Plant Pest Detection Using an Artificial Nose System: A Review
Abstract
:1. Introduction
2. Electronic Nose Detecting Technology
2.1. Gas Sensors
2.1.1. Conductivity Sensors
2.1.2. Gravimetric Sensors
2.1.3. Optical Sensors
2.2. Sampling Methods
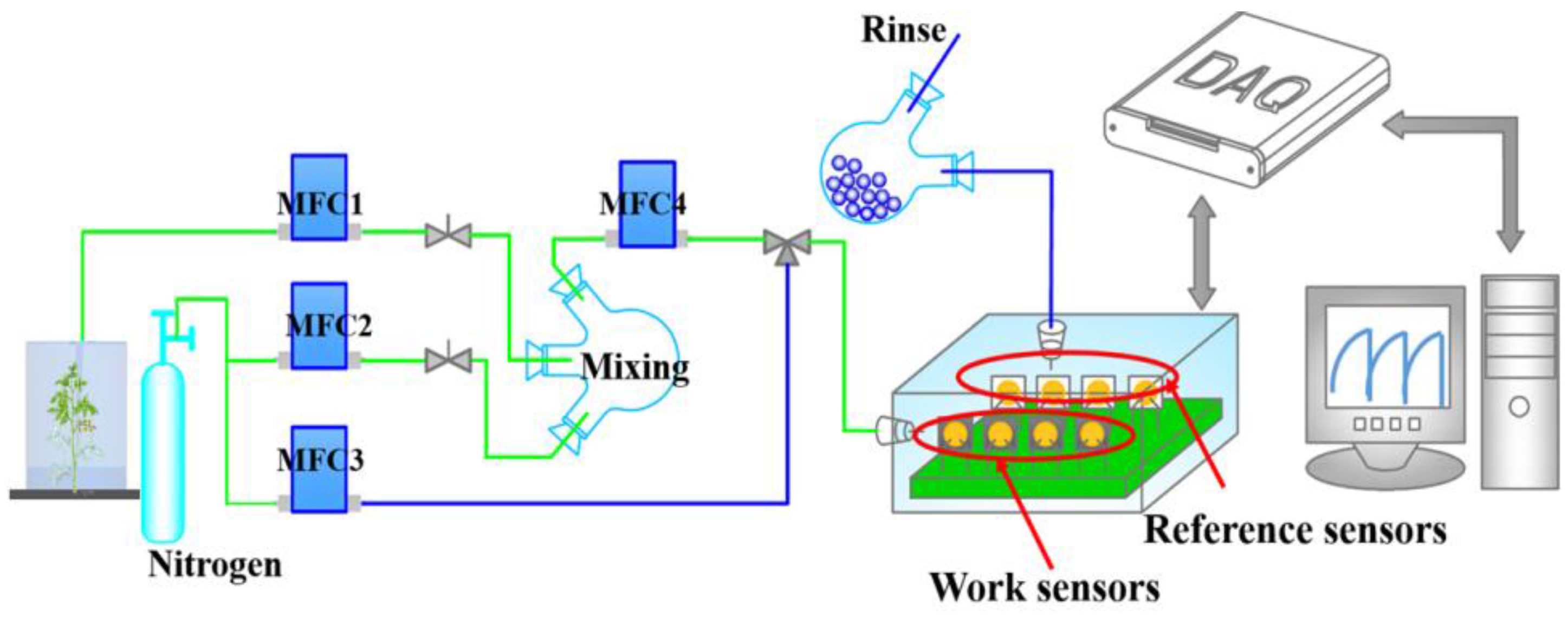
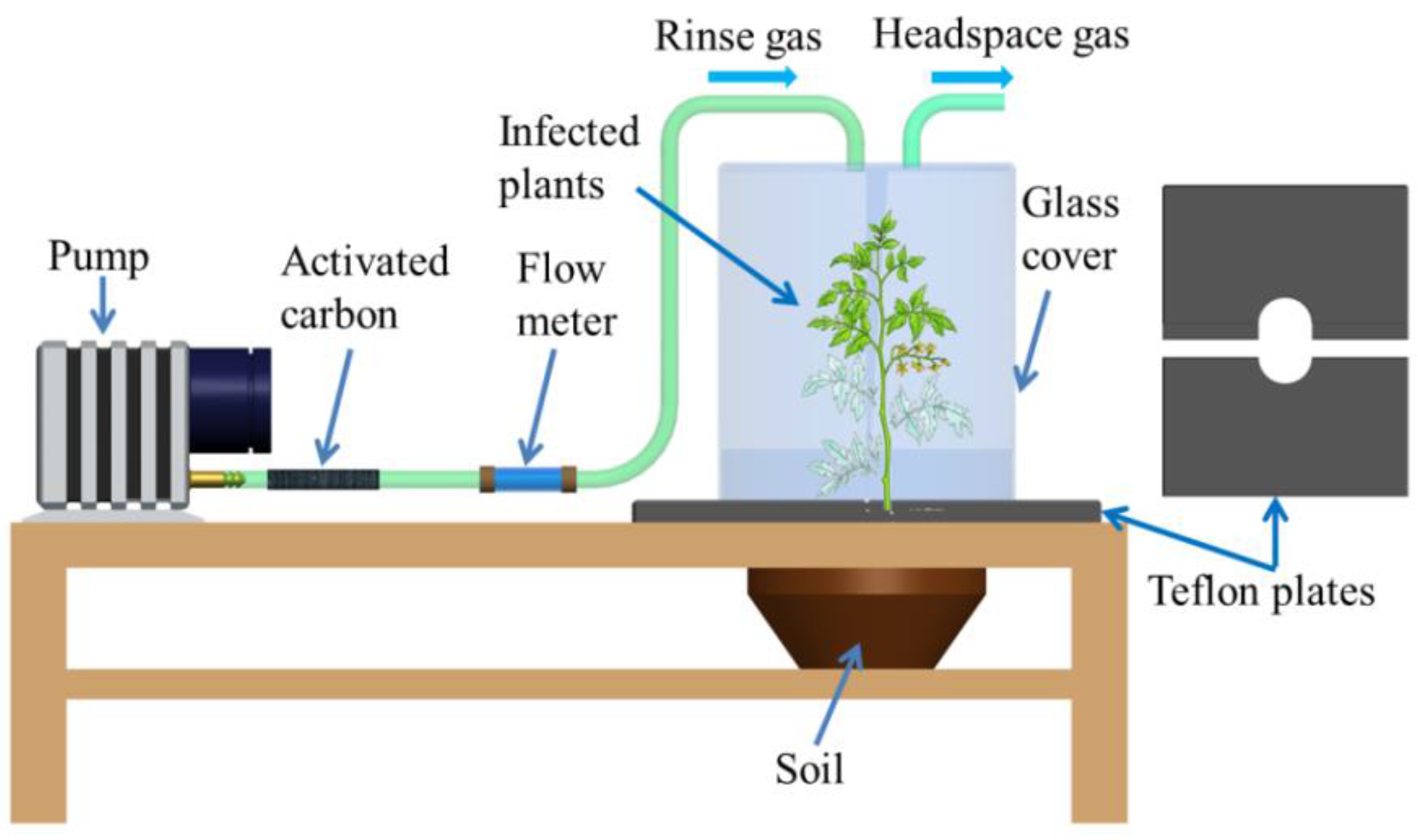
2.2.1. Laboratory Sampling
2.2.2. Field Sampling
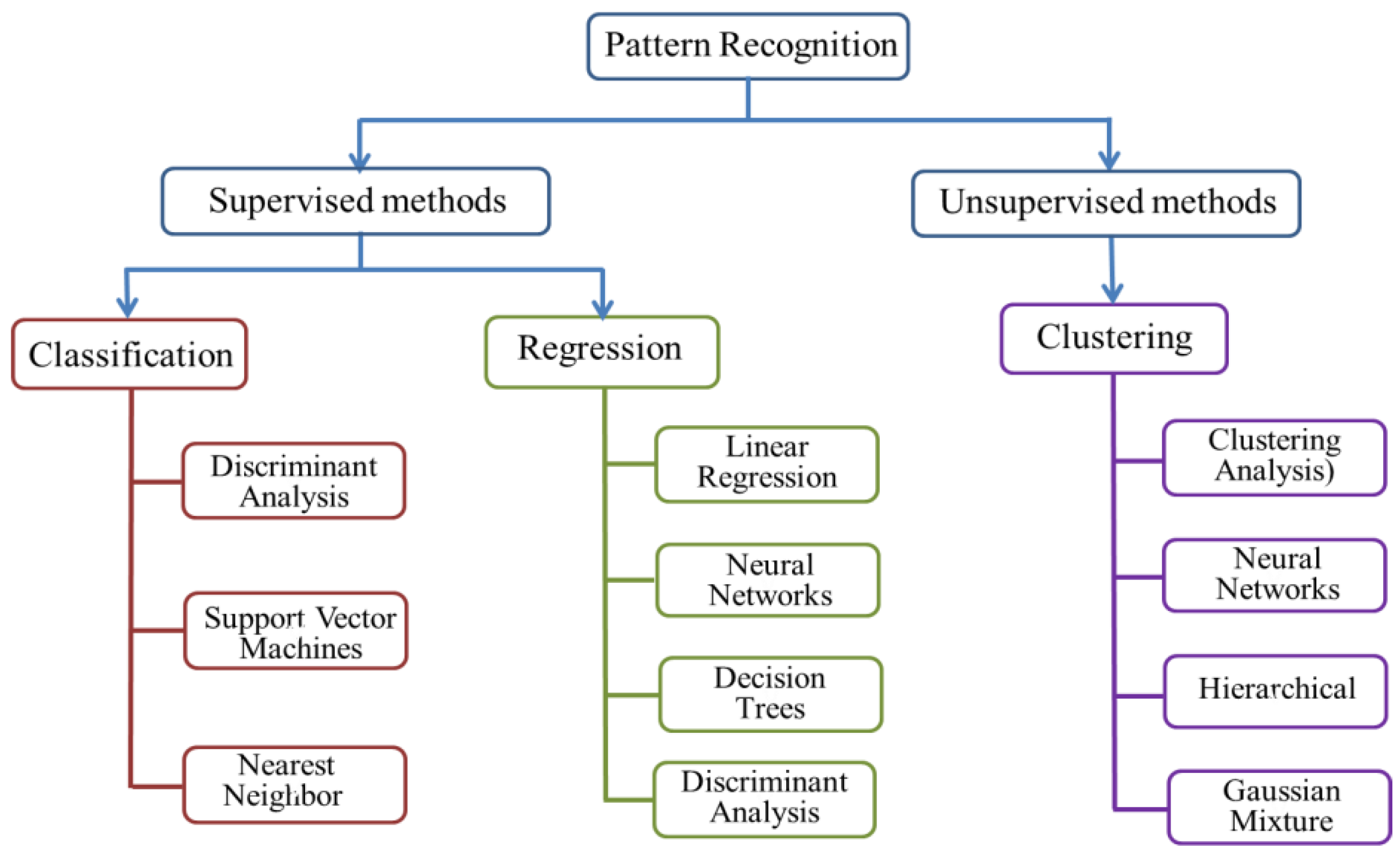
2.3. Data Analysis Methods
2.3.1. Unsupervised Statistical Methods
2.3.2. Supervised Statistical Methods
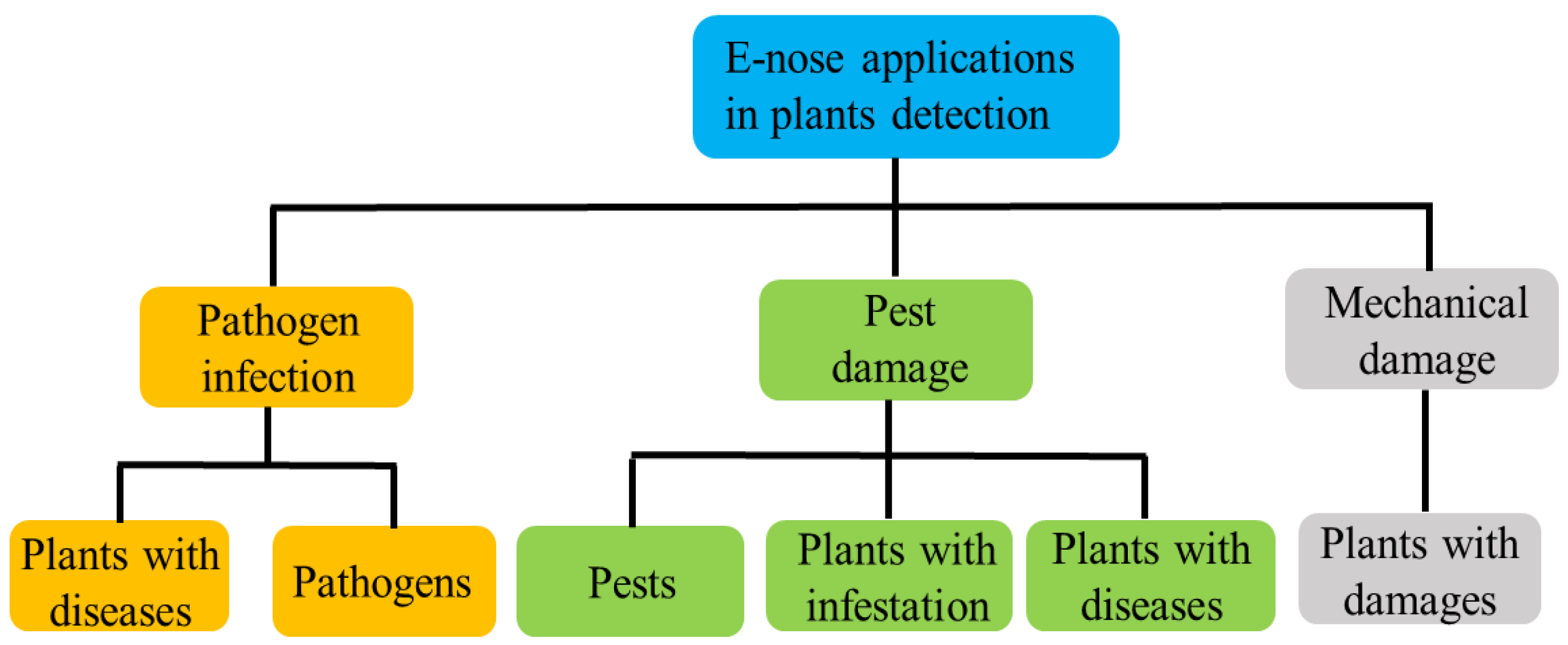
3. Applications in Plant Diagnosis
3.1. Fungal and Bacterial Disease Infections
3.2. Insect Damage
3.3. Mechanical Damage
4. Challenges and Improvements
4.1. Dynamic Nature of VOCs
4.2. Environmental Effects on Sensing
4.3. Detection in Field Conditions
4.4. Plant Pest Specific Detection Technique Optimization
4.5. Combinations with Other Advanced Technologies
4.6. Micro E-Noses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nishi, K.; Isobe, S.-I.; Zhu, Y.; Kiyama, R. Fluorescence-Based Bioassays for the Detection and Evaluation of Food Materials. Sensors 2015, 15, 25831–25867. [Google Scholar] [CrossRef] [PubMed]
- Nassuth, A.; Pollari, E.; Helmeczy, K.; Stewart, S.; Kofalvi, S.A. Improved RNA extraction and one-tube RT-PCR assay for simultaneous detection of control plant RNA plus several viruses in plant extracts. J. Virol. Methods 2000, 90, 37–49. [Google Scholar] [CrossRef]
- Clark, M.F.; Adams, A.N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Mahlein, A.K.; Oerke, E.C.; Steiner, U.; Dehne, H.W. Recent advances in sensing plant diseases for precision crop protection. Eur. J. Plant Pathol. 2012, 133, 197–209. [Google Scholar] [CrossRef]
