Fabrication and Optimization of Bilayered Nanoporous Anodic Alumina Structures as Multi-Point Interferometric Sensing Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Bilayered NAA Films (BL-NAAs) and NAAs
2.3. Surface Chemistry Functionalization of BL-NAAs and NAAs
2.4. Optical Sensitivity Assessment of BL-NAAs and NAAs by RIfS
2.5. Structural Characterization of BL-NAAF and NAAs
3. Results and Discussion
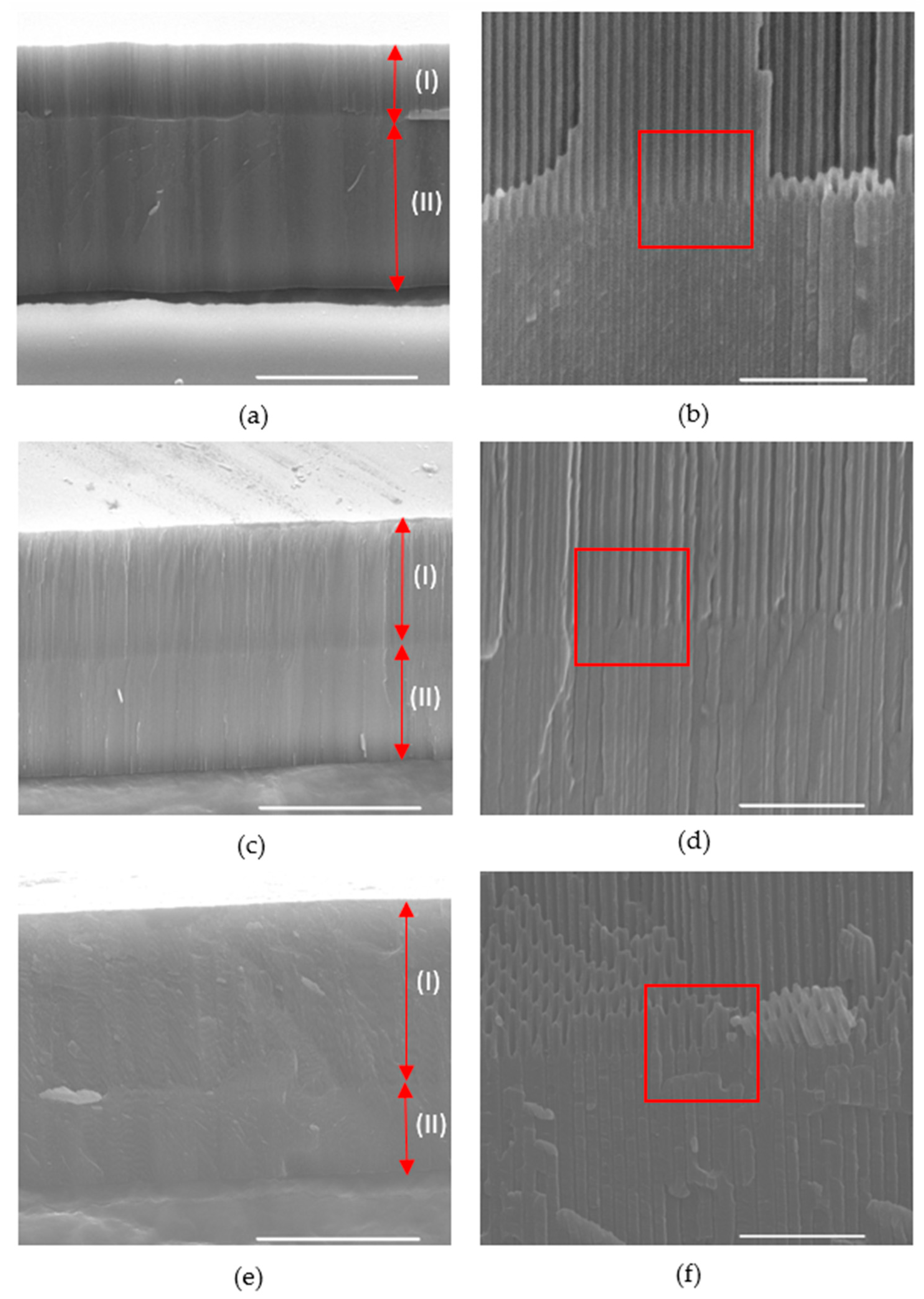
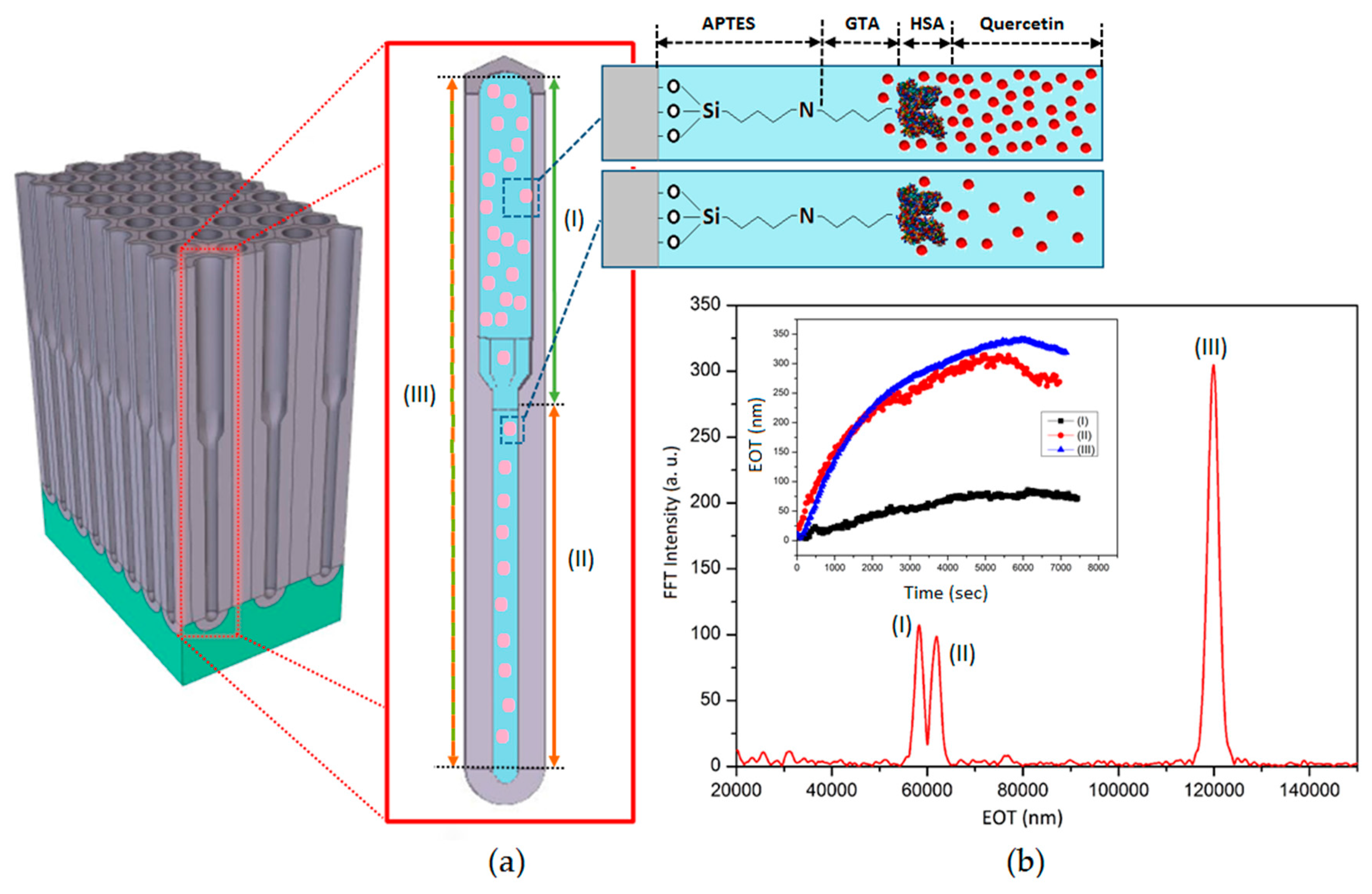
3.1. Structural Characterisation of Prepared BL-NAAs and NAAs
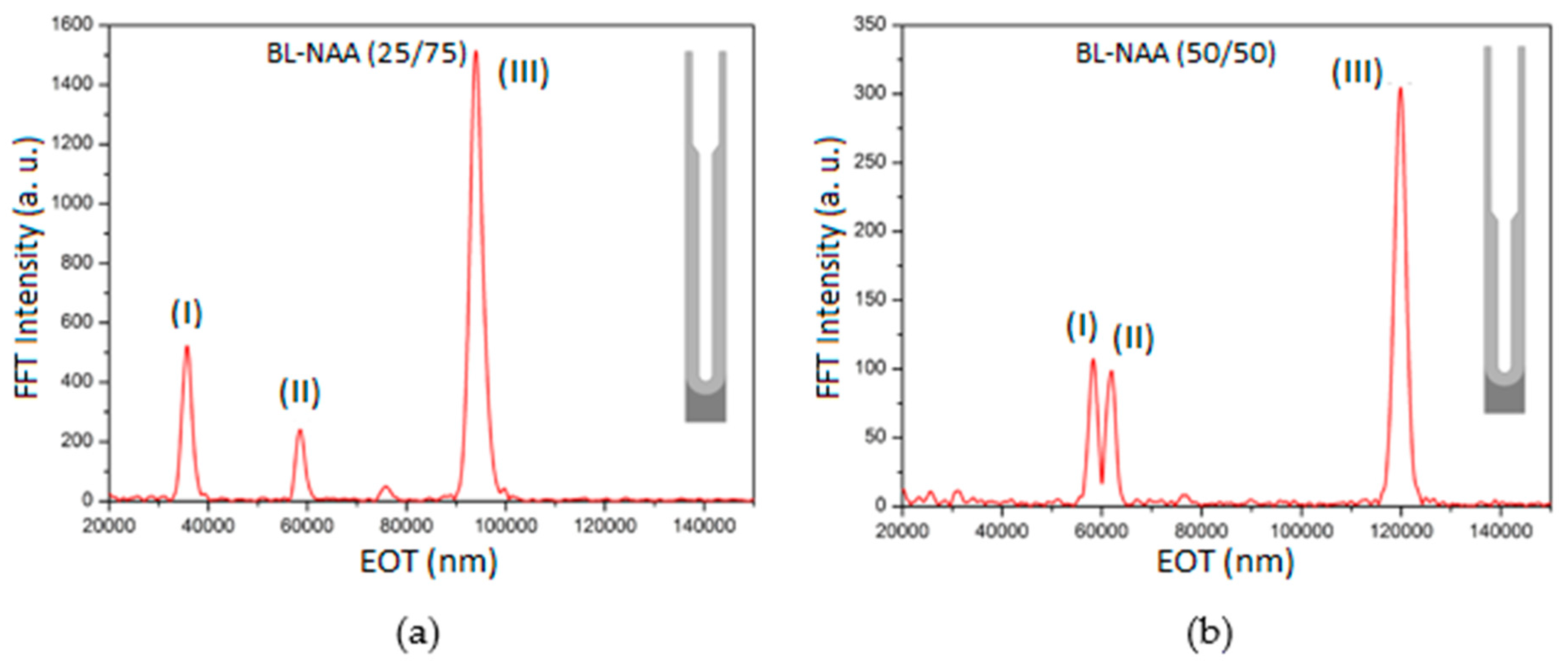
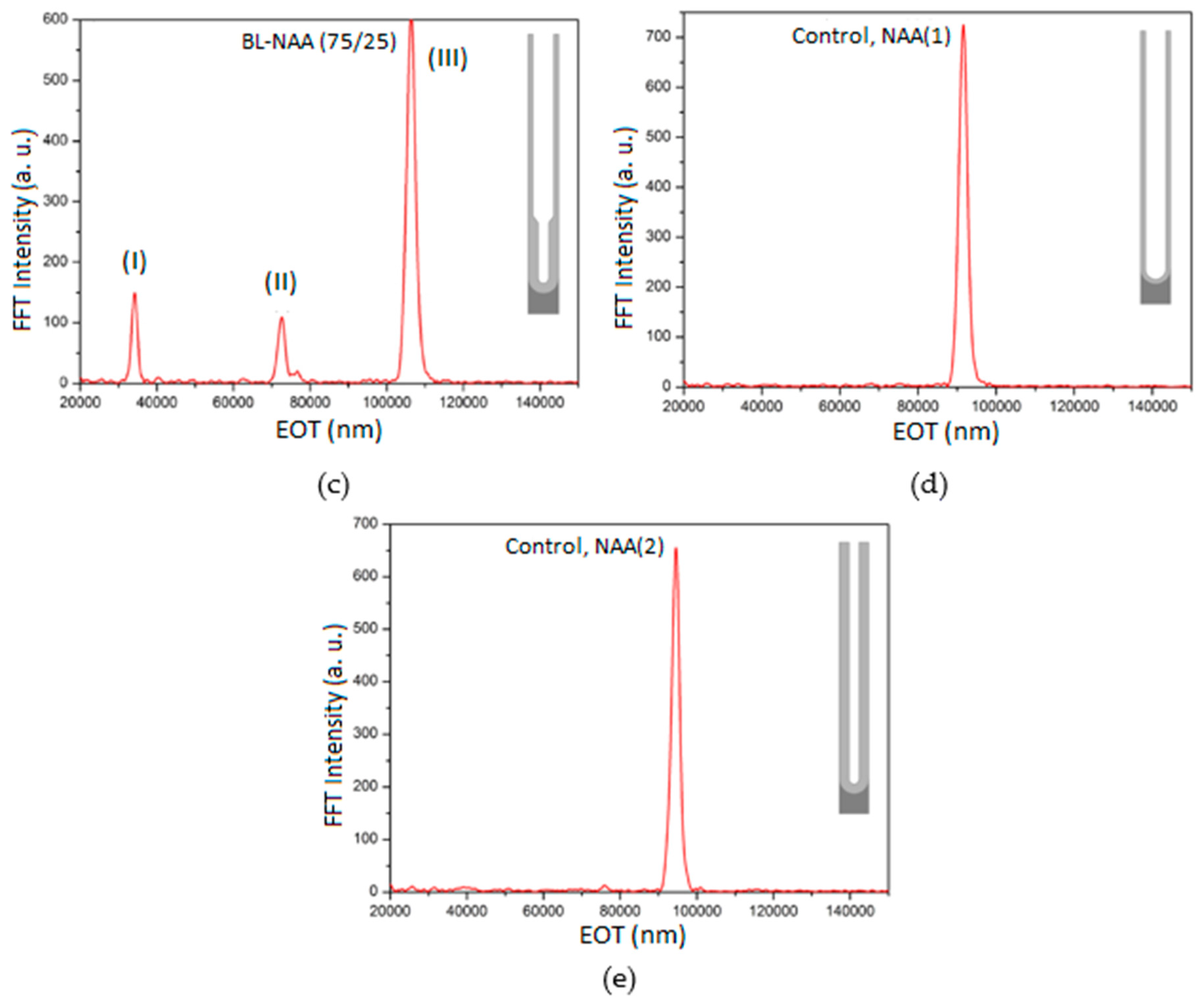
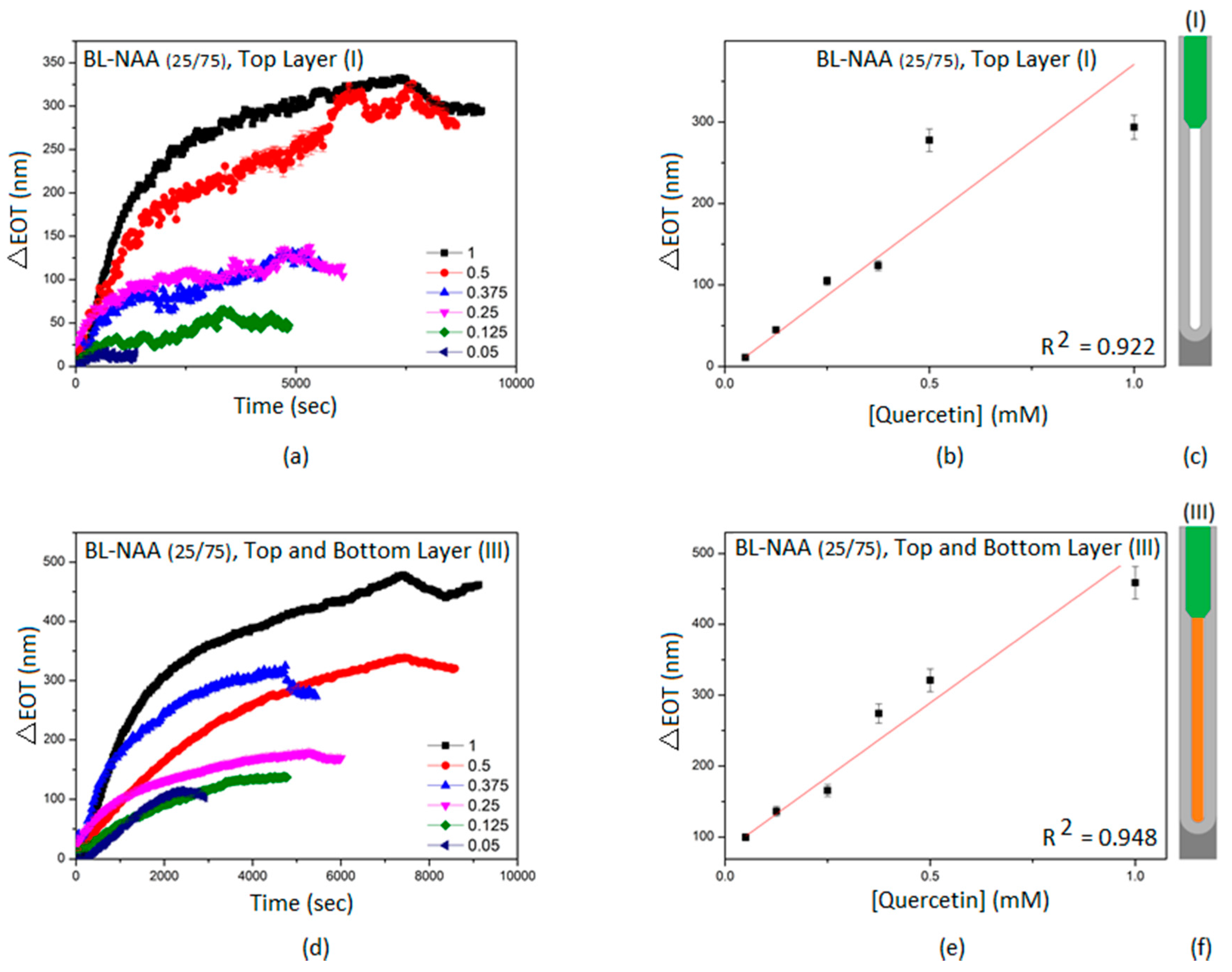
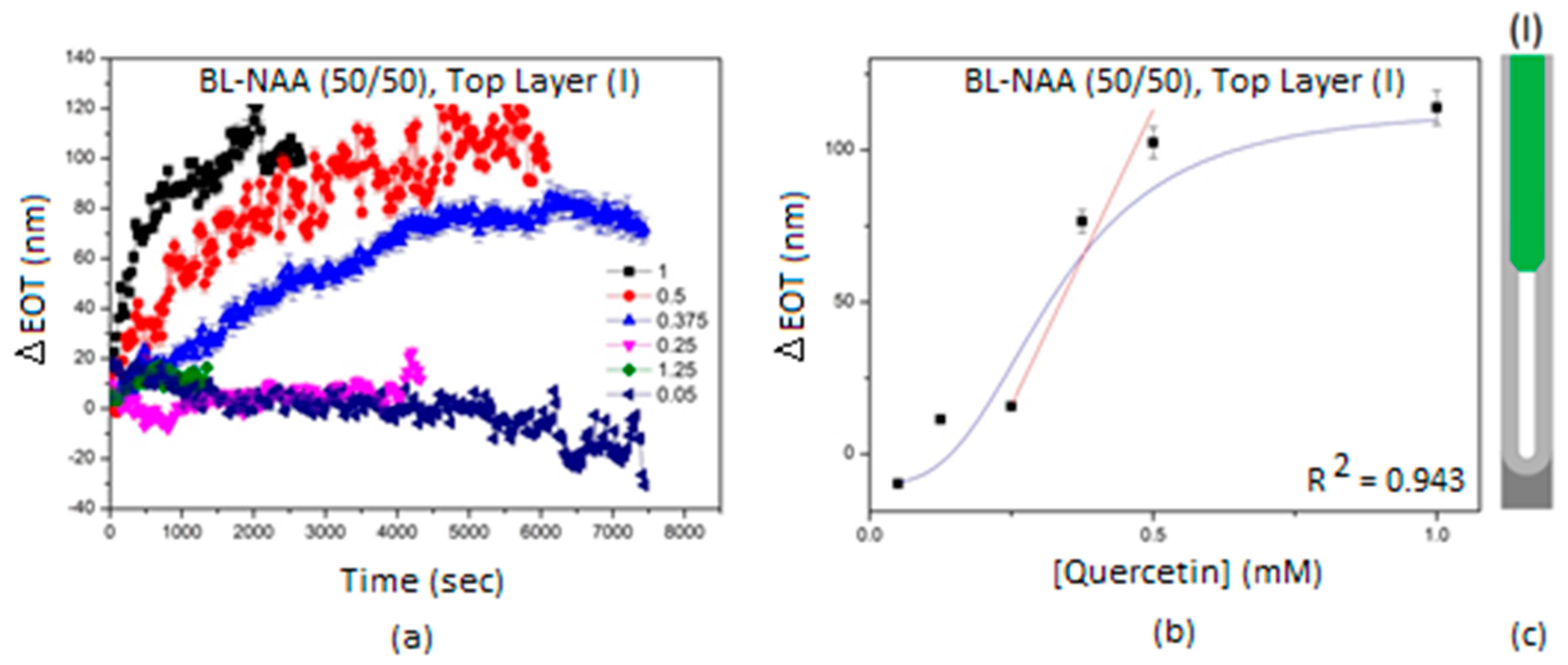
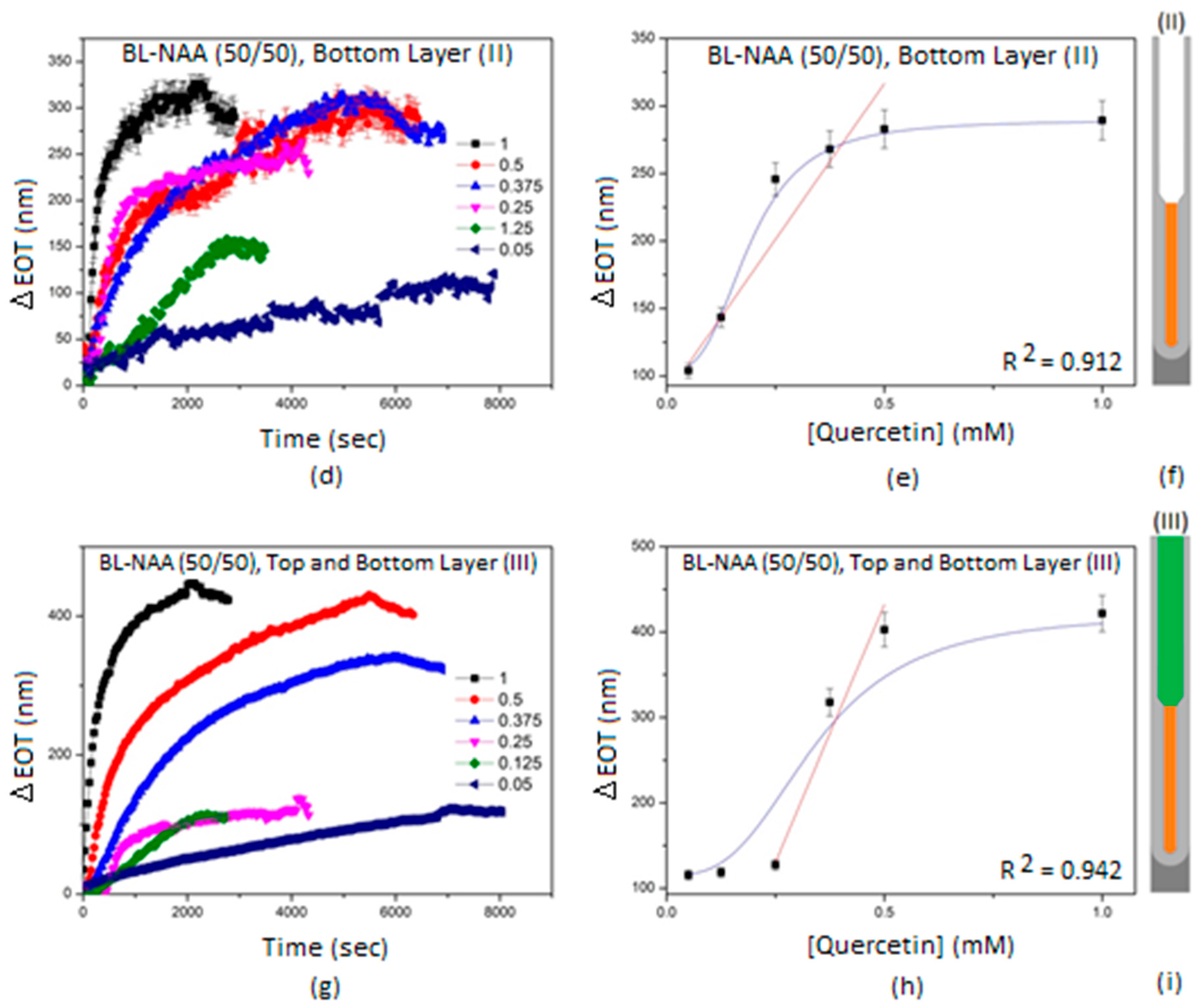
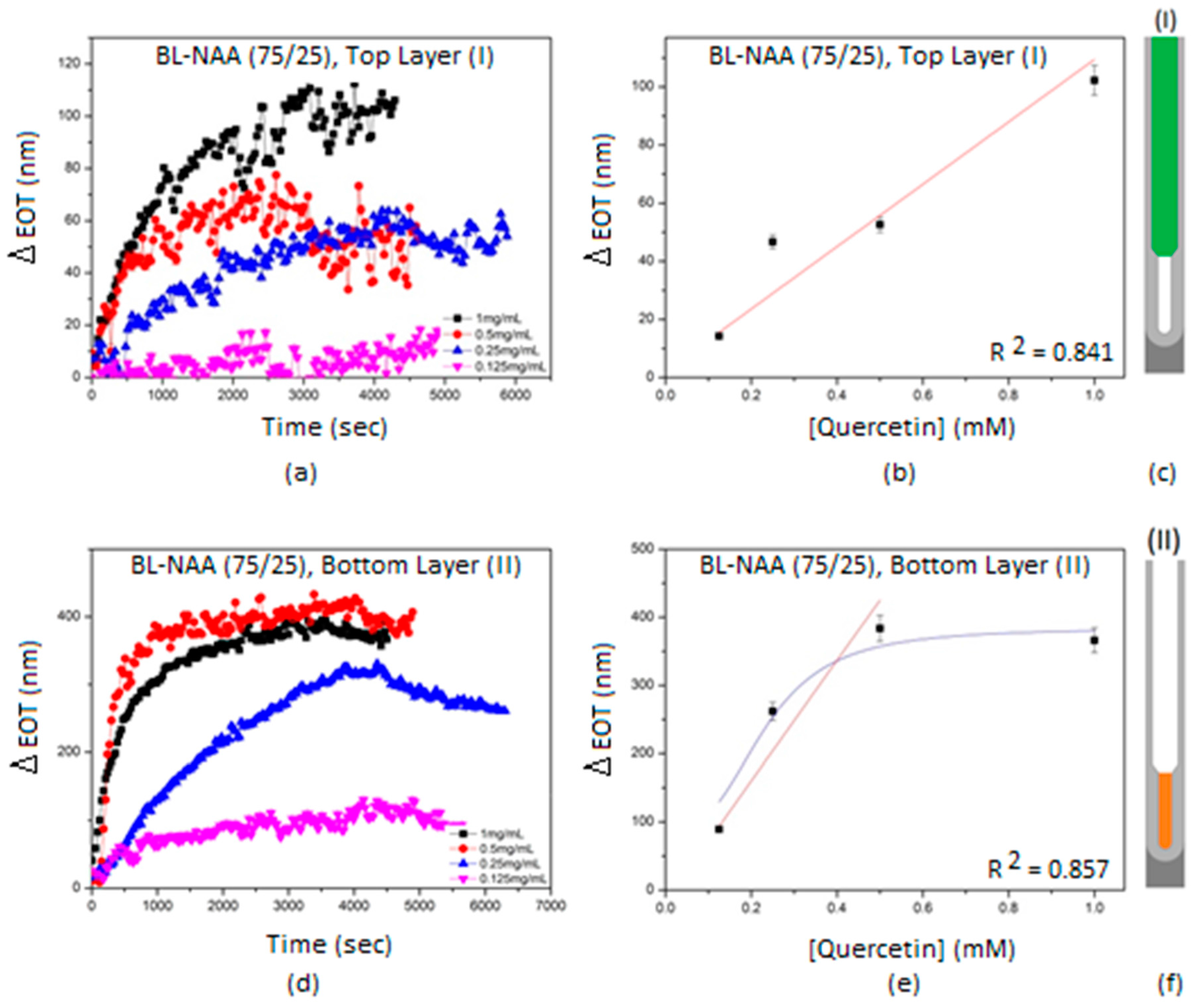
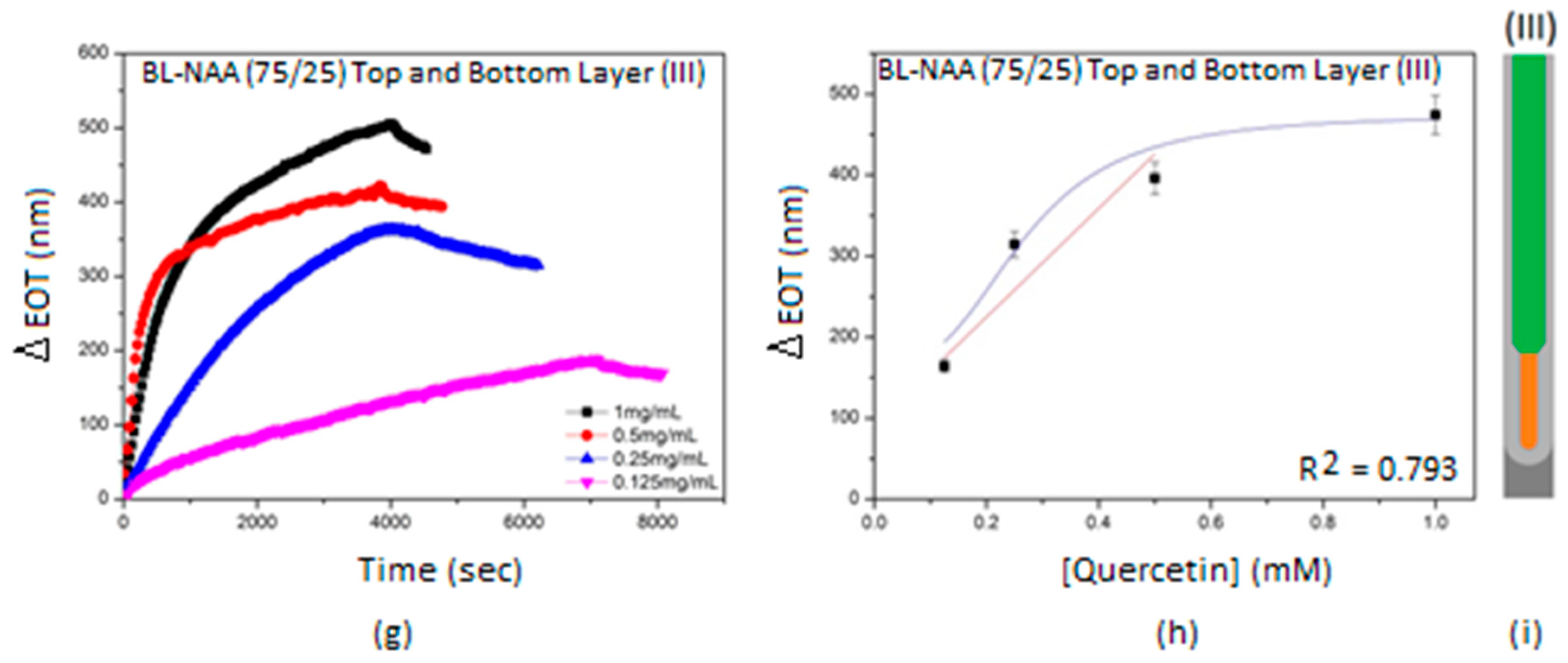
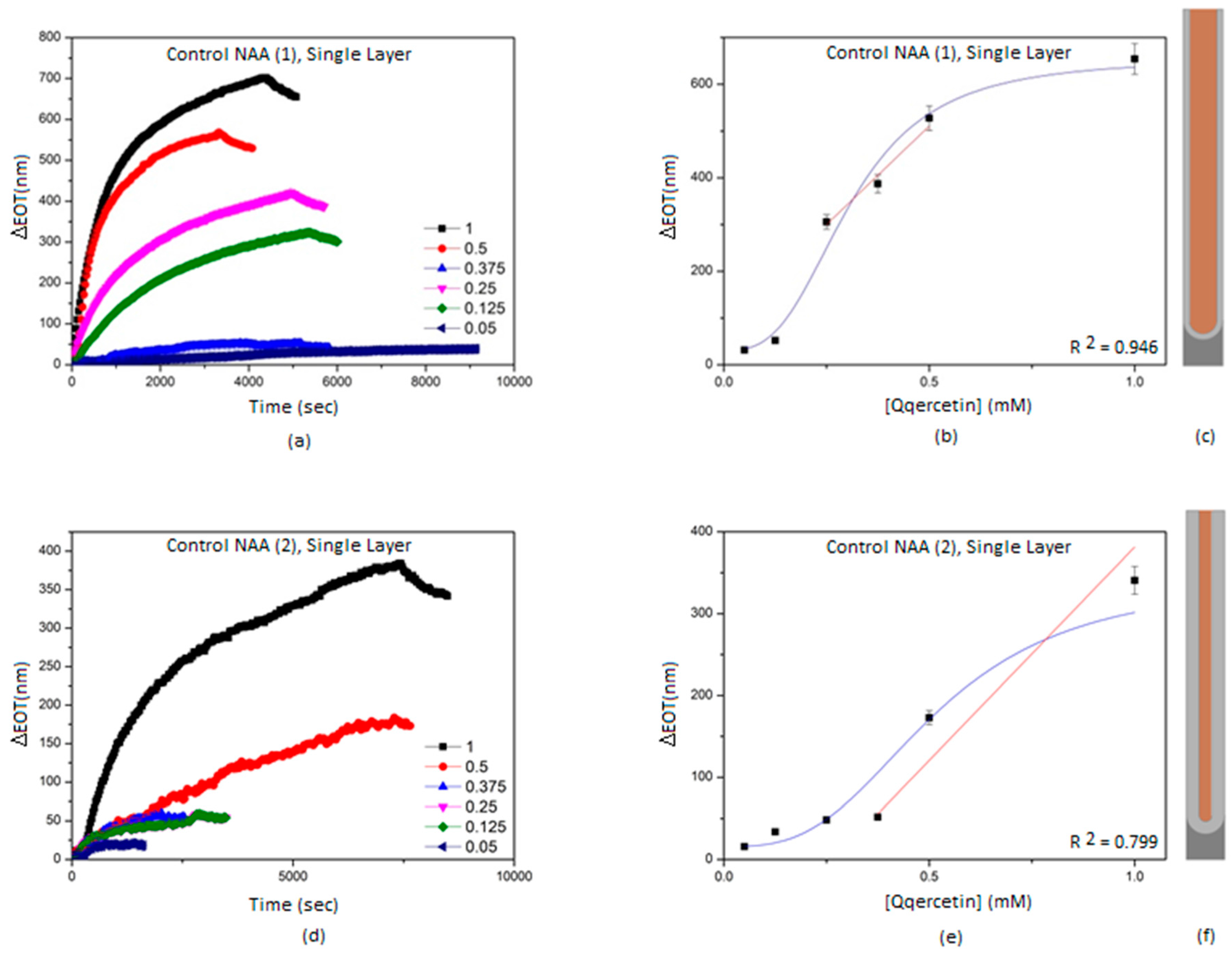
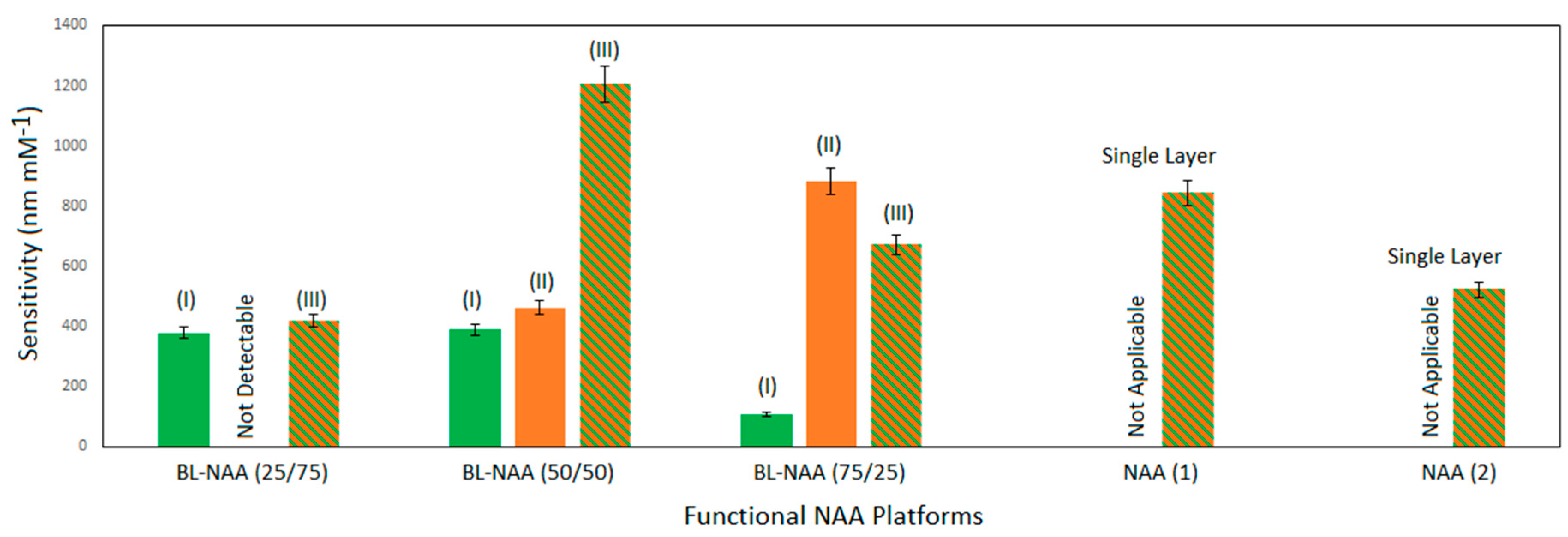
3.2. Characterization and Optimization of RIfS Signals from BL-NAAs and NAAs Platforms
3.3. The Evaluation of Bl-NAA and NAA Sensing Platforms for Biomolecules Sensing
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Md Jani, A.M.; Losic, D.; Voelcker, N.H. Nanoporous anodic aluminium oxide: Advances in surface engineering and emerging applications. Prog. Mater. Sci. 2013, 58, 636–704. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic alumina: A versatile platform for optical biosensors. Materials 2014, 7, 4297–4320. