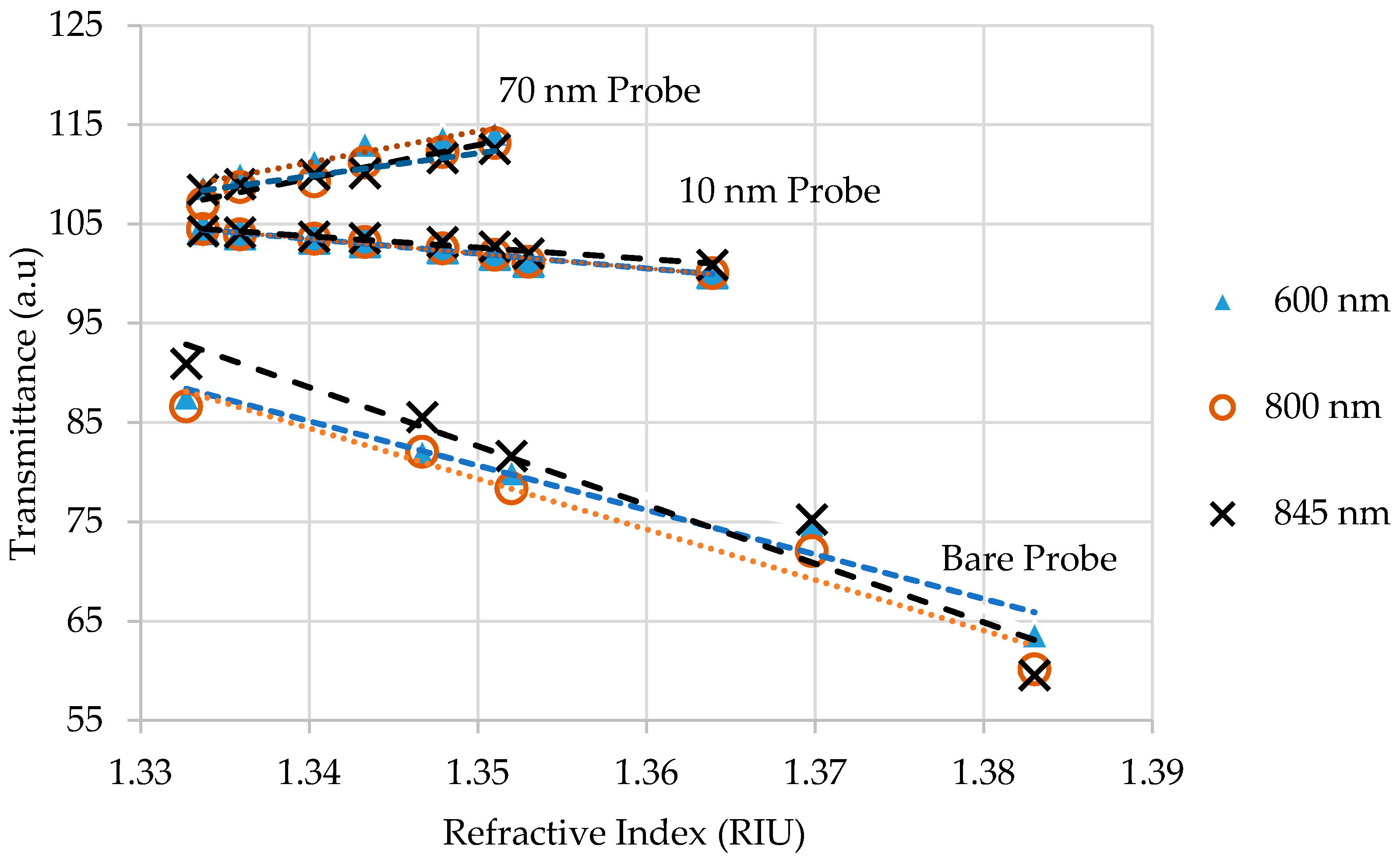

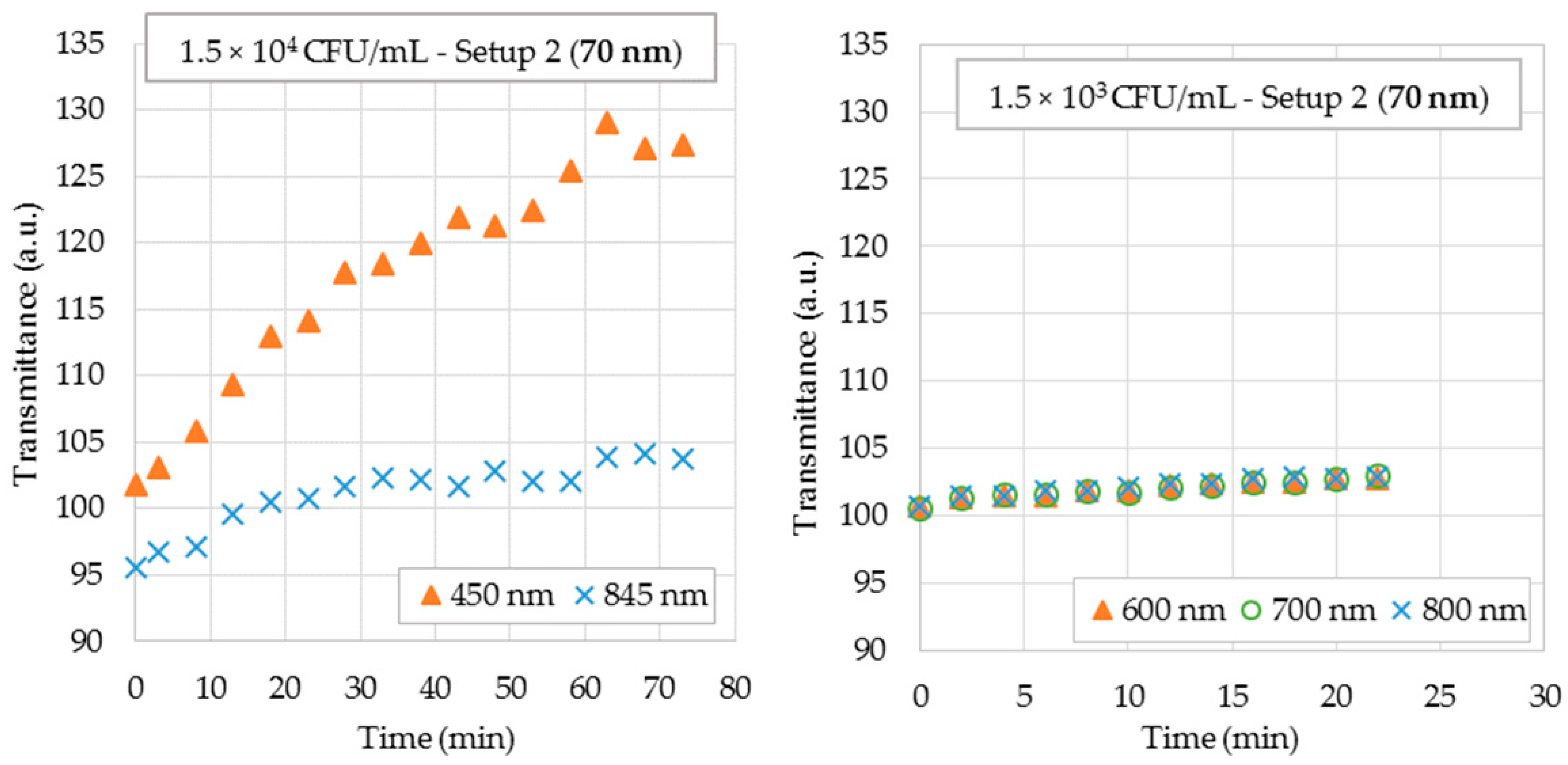
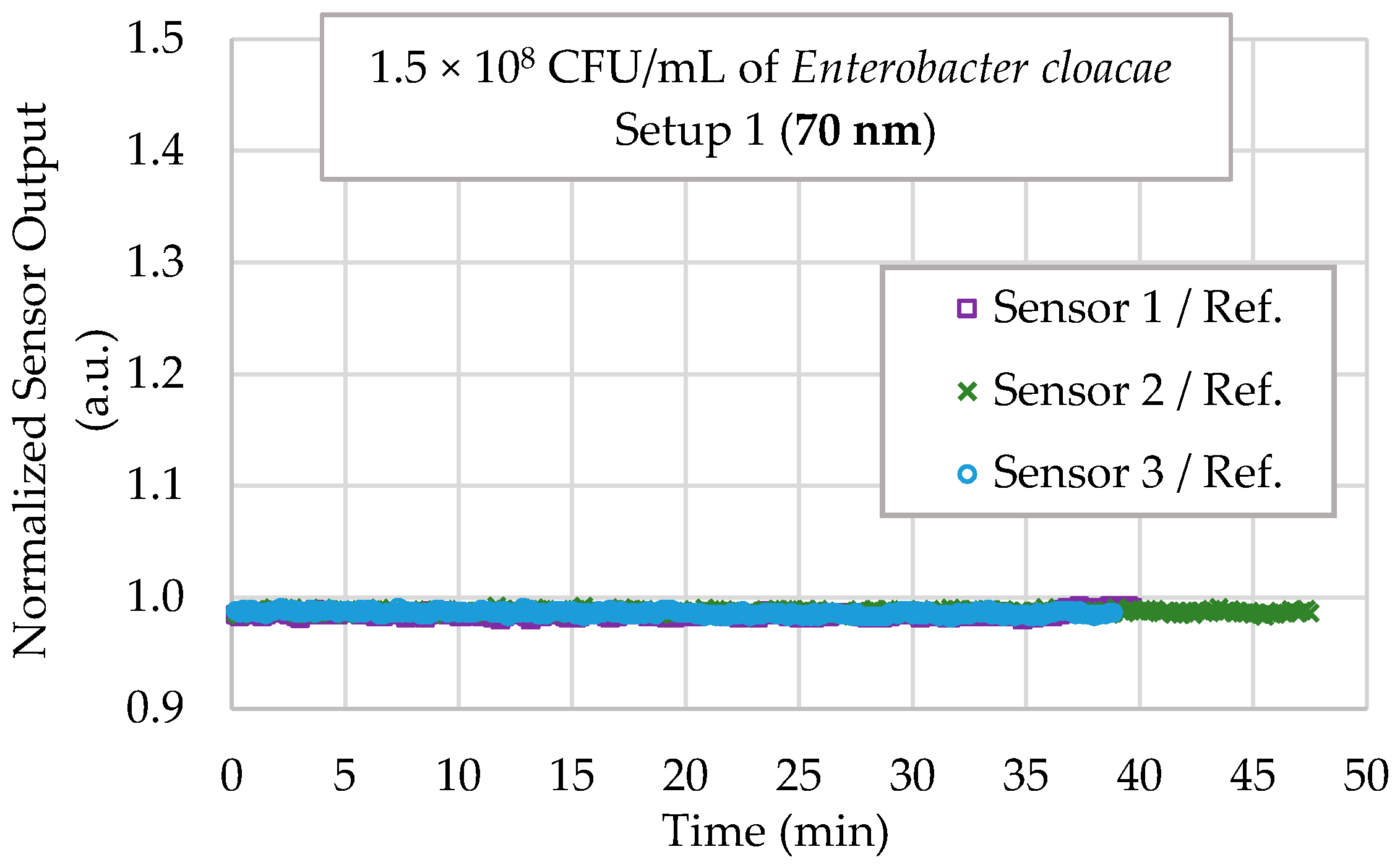

| Old Thickness (nm) | Sensitivity (V/RIU) | Standard Deviation (V/RIU) | Uncertainty (RIU) |
|---|---|---|---|
| 0 | −39.71 | 2.89 | 3.5 × 10−3 |
| 3.5 | −20.34 | 2.25 | 2.9 × 10−3 |
| 7 | −16.73 | 2.11 | 7.4 × 10−3 |
| 10 | −17.57 | 2.43 | 9.8 × 10−3 |
| 18 | −9.53 | 1.06 | 5.5 × 10−3 |
| 30 | −0.88 | -- | -- |
| 50 | 0.57 | -- | -- |
| 70 | 6.01 | 0.65 | 1.9 × 10−2 |
| 100 | 8.25 | 1.05 | 7.7 × 10−2 |