- Bürling, K.; Hunsche, M.; Noga, G. Use of blue-green and chlorophyll fluorescence measurements for differentiation between nitrogen deficiency and pathogen infection in winter wheat. J. Plant Physiol. 2011, 168, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Umasankar, Y.; Ramasamy, R.P. Electrochemical detection of p-ethylguaiacol, a fungi infected fruit volatile using metal oxide nanoparticles. Analyst 2014, 139, 3804–3810. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Ray, A.; Dash, S.; Mishra, A.; Achary, K.G.; Nayak, S.; Singh, S. Fungal disease detection in plants: Traditional assays, novel diagnostic techniques and biosensors. Biosens. Bioelectron. 2017, 87, 708–723. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef]
- Kuzdralinski, A.; Kot, A.; Szczerba, H.; Nowak, M.; Muszyriska, M. A review of conventional PCR Assays for the detection of selected phytopathogens of wheat. J. Mol. Microbiol. Biotechnol. 2017, 27, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, D.K.; Park, E.; Nagendran, R.; Hung, N.B.; Cho, B.-K.; Kim, K.-H.; Lee, Y.H. Visual Analysis for Detection and Quantification of Pseudomonas cichorii Disease Severity in Tomato Plants. Plant Pathol. J. 2016, 32, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Cellini, A.; Biondi, E.; Blasioli, S.; Rocchi, L.; Farneti, B.; Braschi, I.; Savioli, S.; Rodriguez-Estrada, M.T.; Biasioli, F.; Spinelli, F. Early detection of bacterial diseases in apple plants by analysis of volatile organic compounds profiles and use of electronic nose. Ann. Appl. Biol. 2016, 168, 409–420. [Google Scholar] [CrossRef]
- Fang, Y.; Ramasamy, R.P. Current and prospective methods for plant disease detection. Biosensors 2015, 5, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Eun, A.J.C.; Huang, L.; Chew, F.T.; Li, S.F.Y.; Wong, S.M. Detection of two orchid viruses using quartz crystal microbalance (QCM) immunosensors. J. Virol. Methods 2002, 99, 71–79. [Google Scholar] [CrossRef]
- Eun, A.J.-C.; Huang, L.; Chew, F.-T.; Fong-Yau Li, S.; Wong, S.-M. Detection of two orchid viruses using quartz crystal microbalance-based DNA biosensors. Phytopathology 2002, 92, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Umasankar, Y.; Ramasamy, R.P. A novel bi-enzyme electrochemical biosensor for selective and sensitive determination of methyl salicylate. Biosens. Bioelectron. 