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Liu, J.; Coronel, N.; Agarwal, A.M.; Michel, J.; Kimerling, L.C. Integration of self-assembled porous alumina and distributed bragg reflector for light trapping in Si photovoltaic devices. IEEE Photon. Technol. Lett. 2010, 22, 1394–1396. [Google Scholar] [CrossRef]
- Toccafondi, C.; Thorat, S.; Rocca, R.L.; Scarpellini, A.; Salerno, M.; Dante, S.; Das, G. Multifunctional substrates of thin porous alumina for cell biosensors. J. Mater. Sci. Mater. Med. 2014, 25, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Hotta, K.; Teramae, N. Optical waveguide sensor based on a porous anodic alumina/aluminum multilayer film. Anal. Chem. 2009, 81, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sulka, G.D.; Hnida, K. Distributed bragg reflector based on porous anodic alumina fabricated by pulse anodization. Nanotechnol 2012, 23, 075303. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Marsal, L.F.; Pallarès, J.; Ferré-Borrull, J. Tuning the photonic stop bands of nanoporous anodic alumina-based distributed bragg reflectors by pore widening. ACS Appl. Mater. Interfaces 2013, 5, 13375–13381. [Google Scholar] [CrossRef] [PubMed]
- Dronov, R.; Jane, A.; Shapter, J.G.; Hodges, A.; Voelcker, N.H. Nanoporous alumina-based interferometric transducers ennobled. Nanoscale 2011, 3, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Brecht, A.; Gauglitz, G. Recent developments in optical transducers for chemical or biomedical applications. Sens. Actuators B 1997, 38, 1–7. [Google Scholar] [CrossRef]
