Electrochemical Immunoassay Using Open Circuit Potential Detection Labeled by Platinum Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
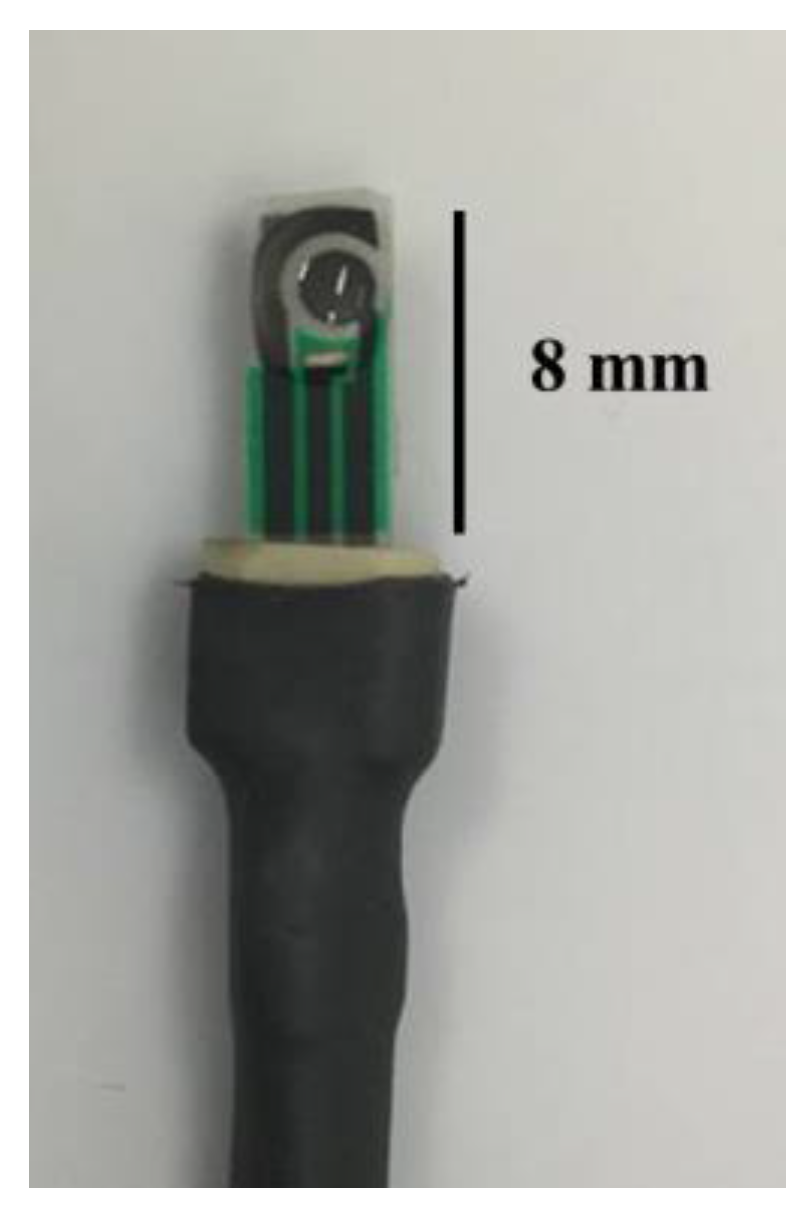
2.2. Instruments
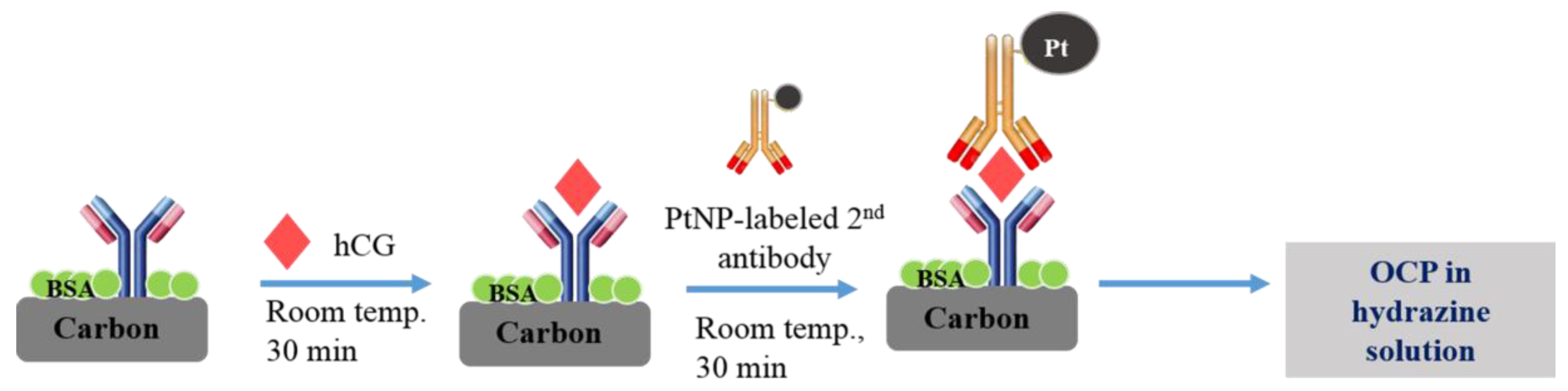
2.3. Sandwiched Immunosensor Procedure
2.3.1. Preparation of PtNP-Labeled hCG Antibody (Pt-Mab-hCG)
2.3.2. Immobilization of Primary Monoclonal Antibodies on the Working Electrode Surface of SPCE (Mab-FSH-Immobilized Immunosensor)
2.3.3. Immobilization of hCG Antigens and Pt-Mab-hCG on the Mab-FSH-Immobilized Immunosensor
2.4. Electrochemical System
3. Results and Discussion
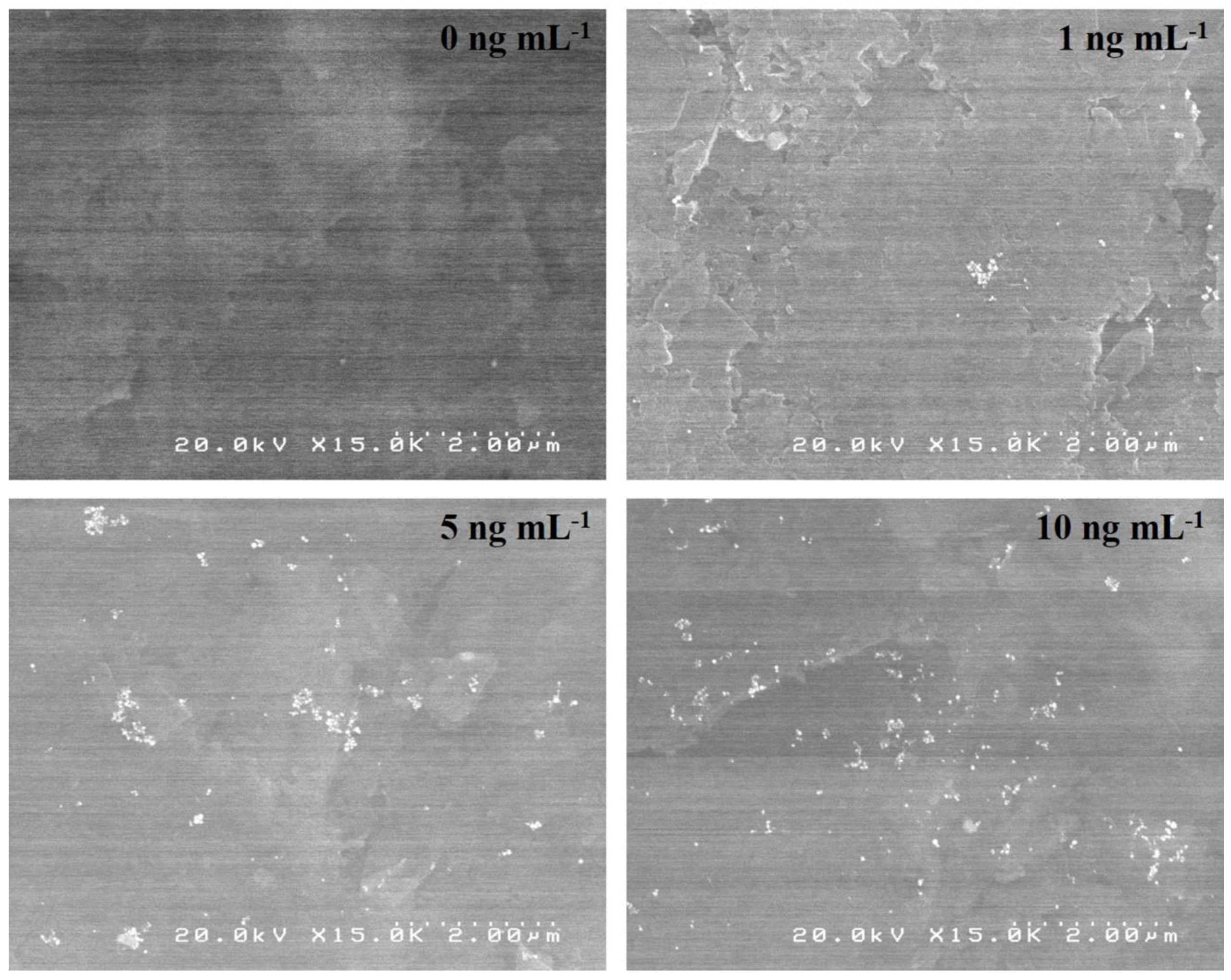
3.1. Surface Morphology of Sandwich-Type Immunosensors Labeled with the PtNPs
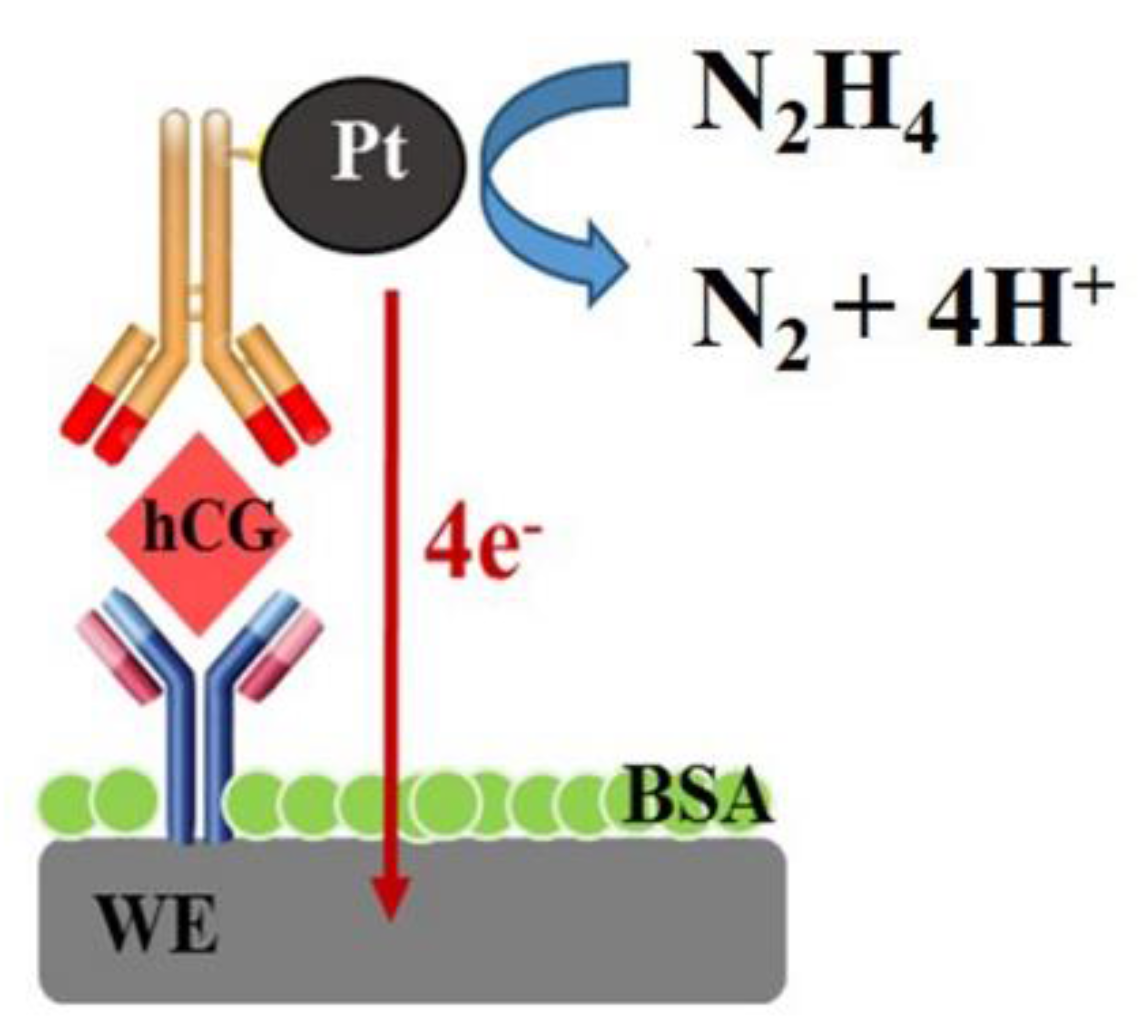
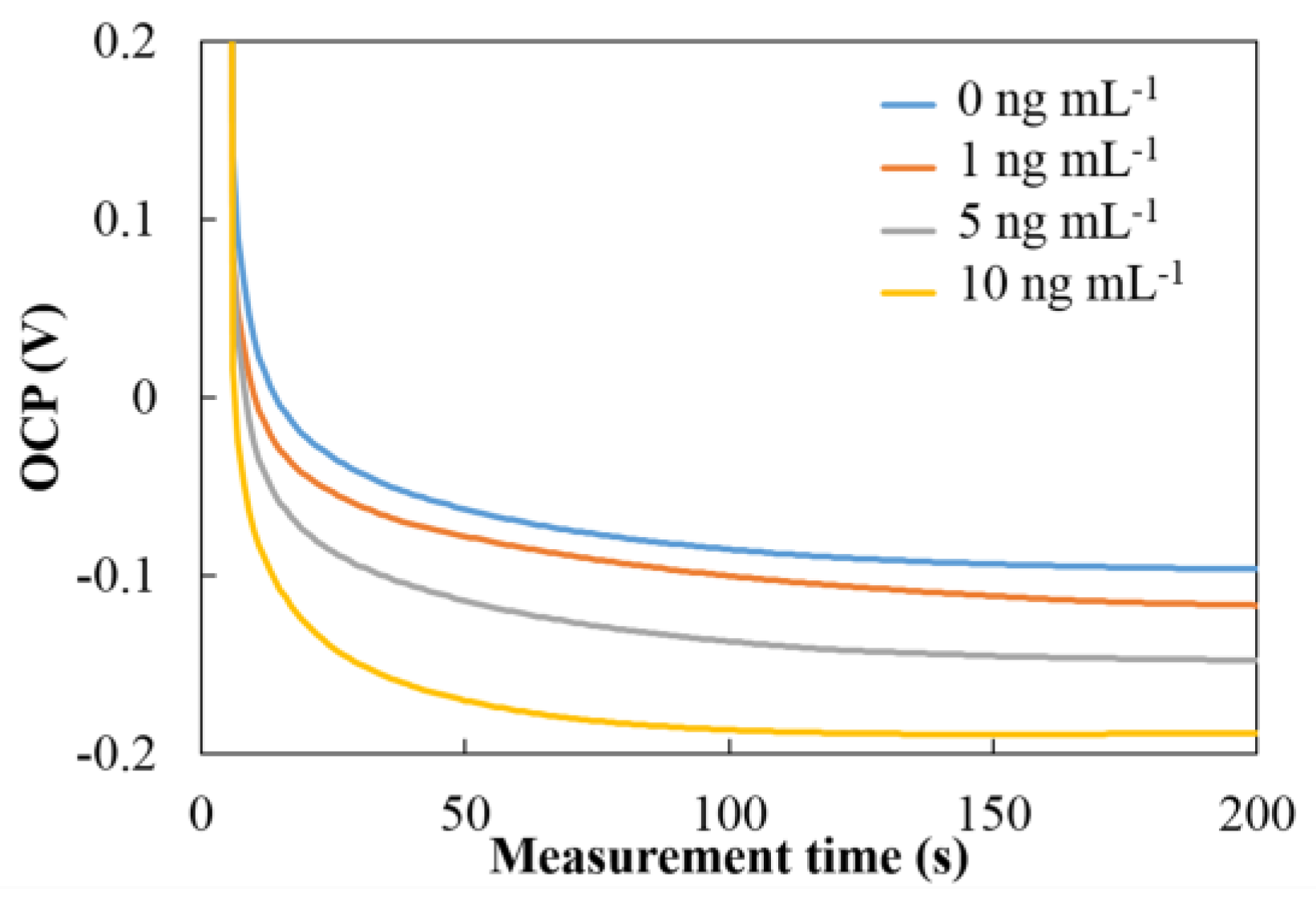
3.2. OCP Behavior of PtNPs Based Immunoassay for hCG Detection
3.3. Optimization of Experimental Parameters
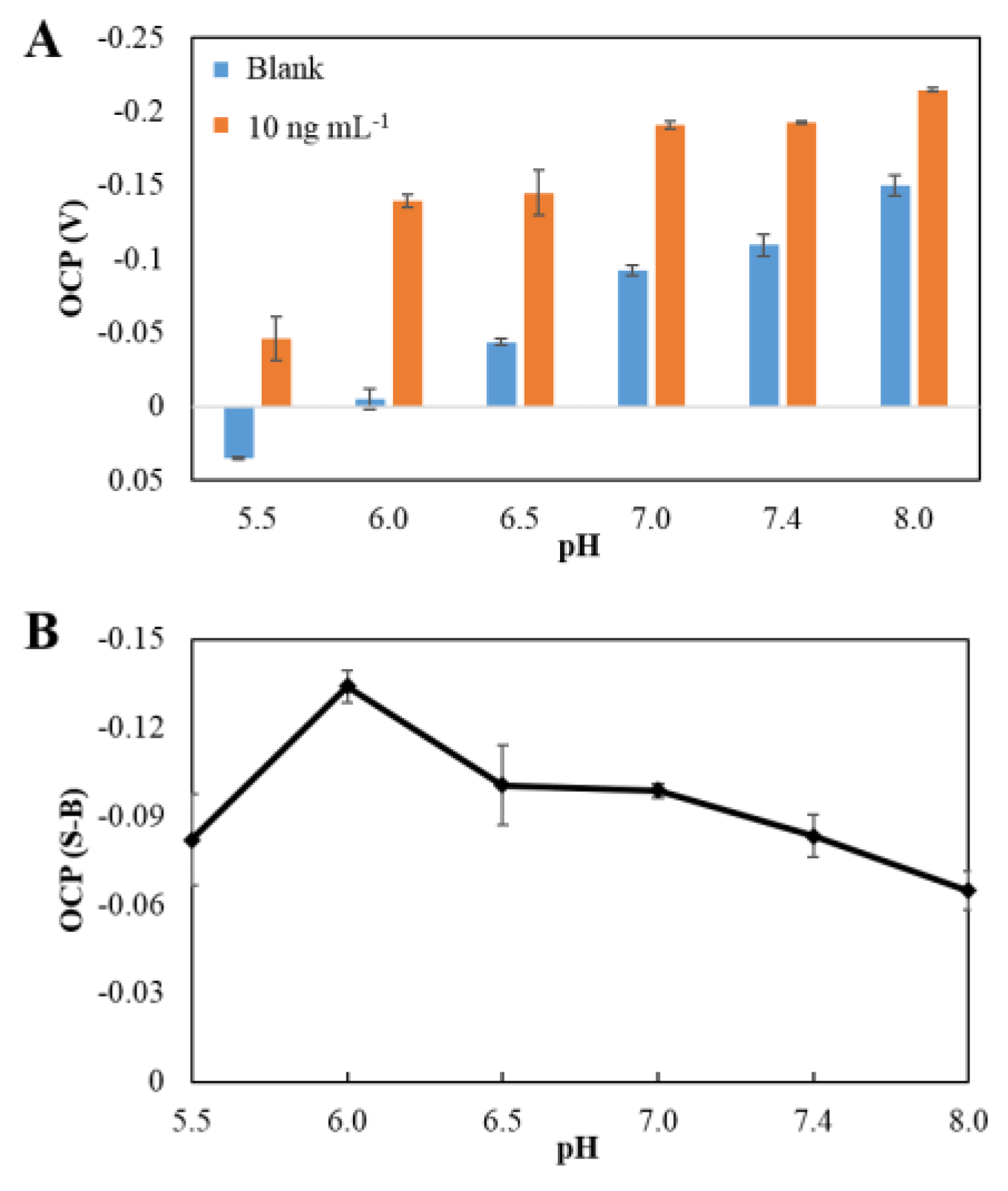