2016, 81, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.M.M.; Marriott, P.J.; Galbally, I.E. Headspace solid-phase microextraction—Comprehensive two-dimensional gas chromatography of wound induced plant volatile organic compound emissions. Analyst 2002, 127, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Kesselmeier, J.; Staudt, M. Biogenic Volatile Organic Compound (VOC): An Overview on Emissions, Physiology and Ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Lowe, A.; Harrison, N.; French, A.P. Hyperspectral image analysis techniques for the detection and classification of the early onset of plant disease and stress. Plant Methods 2017, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Csefalvay, L.; Gaspero, G.D.; Matous, K.; Bellin, D.; Ruperti, B.; Olejnickova, J. Pre-symptomatic detection of Plasmopara viticola infection in grapevine leaves using chlorophyll fluorescence imaging. Eur. J. Physical. Med. Rehab. 2009, 125, 291–302. [Google Scholar] [CrossRef]
- Perez, E.; Saavedra, J.; Crawford, P.; Arias, S. Recovery and creative practices in people with severe mental illness: Evaluating well-being and social inclusion. Disabil. Rehabilit. 2018, 40, 905–911. [Google Scholar] [CrossRef]
- López, M.M.; Bertolini, E.; Olmos, A.; Caruso, P.; Gorris, M.T.; Llop, P.; Penyalver, R.; Cambra, M. Innovative tools for detection of plant pathogenic viruses and bacteria. Int. Microbiol. 2003, 6, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Wallner, G.; Amann, R.; Beisker, W. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 1993, 14, 136–143. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.; Wickham, G.; Pace, N. Phylogenetic stains: Ribosomal RNA-based probes for the identification of single cells. Science 1989, 243, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Schaad, N.W.; Frederick, R.D. Real-time PCR and its application for rapid plant disease diagnostics. Can. J. Plant Pathol. 2002, 24, 250–258. [Google Scholar] [CrossRef]