- Reichl, D.; Krage, R.; Krumme, C.; Gauglitz, G. Sensing of volatile organic compounds using a simplified reflectometric interference spectroscopy set up. Appl. Spectrosc. 2000, 54, 583–586. [Google Scholar] [CrossRef]
- Belmont, A.-S.; Jaeger, S.; Knopp, D.; Niessner, R.; Gauglitz, G.; Haupt, K. Molecularly imprinted polymer films for reflectometric interference spectroscopic sensors. Biosens. Bioelectron. 2007, 22, 3267–3272. [Google Scholar] [CrossRef] [PubMed]
- Leopold, N.; Busche, S.; Gauglitz, G.; Lendl, B. IR absorption and reflectometric interference spectroscopy (RIfS) combined to a new sensing approach for gas analytes absorbed into thin polymer films. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 72, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Santos, A.; Kumeria, T.; Losic, D. Label-free real-time quantification of enzyme levels by interferometric spectroscopy combined with gelatin-modified nanoporous anodic alumina photonic films. Anal. Chem. 2015, 87, 9016–9024. [Google Scholar] [CrossRef] [PubMed]
- Brecht, A.; Gauglitz, G. Optical probes and transducers. Biosens. Bioelectron. 1995, 10, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G. Direct optical sensors: Principles and selected applications. Anal. Bioanal. Chem. 2005, 381, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G. Direct optical detection in bioanalysis: An update. Anal. Bioanal. Chem. 2010, 398, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Dancil, K.-P.S.; Greiner, D.P.; Sailor, M.J. A porous silicon optical biosensor: Detection of reversible binding of IgG to a protein A-modified surface. J. Am. Chem. Soc. 1999, 121, 7925–7930. [Google Scholar] [CrossRef]
