Amyloid Beta Detection by Faradaic Electrochemical Impedance Spectroscopy Using Interdigitated Microelectrodes
Abstract
:1. Introduction
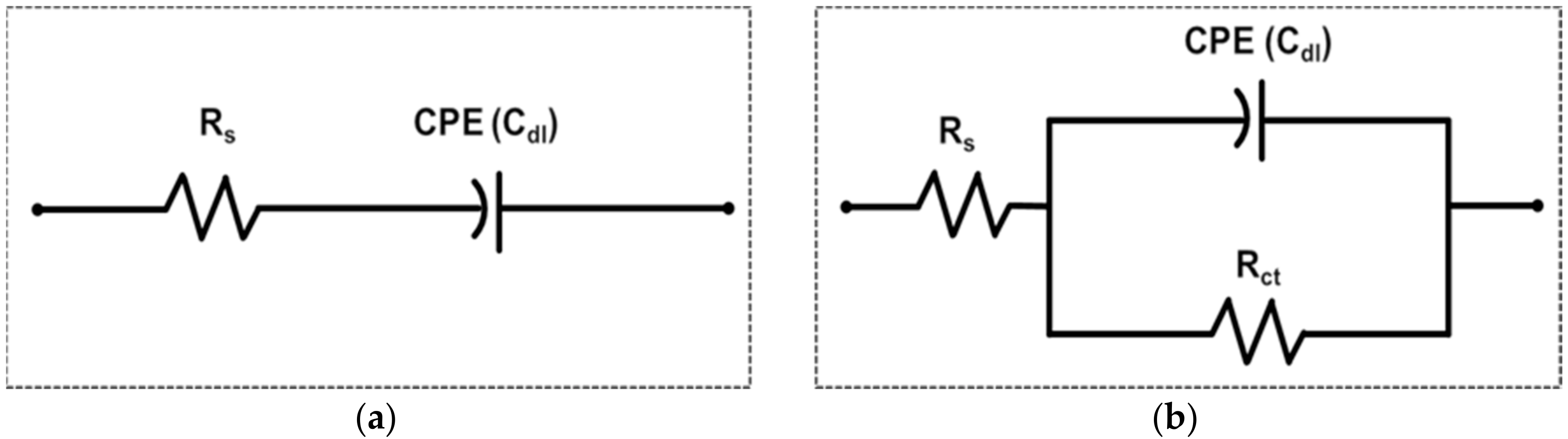
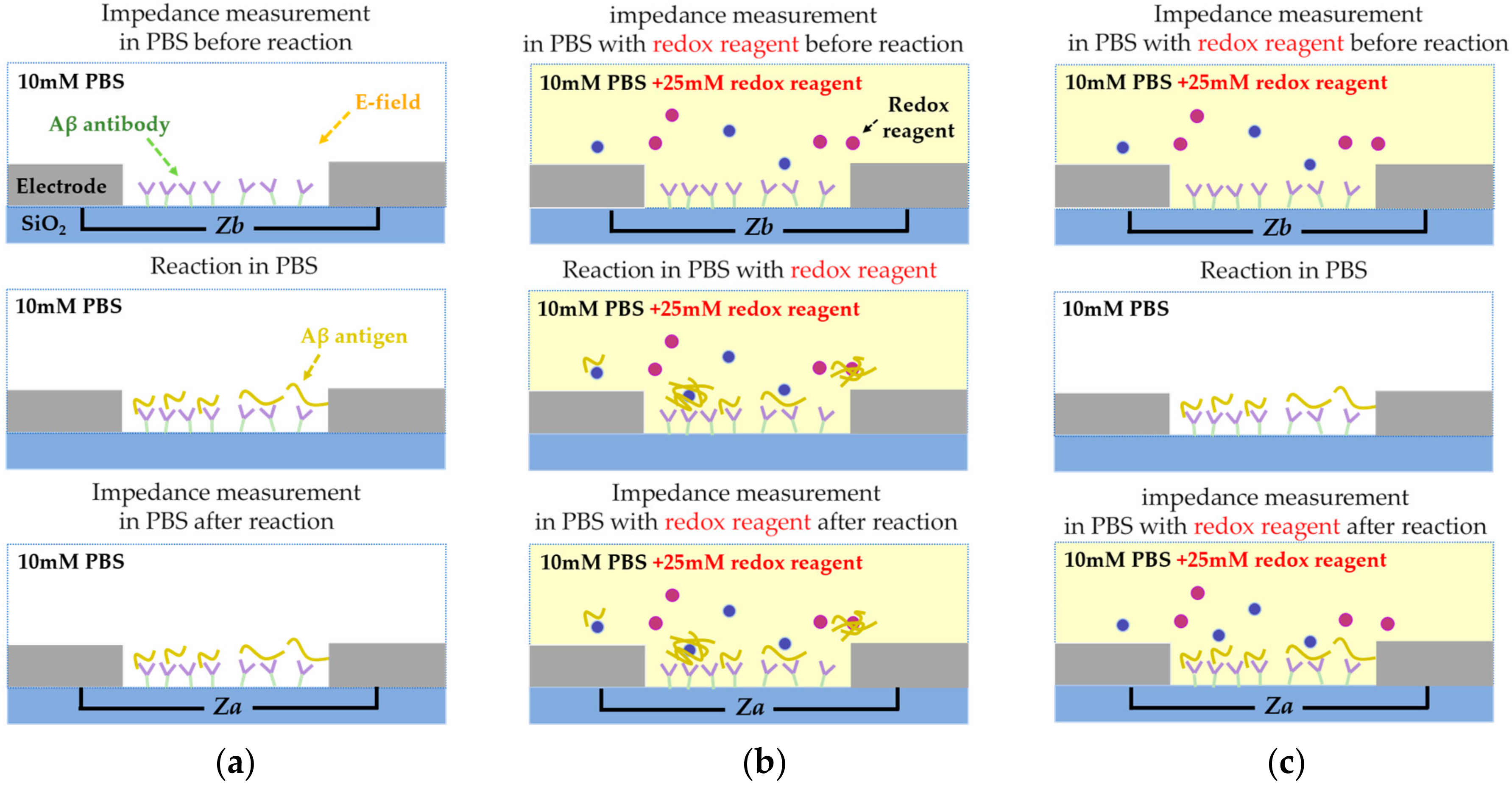
2. Model Evaluation of Each nf-EIS-based and f-EIS-based IME Sensor
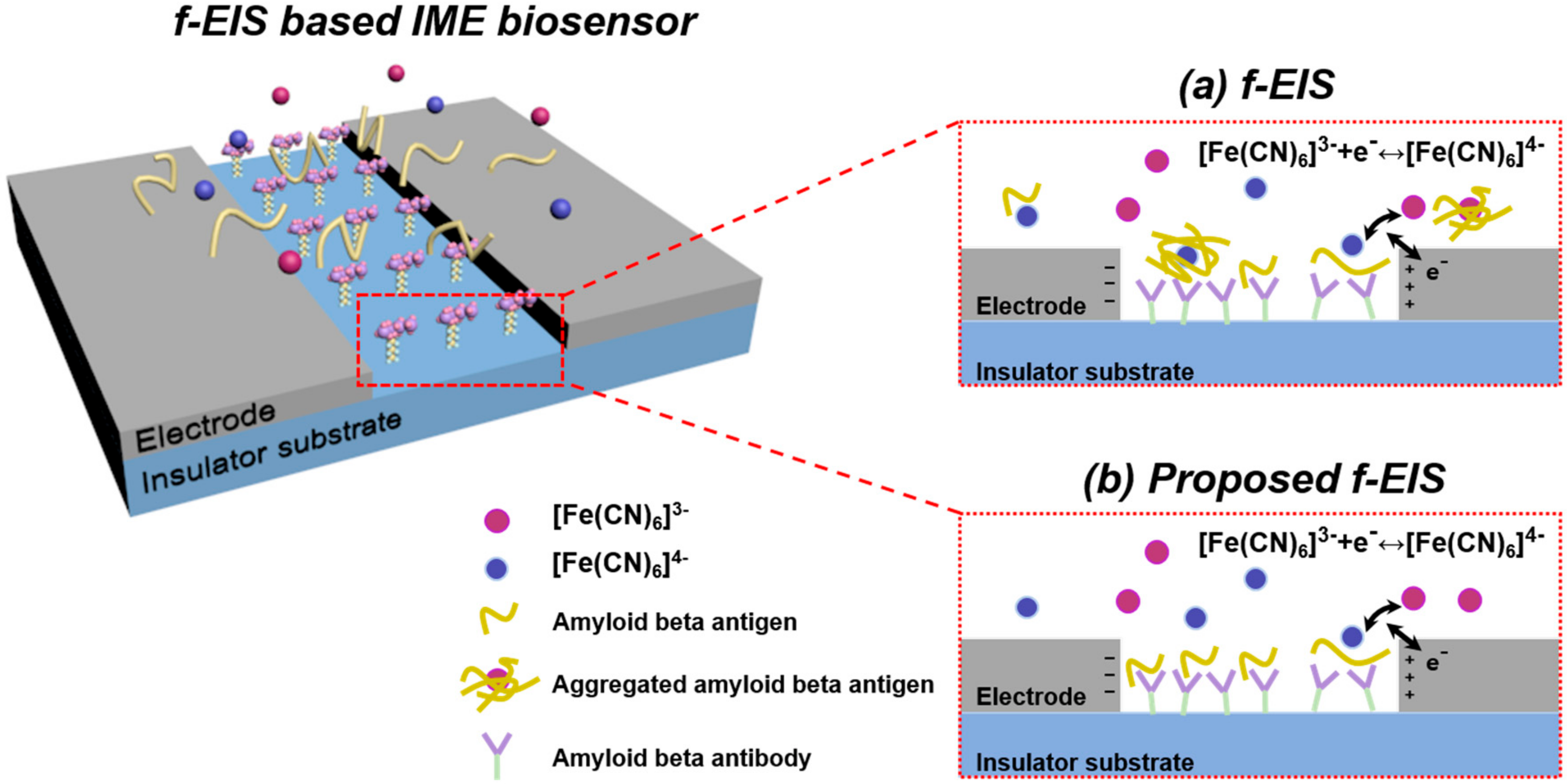
3. The Proposed f-EIS-Based Interdigitated Microelectrode Sensor
4. Methods
4.1. Chemical
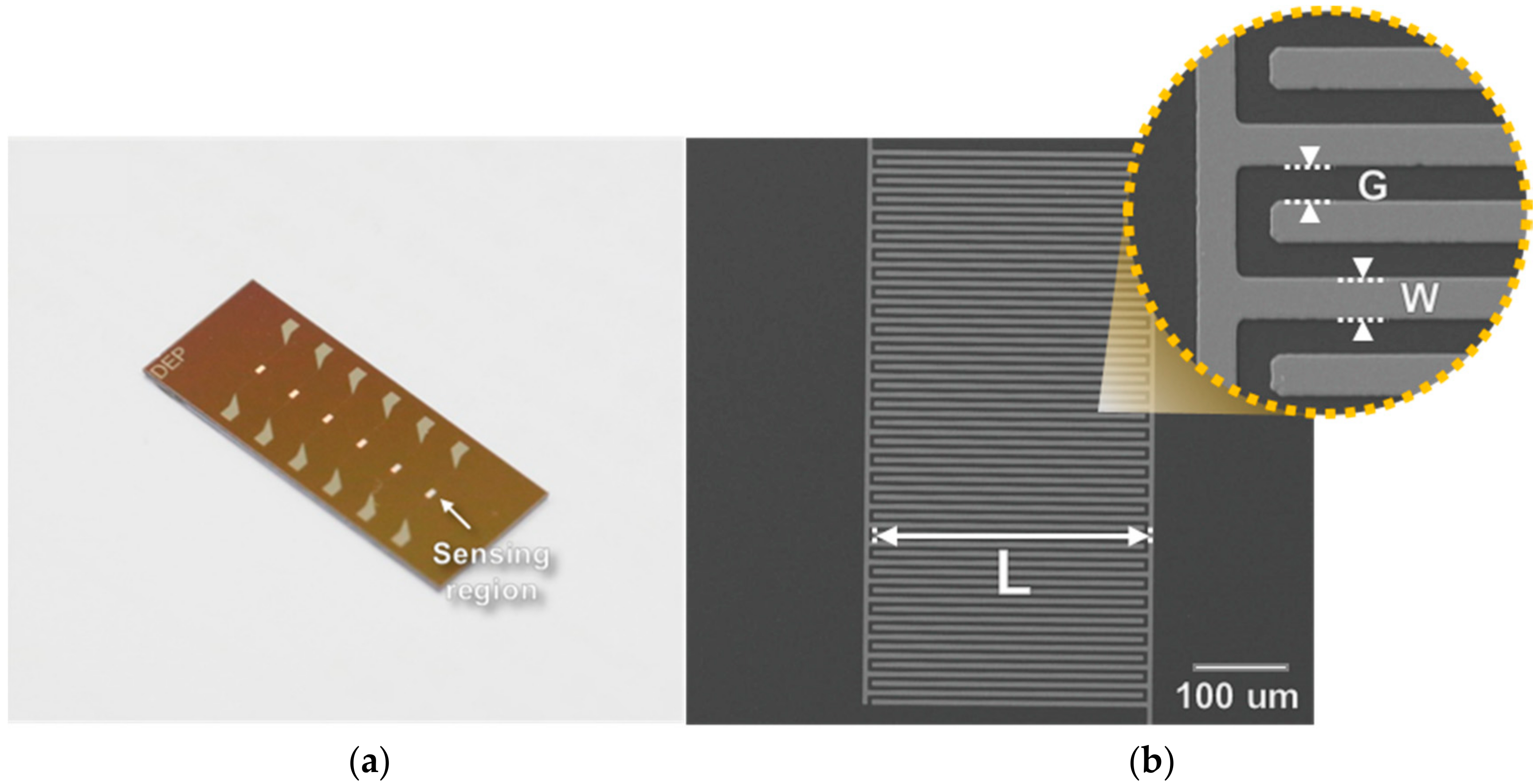
4.2. Interdigitated Microelectrode Sensor
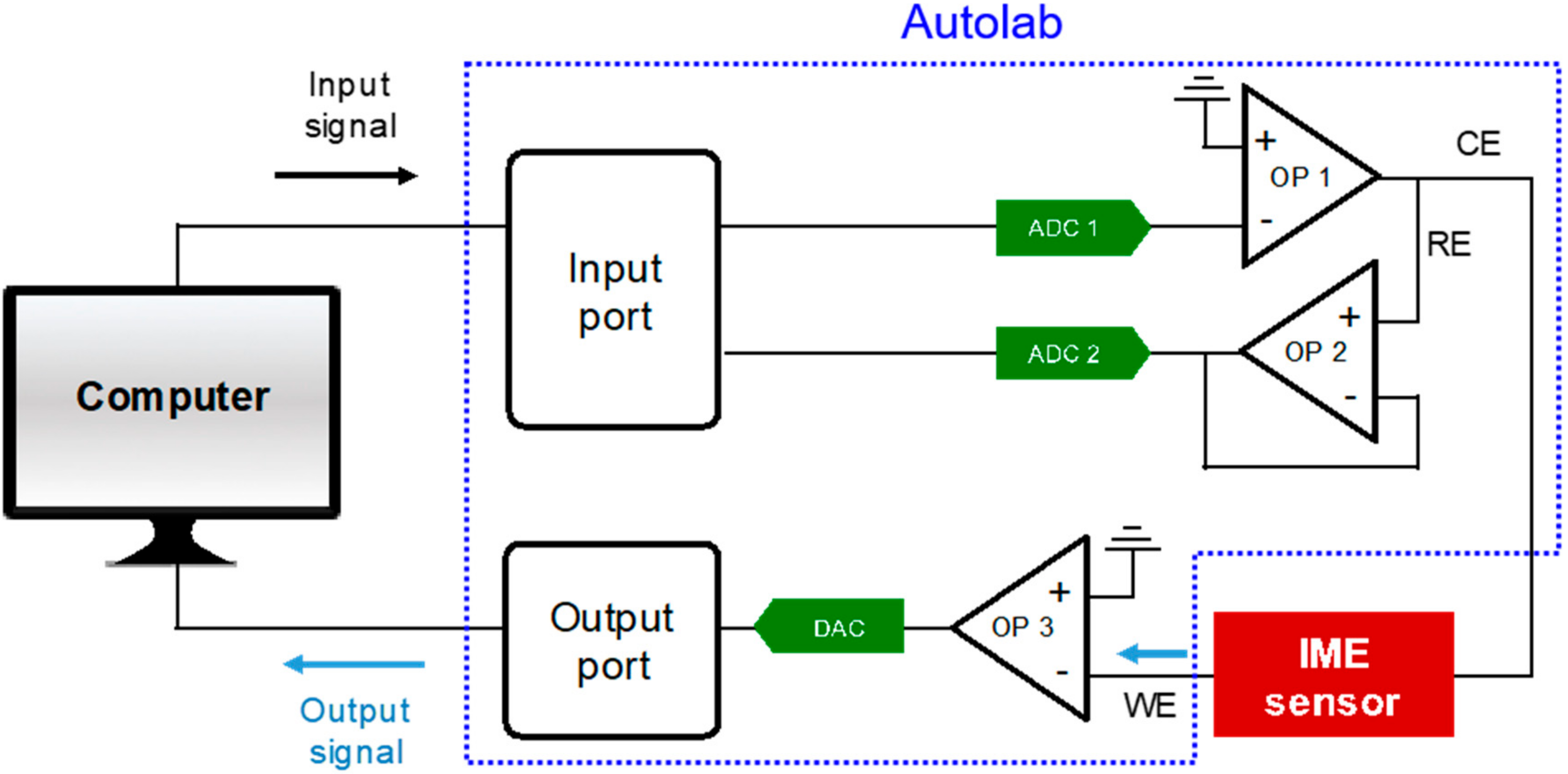
4.3. Impedance Measurement
4.4. Experimental Process