- Lau, H.Y.; Botella, J.R. Advanced DNA-Based Point-of-Care Diagnostic Methods for Plant Diseases Detection. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Moter, A.; Göbel, U.B. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J. Microbiol. Methods 2000, 41, 85–112. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Shaikh, P.M.M.; Butala, N.; Sharma, S.; Sukumaran, S. Review on Diagnosis the Plant Disease at Early Stage. Int. J. Adv. Res. Comput. Commun. Eng. 2017, 6, 321–324. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Preston, C.A. Volatile Signaling in Plant-Plant Interactions: “Talking Trees” in the Genomics Era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.K.; Johri, B.N.; Prakash, A. Volatiles as priming agents that initiate plant growth and defence responses. Curr. Sci. 2008, 94, 595–604. [Google Scholar]
- Pare, P.W.; Tumlinson, J.H. Update on plant-insect interactions plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Sites of synthesis, biochemistry and functional role of plant volatiles. S. Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef]
- Nusaibah, S.A.; Siti Nor Akmar, A.; Idris, A.S.; Sariah, M.; Mohamad Pauzi, Z. Involvement of metabolites in early defense mechanism of oil palm (Elaeis guineensis Jacq.) against Ganoderma disease. Plant Physiol. Biochem. 2016, 109, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Flematti, G.R.; Davis, T.M.; Suzuki, M.; Homig, H.; Benz-Bohm, G.; Kreuder, J.; Younossi-Hartenstein, A.; Sauvanet, A.; Couvelard, A.; et al. Detection of carbon disulfide in breath and air: A possible new risk factor for coronary artery disease. Int. Arch. Occup. Environ. Health 1992, 64, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, R.; Laothawornkitkul, J.; Iliescu, D.; Hines, E.; Leeson, M.; Napier, R.; Moore, J.P.; Paul, N.D.; Hewitt, C.N.; Taylor, J.E. Plant pest and disease diagnosis using electronic nose and support vector machine approach. J. Plant Dis. Prot. 2012, 119, 200–207. [Google Scholar] [CrossRef]