- Kumeria, T.; Santos, A.; Losic, D. Nanoporous Anodic Alumina Platforms: Engineered Surface Chemistry and Structure for Optical Sensing Applications. Sensors 2014, 14, 11878–11918. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Santos, A.; Law, C.S.; Losic, D. Assessment of binding affinity between drugs and human serum albumin using nanoporous anodic alumina photonic crystals. Anal. Chem. 2016, 88, 5971–5980. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Balderrama, V.; Alba, M.; Formentín, P.; Ferré- Borrull, J.; Pallarès, J.; Marsal, L.F. Nanoporous anodic alumina barcodes: Toward smart optical biosensors. Adv. Mater. 2012, 24, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Kumeria, T.; Kurkuri, M.D.; Diener, K.R.; Parkinson, L.; Losic, D. Label-free reflectometric interference microchip biosensor based on nanoporous alumina for detection of circulating tumor cells. Biosens. Bioelectron. 2012, 35, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Kumeria, T.; Parkinson, L.; Losic, D. A nanoporous interferometric micro-sensor for biomedical detection of volatile Sulphur compounds. Nanoscale Res. Lett. 2011, 6, 634. [Google Scholar] [CrossRef] [PubMed]
- Law, C.S.; Santos, A.; Nemati, M.; Losic, D. Structural engineering of nanoporous anodic alumina photonic crystals by sawtooth-like pulse anodization. ACS Appl. Mater. Interfaces 2016, 8, 13542–13554. [Google Scholar] [CrossRef] [PubMed]