| Gold Thickness (nm) | Sensitivity (1/RIU) | Uncertainty (RIU) |
|---|---|---|
| 0 | −591.78 (845 nm) | 5.8 × 10−4 |
| 10 | −148.66 (600 nm) | 5.1 × 10−4 |
| 70 | 338.26 (800 nm) | 5.6 × 10−4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcas, A.D.S.; Dutra, F.D.S.; Allil, R.C.S.B.; Werneck, M.M. Surface Plasmon Resonance and Bending Loss-Based U-Shaped Plastic Optical Fiber Biosensors. Sensors 2018, 18, 648. https://doi.org/10.3390/s18020648
Arcas ADS, Dutra FDS, Allil RCSB, Werneck MM. Surface Plasmon Resonance and Bending Loss-Based U-Shaped Plastic Optical Fiber Biosensors. Sensors. 2018; 18(2):648. https://doi.org/10.3390/s18020648
Chicago/Turabian StyleArcas, Ariadny Da S., Fábio Da S. Dutra, Regina C. S. B. Allil, and Marcelo M. Werneck. 2018. "Surface Plasmon Resonance and Bending Loss-Based U-Shaped Plastic Optical Fiber Biosensors" Sensors 18, no. 2: 648. https://doi.org/10.3390/s18020648
APA StyleArcas, A. D. S., Dutra, F. D. S., Allil, R. C. S. B., & Werneck, M. M. (2018). Surface Plasmon Resonance and Bending Loss-Based U-Shaped Plastic Optical Fiber Biosensors. Sensors, 18(2), 648. https://doi.org/10.3390/s18020648