- Wilson, A.D. Diverse applications of electronic-nose technologies in agriculture and forestry. Sensors 2013, 13, 2295–2348. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tian, F.; Yang, S.X.; Li, Q.; Yan, J.; Ma, J. A solid trap and thermal desorption system with application to a medical electronic nose. Sensors 2008, 8, 6885–6898. [Google Scholar] [CrossRef] [PubMed]
- Estefania Nunez Carmona, E.; Sberveglieri, V.; Ponzoni, A.; Galstyan, V.; Zappa, D.; Pulvirenti, A.; Comini, E. Detection of food and skin pathogen microbiota by means of an electronic nose based on metal oxide chemiresistors. Sens. Actuators B Chem. 2017, 238, 1224–1230. [Google Scholar] [CrossRef]
- Xu, S.; Lü, E.; Lu, H.; Zhou, Z.; Wang, Y.; Yang, J.; Wang, Y. Quality Detection of Litchi Stored in Different Environments Using an Electronic Nose. Sensors 2016, 16, 852. [Google Scholar] [CrossRef] [PubMed]
- Messina, V.; Domínguez, P.G.; Sancho, A.M.; Walsöe de Reca, N.; Carrari, F.; Grigioni, G. Tomato Quality during Short-Term Storage Assessed by Colour and Electronic Nose. Int. J. Electrochem. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Baietto, M.; Wilson, A.D.; Bassi, D.; Ferrini, F. Evaluation of Three Electronic Noses for Detecting Incipient Wood Decay. Sensors. 2010, 10, 1062–1092. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.Q.; Li, S.; Huang, Y.H.; Liu, Y.; Bauer, R.; Peng, L.; Tao, O.; Yan, S.R.; Yan, Y.H. Rapid identification of Asteraceae plants with improved RBF-ANN classification models based on MOS sensor E-nose. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, F.; Cellini, A.; Vanneste, J.L.; Rodriguez-Estrada, M.T.; Costa, G.; Savioli, S.; Harren, F.J.M.; Cristescu, S.M. Emission of volatile compounds by Erwinia amylovora: Biological activity in vitro and possible exploitation for bacterial identification. Trees Struct. Funct. 2012, 26, 141–152. [Google Scholar] [CrossRef]