- Law, C.S.; Sylvia, G.M.; Nemati, M.; Yu, J.; Losic, D.; Abell, A.D.; Santos, A. Engineering of surface chemistry for enhanced sensitivity in nanoporous interferometric sensing platforms. ACS Appl. Mater. Interfaces 2017, 9, 8929–8940. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Lillo, M.; Losci, D., Jr. Porous alumina with shaped pore geometries and complex pore architectures fabricated by cyclic anodization. Small 2009, 5, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Losci, D., Jr. preparation of porous anodic alumina with periodically perforated pores. Langmuir 2009, 25, 5426–5431. [Google Scholar] [CrossRef] [PubMed]
- Kumeria, T.; Rahman, M.M.; Santos, A.; Ferré-Borrull, J.; Marsal, F.L.; Losic, D. Structural and optical nanoengineering of nanoporous anodic alumina rugate filters for real-time and label-free biosensing applications. Anal. Chem. 2014, 86, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Macias, G.; Ferré-Borrull, J.; Pallares, J.; Marsal, L.F. 1-D nanoporous anodic alumina rugate filters by means of small current variations for real-time sensing applications. Nanoscale Res. Lett. 2014, 9, 315. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.J.; Fei, G.T.; Wang, B. Modulation of transmission spectra of anodized alumina membrane distributed Bragg reflector by controlling anodization temperature. Nanoscale Res. Lett. 2009, 4, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Formentín, P.; Pallarès, J.; Ferré-Borrull, J.; Marsal, L.F. Structural engineering of nanoporous anodic alumina funnels with high aspect ratio. J. Electroanal. Chem. 2011, 655, 73–78. [Google Scholar] [CrossRef]