3.3.1. Effect of pH of the Buffer Solution
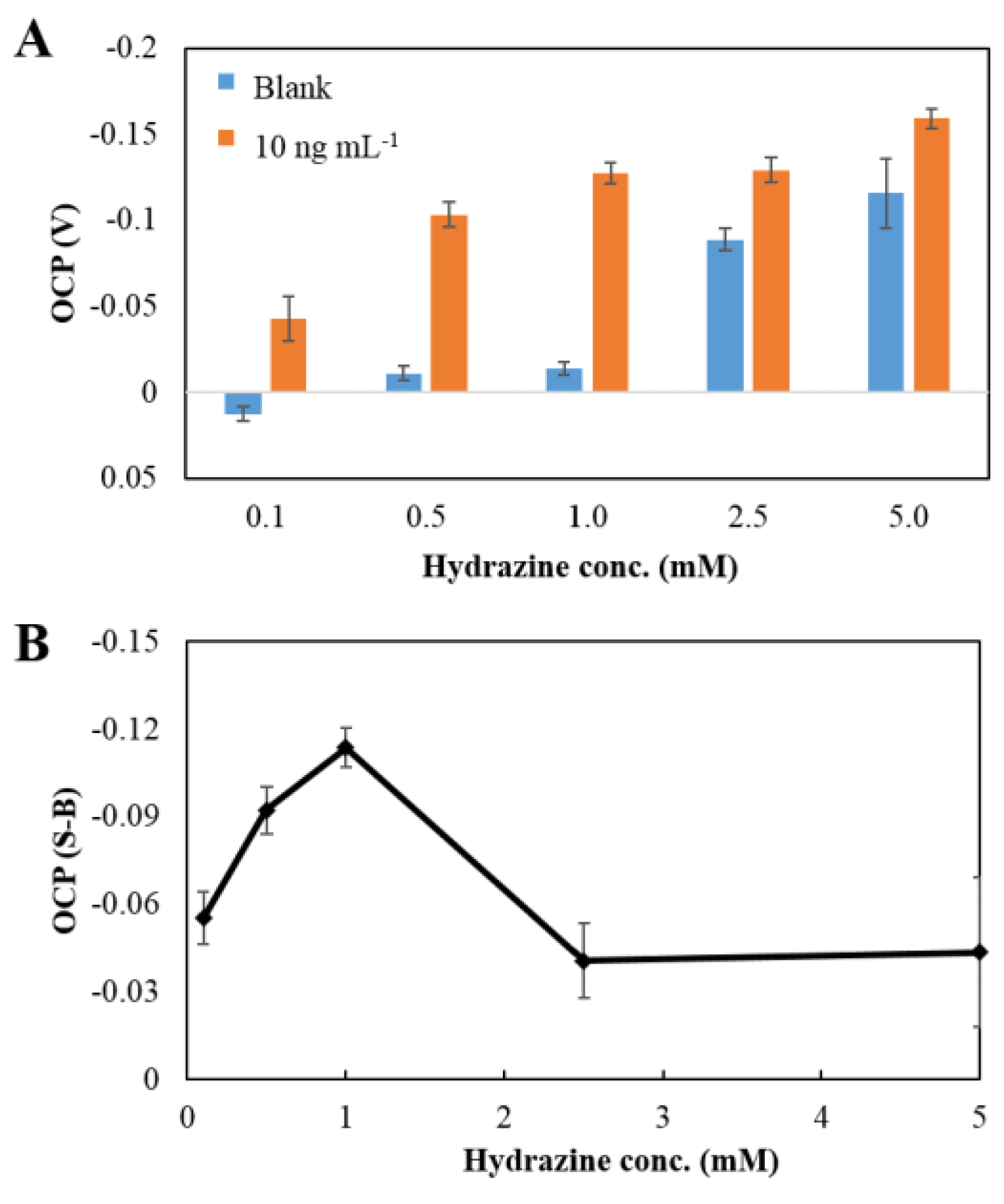
3.3.2. Concentration of Hydrazine Solution
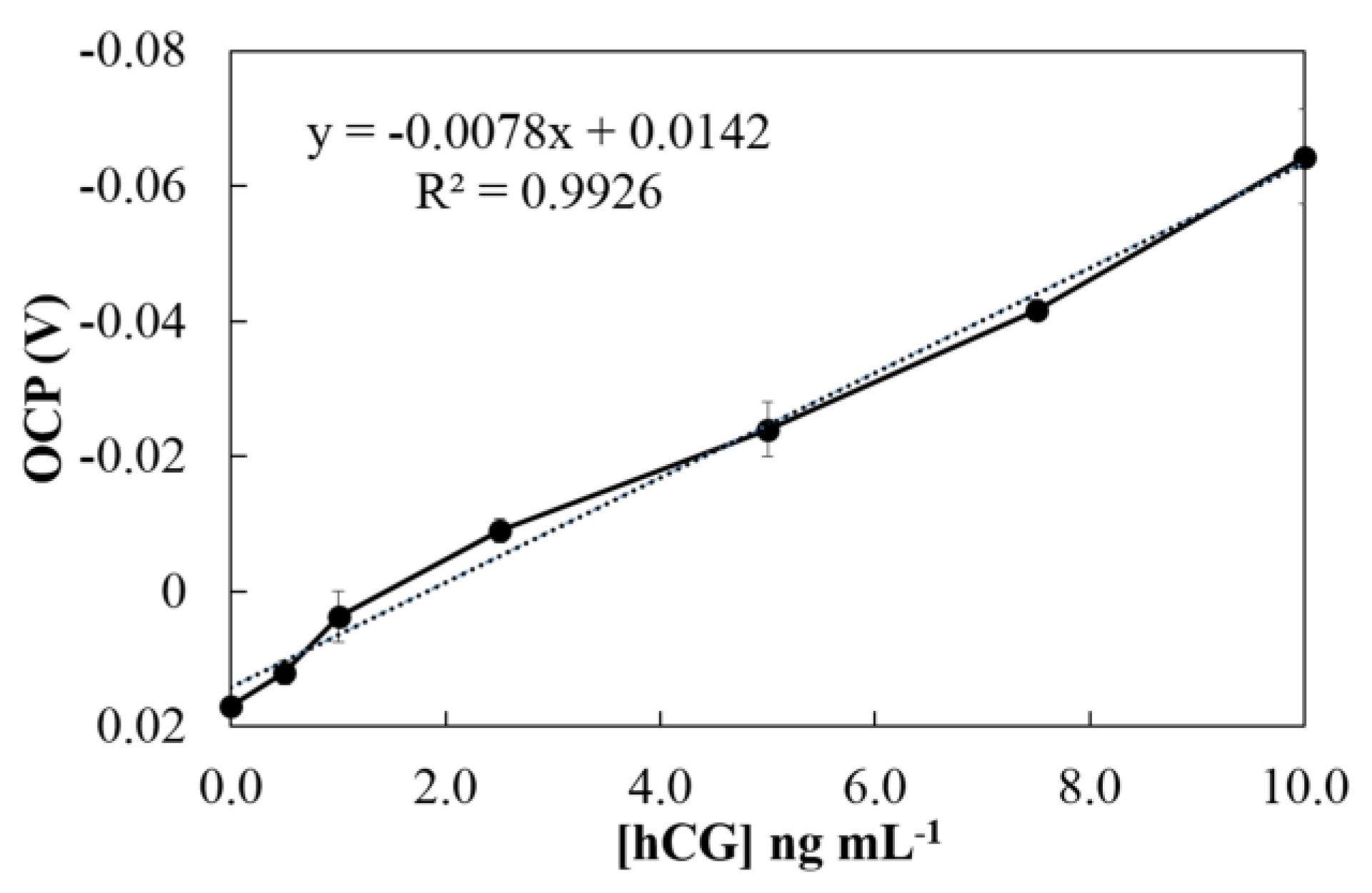
3.4. Analytical Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bakker, E.; Qin, Y. Electrochemical Sensors. Anal. Chem. 2006, 78, 3965–3984. [Google Scholar] [CrossRef] [PubMed]
- Bannister, J.V. Development of biosensors for immunoassays. Annali Dell’istituto Superiore di Sanita 1991, 27, 145–147. [Google Scholar] [PubMed]
- Halsall, H.B.; Heineman, W.R. Electrochemical immunoassay: An ultrasensitive method. J. Int. Fed. Clin. Chem. 1990, 2, 179–187. [Google Scholar] [PubMed]
- Wan, Y.; Su, Y.; Zhu, X.; Liu, G.; Fan, C. Development of electrochemical immunosensors towards point of care diagnostics. Biosens. Bioelectron. 2013, 47 (Suppl. C), 1–11. [Google Scholar] [CrossRef] [PubMed]
- Warsinke, A.; Benkert, A.; Scheller, F.W. Electrochemical immunoassays. Fresenius’ J. Anal. Chem. 2000, 366, 622–634. [Google Scholar] [CrossRef]