5. Results and Discussion
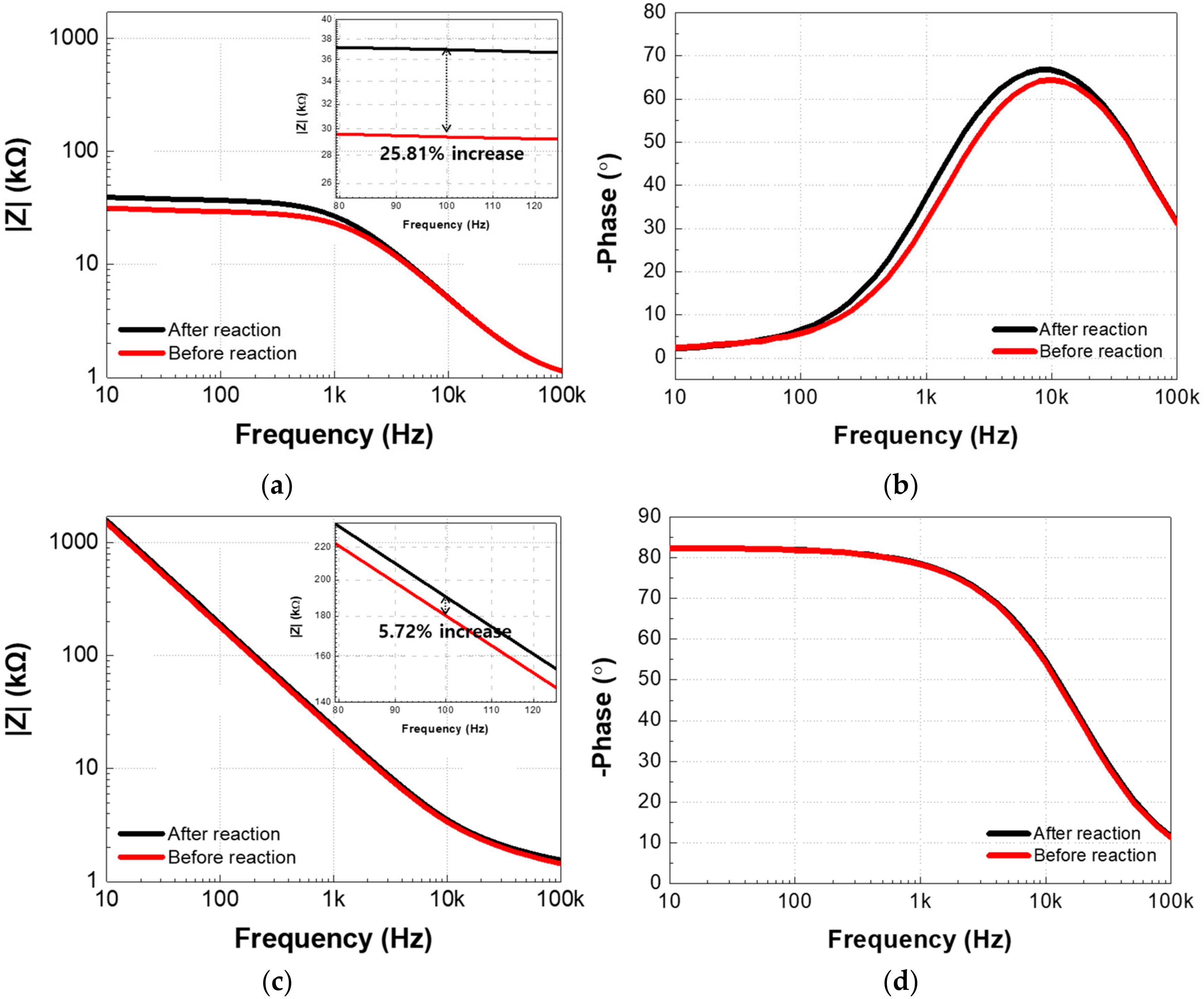
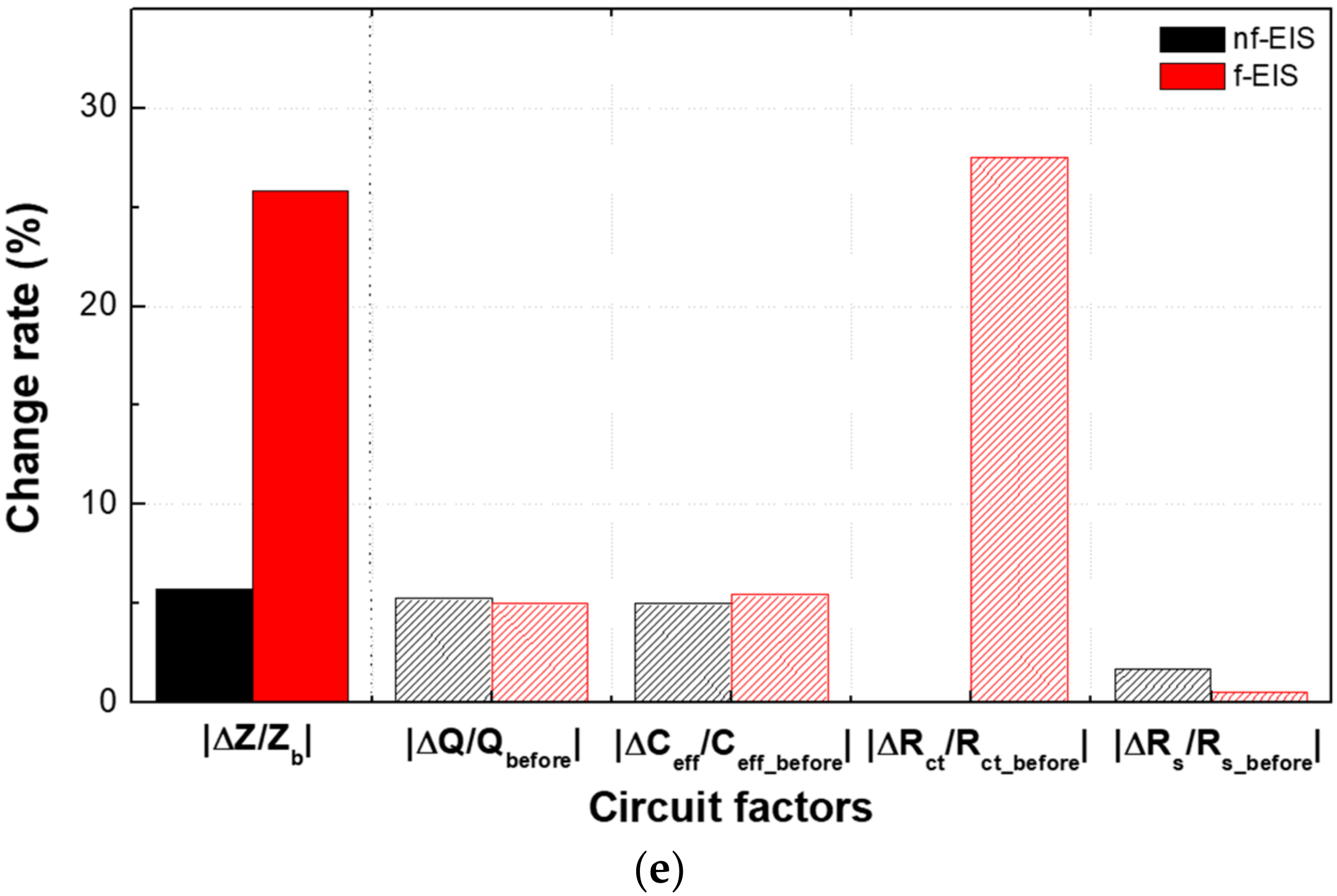
5.1. The Non-Faradaic and Faradaic Electrochemical Impedance Spectroscopy-Based IME Sensors
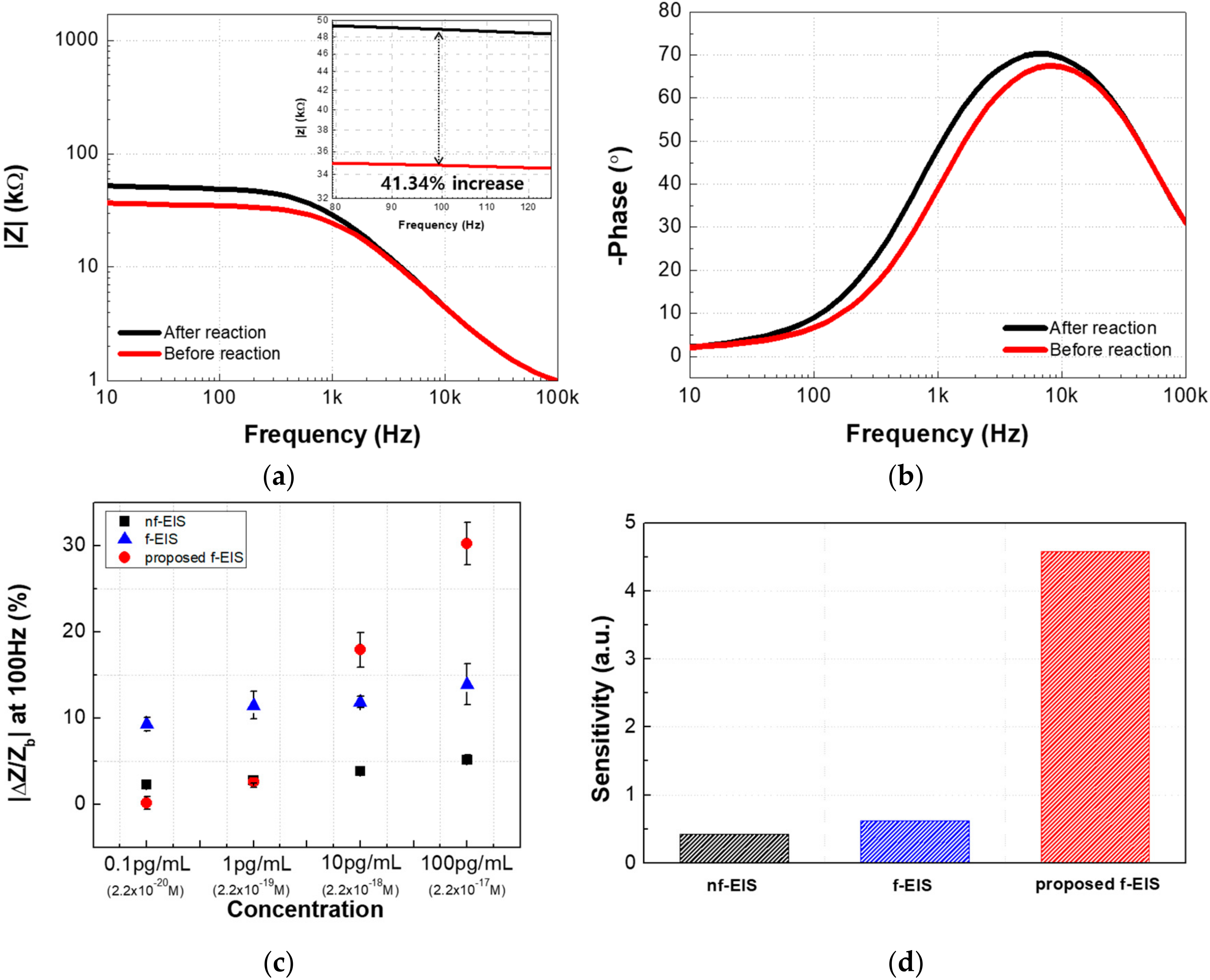
5.2. The Proposed Faradaic Electrochemical Impedance Spectroscopy -Based IME Sensor
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Probing biomolecular interactions at conductive and semiconductive surface by impedance spectroscopy: Routes to impedimetric immunosensors, DNA-sensors, and enzyme biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Yoetz-Kopelman, T.; Ram, Y.; Freeman, A.; Shacham-Diamand, Y. Faradaic Impedance Spectroscopy for Detection of Small Molecules Binding using the Avidin-Biotin Model. Electrochim. Acta 2015, 173, 630–635. [Google Scholar] [CrossRef]
- Michaels, P.; Alam, M.T.; Ciampi, S.; Rouesnel, W.; Parker, S.G.; Choudhury, M.H.; Gooding, J.J. A robust DNA interface on a silicon electrode. Chem. Commun. 2014, 50, 7878–7880. [Google Scholar] [CrossRef] [PubMed]
- Hardly, J.; Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Chen, W.T.; Liao, Y.H.; Yu, H.M.; Cheng, I.H.; Chen, Y.R. Distinct effects of Zn2+, Cu2+, Fe3+, and Al3+ on amyloid-beta stability, oligomerization, and aggregation: Amyloid-β destabilization promotes annular protofibril formation. J. Biol. Chem. 2011, 286, 9646–9656. [Google Scholar] [CrossRef] [PubMed]
- Hung, V.W.S.; Cheng, X.R.; Li, N.; Veloso, A.J.; Keramn, K. Electrochemical Detection of Amyloid-β Aggregation in the Presence of Resveratrol. J. Electrochem. Soc. 2013, 160, G3097–G3101. [Google Scholar] [CrossRef]
- Randles, J.E.B. Kinetics of rapid electrode reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfa. Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Cordoba-Torres, P.; Mesquia, T.J.; Nogueira, R.P. Relationship between the Origin of Constant-Phase Element Behavior in Electrochemical Impedance Spectroscopy and Electrode Surface Structure. J. Phys. Chem. C 2015, 119, 4136–4147. [Google Scholar] [CrossRef]
- Wang, H.; Pilon, L. Accurate Simulation of Electric Double Layer Capacitance of Ultramicroelectrodes. J. Phys. Chem. C 2011, 115, 16711–16719. [Google Scholar] [CrossRef]
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive Biosensors. Electroanalysis 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Liu, J.; Chisti, M.M.; Zeng, X. General Signal Amplification Strategy for Nonfaradaic Impedimetric Sensing: Trastuzumab Detection Employing a Peptide Immunosensor. Anal. Chem. 2017, 89, 4013–4020. [Google Scholar] [CrossRef] [PubMed]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 9781118209943. [Google Scholar]
- Huang, Y.; Bell, M.C.; Suni, I.I. Impedance Biosensor for Peanut Protein Ara h 1. Anal. Chem. 2008, 80, 9157–9161. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, J.; Yoo, Y.K.; Lee, J.H.; Park, J.H.; Hwang, K.S. Sensitivity improvement of an electrical sensor achieved by control of biomolecules based on the negative dielectrophoretic force. Biosens. Bioelectron. 