- Macias, G.; Hernández-Eguía, L.P.; Ferreé-Borrull, J.; Pallares, J.; Marsal, L.F. Gold-coated ordered nanoporous anodic alumina bilayers for future label-free interferometric biosensors. ACS Appl. Mater. Interfaces 2013, 5, 8093–8098. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Kumeria, T.; Wang, Y.; Losic, D. In situ monitoring engineering of inverted nanoporous anodic alumina funnels: On the precise generation of 3D optical nanostructures. Nanoscale 2014, 6, 9991–9999. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, G. Enhanced the optical transmission efficiency by funnel-shaped nanopore. In Proceedings of the International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale (3M-NANO), Chongqing, China, 18–22 July 2016; pp. 55–58. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémécy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, N.; Zhu, Z.; Huang, J.; Li, G. Detection of flavonoids and assay for their antioxidant activity based on enlargement of gold nanoparticles. Anal. Bioanal. Chem. 2007, 388, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Lamson, D.W.; Brignall, M.S. Antioxidants and cancer III: Quercetin. Altern Med. Rev. 2000, 5, 196–208. [Google Scholar] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.; Ransom, T.P.P. Dietary fiber, the evolution of the human diet and coronary heart disease. Nutr. Res. 1998, 18, 633–652. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Singh, A.; Sachchidanand, S.; Berta, A.K. Quercetin inhibits acid-sensing ion channels through a putative binding site in the central vestibular region. Neuroscience 2017, 348, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Velleman, L.; Triani, G.; Evans, P.J.; Shapter, J.G.; Losic, D. Structural and chemical modification of porous alumina membranes. Micropor. Mesopor. Mater. 2009, 126, 87–94. [Google Scholar] [CrossRef]
- Kumeria, T.; Losic, D. Controlling interferometric properties of nanoporous anodic aluminium oxide. Nanoscale Res. Lett. 2012, 7, 88. [Google Scholar] [CrossRef] [PubMed]
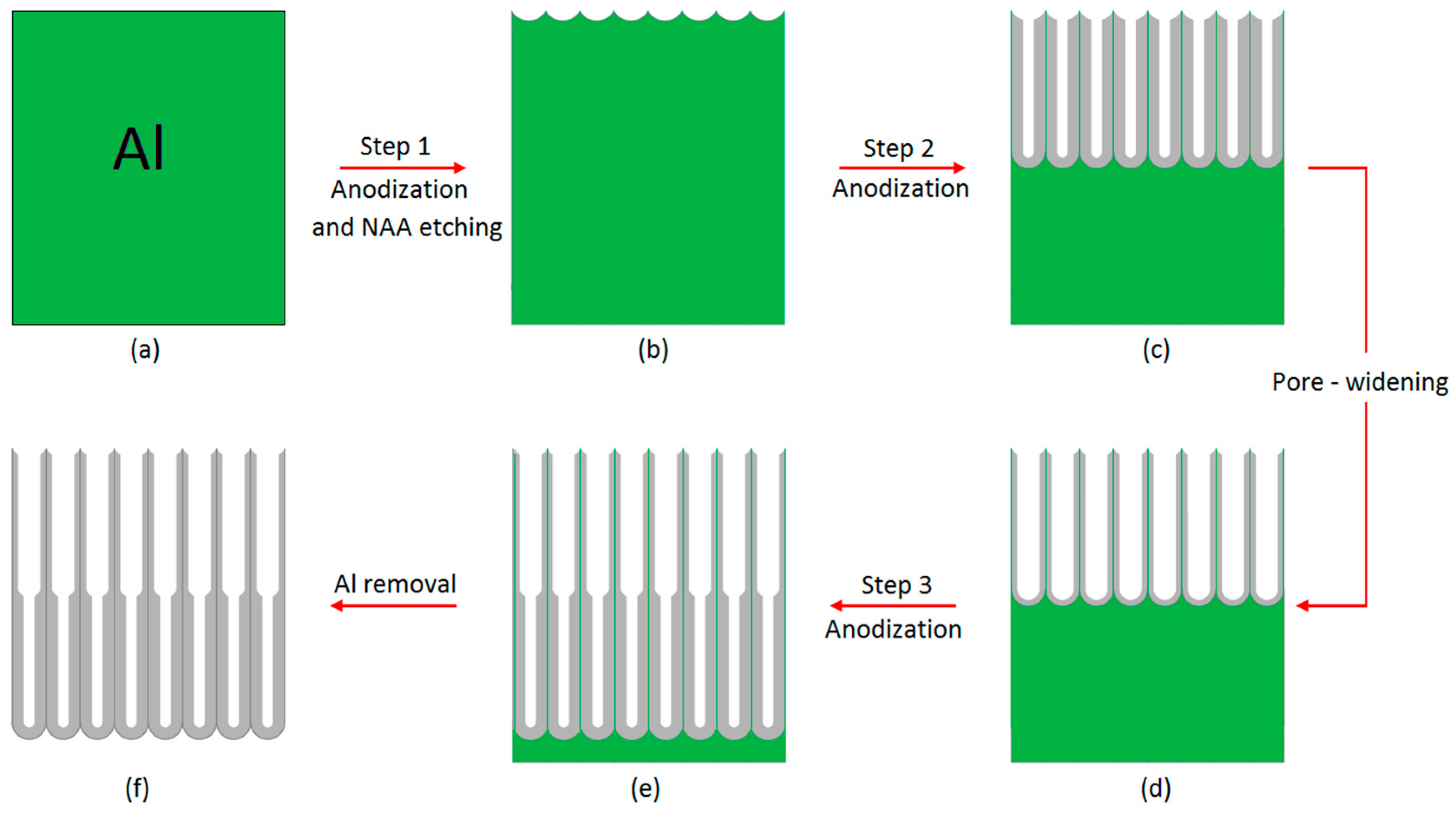
| Anodized Anodic Aluminum Platforms | Step (1) Anodization (h) | Step (2) Anodization (h) | Pore Widening (min) | Step (3) Anodization (h) | |
|---|---|---|---|---|---|
| NAAF | BL-NAA(25/75) | 20 | 3 | 15 | 6 |
| BL-NAA(50/50) | 20 | 4.5 | 15 | 4.5 | |
| BL-NAA(75/25) | 20 | 6 | 15 | 3 | |
| NAA | NAA(1) | 20 | 9 | 15 | N/A 1 |
| NAA(2) | 20 | 9 | N/A1 | N/A 1 | |
| Anodized Anodic Aluminum Platforms | Top Layer Thickness (μm) | Bottom Layer Thickness (μm) | Nanopore thickness (μm) | Pore Diameter (Top/Bottom Layer) (nm) | |
|---|---|---|---|---|---|
| NAAF | BL-NAA(25/75) | 9 ± 0.45 | 22 ± 1.1 | 30 ± 1.5 | 55 ± 2.75/45 ± 2.25 |
| BL-NAA(50/50) | 15 ± 0.75 | 15 ± 0.75 | 30 ± 1.5 | 55 ± 2.75/45 ± 2.25 | |
| BL-NAA(75/25) | 22 ± 1.1 | 10 ± 0.5 | 32 ± 1.6 | 55 ± 2.75/45 ± 2.25 | |
| NAA | NAA(1) | N/A1 | N/A1 | 22 ± 1.1 | 55 ± 2.75 |
| NAA(2) | N/A1 | N/A1 | 23.5 ± 1.17 | 45 ± 2.25 | |