- Chang, K.P.P.; Zakaria, A.; Nasir, A.S.A.; Yusuf, N.; Thriumani, R.; Shakaff, A.Y.M.; Adom, A.H. Analysis and Feasibility Study of Plant Disease using E-Nose. In Proceedings of the 2014 IEEE International Conference on Control System, Computing and Engineering (ICCSCE 2014), Batu Ferringhi, Malaysia, 28–30 November 2014. [Google Scholar]
- Lan, Y.-B.; Zheng, X.-Z.; Westbrook, J.K.; Lopez, J.; Lacey, R.; Hoffmann, W.C. Identification of Stink Bugs Using an Electronic Nose. J. Bionic Eng. 2008, 5, 172–180. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J. Discrimination of different types damage of rice plants by electronic nose. Biosyst. Eng. 2011, 109, 250–257. [Google Scholar] [CrossRef]
- Costa, A.; Tangorra, F.M.; Zaninelli, M.; Oberti, R.; Cavalchini, A.G.; Savoini, G.; Lazzari, M. Evaluating an e-nose ability todetect biogas plant efficiency: a case study. Ital. J. Anim. Sci. 2016, 15, 116–123. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G.; Tortorella, F. Gas recognition by activated WO3 thin-film sensors array. Sens. Actuators B Chem. 2001, 81, 115–121. [Google Scholar] [CrossRef]
- Gebicki, J. Application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. TrAC Trends Anal. Chem. 2016, 77, 1–13. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Pham, C.; Chang, J.P. Potentials and challenges of integration for complex metal oxides in CMOS devices and beyond. J. Phys. D Appl. Phys. 2015, 48, 63001. [Google Scholar] [CrossRef]
- Albert, K.J.; Lewis, N.S.; Schauer, C.L.; Sotzing, G.A.; Stitzel, S.E.; Vaid, T.P.; Walt, D.R. Cross-Reactive Chemical Sensor Arrays. Chem. Rev. 2000, 100, 2595–2626. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Yang, L.; Wang, J.; Wang, X. Fabrication of a sensitive gas sensor based on PPy/TiO2 nanocomposites films by layer-by-layer self-assembly and its application in food storage. Sensors Actuators B Chem. 2016, 233, 337–346. [Google Scholar] [CrossRef]