- Yan, F.; Wu, J.; Tan, F.; Yan, Y.; Ju, H. A rapid and simple method for ultrasensitive electrochemical immunoassay of protein by an electric field-driven strategy. Anal. Chim. Acta 2009, 644, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAC Trends Anal. Chem. 2016, 79 (Suppl. C), 88–105. [Google Scholar] [CrossRef]
- Ding, L.; Bond, A.M.; Zhai, J.; Zhang, J. Utilization of nanoparticle labels for signal amplification in ultrasensitive electrochemical affinity biosensors: A review. Anal. Chim. Acta 2013, 797 (Suppl. C), 1–12. [Google Scholar] [CrossRef] [PubMed]
- Idegami, K.; Chikae, M.; Kerman, K.; Nagatani, N.; Yuhi, T.; Endo, T.; Tamiya, E. Gold Nanoparticle-Based Redox Signal Enhancement for Sensitive Detection of Human Chorionic Gonadotropin Hormone. Electroanalysis 2008, 20, 14–21. [Google Scholar] [CrossRef]
- Tao, M.; Li, X.; Wu, Z.; Wang, M.; Hua, M.; Yang, Y. The preparation of label-free electrochemical immunosensor based on the Pt–Au alloy nanotube array for detection of human chorionic gonadotrophin. Clin. Chim. Acta 2011, 412, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, H.; Fan, S.; Deng, S.; Lv, Q.; Lin, J.; Li, C.-P. Label-free electrochemical immunosensor based on gold–silicon carbide nanocomposites for sensitive detection of human chorionic gonadotrophin. Biosens. Bioelectron. 2014, 57 (Suppl. C), 199–206. [Google Scholar] [CrossRef] [PubMed]
- Videla, H.A. Manual of Biocorrosion; CRC Press, Inc.: Boca Raton, FL, USA, 1996. [Google Scholar]
- Baker, P.W.; Ito, K.; Watanabe, K. Marine prosthecate bacteria involved in the ennoblement of stainless steel. Environ. Microbiol. 2003, 5, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Dumańska, J.; Maksymiuk, K. Studies on Spontaneous Charging/Discharging Processes of Polypyrrole in Aqueous Electrolyte Solutions. Electroanalysis 2001, 13, 567–573. [Google Scholar] [CrossRef]