| Sample | Optical Layer | S (nm mM−1) | LLOD (mM) | R2 |
|---|---|---|---|---|
| BL-NAA(25/75) | (I) | 378 ± 48 | 0.034 | 0.922 |
| (II) | ND1 | ND1 | ND1 | |
| (III) | 418 ± 43 | 0.074 | 0.948 | |
| BL-NAA(50/50) | (I) | 389 ± 66 | 0.148 | 0.943 |
| (II) | 462 ± 70 | 0.1 | 0.912 | |
| (III) | 1207 ± 208 | 0.168 | 0.942 | |
| BL-NAA(75/25) | (I) | 107 ± 26 | 0.179 | 0.841 |
| (II) | 884 ± 245 | 0.160 | 0.857 | |
| (III) | 670 ± 227 | 0.256 | 0.793 | |
| NAA(1) | Single layer | 842 ± 139 | 0.188 | 0.946 |
| NAA(2) | Single layer | 521 ± 174 | 0.449 | 0.799 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemati, M.; Santos, A.; Losic, D. Fabrication and Optimization of Bilayered Nanoporous Anodic Alumina Structures as Multi-Point Interferometric Sensing Platform. Sensors 2018, 18, 470. https://doi.org/10.3390/s18020470
Nemati M, Santos A, Losic D. Fabrication and Optimization of Bilayered Nanoporous Anodic Alumina Structures as Multi-Point Interferometric Sensing Platform. Sensors. 2018; 18(2):470. https://doi.org/10.3390/s18020470
Chicago/Turabian StyleNemati, Mahdieh, Abel Santos, and Dusan Losic. 2018. "Fabrication and Optimization of Bilayered Nanoporous Anodic Alumina Structures as Multi-Point Interferometric Sensing Platform" Sensors 18, no. 2: 470. https://doi.org/10.3390/s18020470
APA StyleNemati, M., Santos, A., & Losic, D. (2018). Fabrication and Optimization of Bilayered Nanoporous Anodic Alumina Structures as Multi-Point Interferometric Sensing Platform. Sensors, 18(2), 470. https://doi.org/10.3390/s18020470