2016, 85, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.S.; Kim, H.J.; Lee, J.-H.; Park, J.H.; Kim, J.; Hwang, K.S.; Lee, B.C. Amyloid Beta Detection by Faradaic Electrochemical Impedance Spectroscopy Using Interdigitated Microelectrodes. Sensors 2018, 18, 426. https://doi.org/10.3390/s18020426
Park JS, Kim HJ, Lee J-H, Park JH, Kim J, Hwang KS, Lee BC. Amyloid Beta Detection by Faradaic Electrochemical Impedance Spectroscopy Using Interdigitated Microelectrodes. Sensors. 2018; 18(2):426. https://doi.org/10.3390/s18020426
Chicago/Turabian StylePark, Jin Soo, Hye Jin Kim, Ji-Hoon Lee, Jung Ho Park, Jinsik Kim, Kyo Seon Hwang, and Byung Chul Lee. 2018. "Amyloid Beta Detection by Faradaic Electrochemical Impedance Spectroscopy Using Interdigitated Microelectrodes" Sensors 18, no. 2: 426. https://doi.org/10.3390/s18020426
APA StylePark, J. S., Kim, H. J., Lee, J.-H., Park, J. H., Kim, J., Hwang, K. S., & Lee, B. C. (2018). Amyloid Beta Detection by Faradaic Electrochemical Impedance Spectroscopy Using Interdigitated Microelectrodes. Sensors, 18(2), 426. https://doi.org/10.3390/s18020426