- Song, Y.; Su, D.; Shen, Y.; Liu, H.; Wang, L. Design and preparation of open circuit potential biosensor for in vitro and in vivo glucose monitoring. Anal. Bioanal. Chem. 2017, 409, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ahammad, A.J.S.; Choi, Y.-H.; Koh, K.; Kim, J.-H.; Lee, J.-J.; Lee, M. Electrochemical Detection of Cardiac Biomarker Troponin I at Gold Nanoparticle-Modified ITO Electrode by Using Open Circuit Potential. Int. J. Electrochem. Sci. 2011, 6, 1906–1916. [Google Scholar]
- Wong, L.C.C.; Jolly, P.; Estrela, P. Development of a Sensitive Multiplexed Open Circuit Potential System for the Detection of Prostate Cancer Biomarkers. BioNanoScience 2017, 1–6. [Google Scholar] [CrossRef]
- Zhou, H.; Park, J.H.; Fan, F.-R.F.; Bard, A.J. Observation of Single Metal Nanoparticle Collisions by Open Circuit (Mixed) Potential Changes at an Ultramicroelectrode. J. Am. Chem. Soc. 2012, 134, 13212–13215. [Google Scholar] [CrossRef] [PubMed]
- Xuan Viet, N.; Chikae, M.; Ukita, Y.; Maehashi, K.; Matsumoto, K.; Tamiya, E.; Hung Viet, P.; Takamura, Y. Gold-linked electrochemical immunoassay on single-walled carbon nanotube for highly sensitive detection of human chorionic gonadotropinhormone. Biosens. Bioelectron. 2013, 42 (Suppl. C), 592–597. [Google Scholar] [CrossRef] [PubMed]
- Nagatani, N.; Tanaka, R.; Yuhi, T.; Endo, T.; Kerman, K.; Takamura, Y.; Tamiya, E. Gold nanoparticle-based novel enhancement method for the development of highly sensitive immunochromatographic test strips. Sci. Technol. Adv. Mater. 2006, 7, 270–275. [Google Scholar] [CrossRef]
- Fu, E.; Liang, T.; Houghtaling, J.; Ramachandran, S.; Ramsey, S.A.; Lutz, B.; Yager, P. Enhanced sensitivity of lateral flow tests using a two-dimensional paper network format. Anal. Chem. 2011, 83, 7941–7946. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.; Sturgeon, C. Pregnancy testing with hCG—Future prospects. Trends Endocrinol. Metab. 2014, 25, 637–648. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Optimal |
|---|---|
| pH | 6.0 |
| Concentration of hydrazine | 1 mM |
| Ref. | Method | Electrode | Linearity (ng mL−1) | LoD (ng mL−1) | Sample Volume (μL) |
|---|---|---|---|---|---|
| This work | OCP | SPCE | 0.05–10 | 0.28 | 2 |
| [9] | DPV | SPCE | 0–2 | 0.036 | 2 |
| [10] | Amperometry | Pt–Au alloy nanotube array | 2.5–40 | 1.2 | 500 |
| [11] | DPV | gold–silicon carbide nanocomposites | 0.01–55–100 | 0.0042 | ≥500 |
| Sample | Added (ng mL−1) | Detected (ng mL−1) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Urine | 1 | 1.04 ± 0.02 | 2.1 | 103.8 |
| 3 | 3.12 ± 0.10 | 3.1 | 104.1 | |
| 5 | 5.02 ± 0.22 | 4.4 | 100.5 | |
| 7 | 7.08 ± 0.13 | 1.8 | 101.1 | |
| 9 | 9.08 ± 0.20 | 2.2 | 100.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoenkitamorn, K.; Tue, P.T.; Kawai, K.; Chailapakul, O.; Takamura, Y. Electrochemical Immunoassay Using Open Circuit Potential Detection Labeled by Platinum Nanoparticles. Sensors 2018, 18, 444. https://doi.org/10.3390/s18020444
Charoenkitamorn K, Tue PT, Kawai K, Chailapakul O, Takamura Y. Electrochemical Immunoassay Using Open Circuit Potential Detection Labeled by Platinum Nanoparticles. Sensors. 2018; 18(2):444. https://doi.org/10.3390/s18020444
Chicago/Turabian StyleCharoenkitamorn, Kanokwan, Phan Trong Tue, Keiko Kawai, Orawon Chailapakul, and Yuzuru Takamura. 2018. "Electrochemical Immunoassay Using Open Circuit Potential Detection Labeled by Platinum Nanoparticles" Sensors 18, no. 2: 444. https://doi.org/10.3390/s18020444
APA StyleCharoenkitamorn, K., Tue, P. T., Kawai, K., Chailapakul, O., & Takamura, Y. (2018). Electrochemical Immunoassay Using Open Circuit Potential Detection Labeled by Platinum Nanoparticles. Sensors, 18(2), 444. https://doi.org/10.3390/s18020444