- Cui, S.; Wang, J.; Wang, X. Fabrication and design of a toxic gas sensor based on polyaniline/titanium dioxide nanocomposite film by layer-by-layer self-assembly. RSC Adv. 2015, 5, 58211–58219. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A Review of Gas Sensors Employed in Electronic Nose Applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Askim, J.R.; Mahmoudi, M.; Suslick, K.S. Optical sensor arrays for chemical sensing: The optoelectronic nose. Chem. Soc. Rev. 2013, 42, 8649–8682. [Google Scholar] [CrossRef] [PubMed]
- Rakow, N.A.; Suslick, K.S. A colorimetric sensor array for odour visualization. Nature 2000, 406, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. An optoelectronic nose: “Seeing” smells by means of colorimetric sensor arrays. MRS Bull. 2004, 29, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Janzen, M.C.; Ponder, J.B.; Bailey, D.P.; Ingison, C.K.; Suslick, K.S. Colorimetric sensor Arrays for volatile organic compounds. Anal. Chem. 2006, 78, 3591–3600. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Musto, C.J.; Kemling, J.W.; Lim, S.H.; Zhong, W.; Suslick, K.S. Colorimetric sensor array for determination and identification of toxic industrial chemicals. Anal. Chem. 2010, 82, 9433–9440. [Google Scholar] [CrossRef] [PubMed]
- Kemling, J.W.; Suslick, K.S. Nanoscale porosity in pigments for chemical sensing. Nanoscale 2011, 3, 1971–1973. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J.; Wang, X.F.; Xu, Y.; Mekhail, T.; Beukemann, M.C.; Na, J.; Kemling, J.W.; Suslick, K.S.; Sasidhar, M. Exhaled breath analysis with a colorimetric sensor array for the identification and characterization of lung cancer. J. Thorac. Oncol. 2012, 7, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.; Minaei, S.; Ghasemi-Varnamkhasti, M. Application of electronic nose systems for assessing quality of medicinal and aromatic plant products: A review. J. Appl. Res. Med. Aromat. Plants 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Advances in electronic-nose technologies developed for biomedical applications. Sensors 2011, 11, 1105–1176. [Google Scholar] [CrossRef] [PubMed]
- Filenko, D.D. Chemical Gas Sensors based on Functionalized Self-Actuated Piezo-Resistive Cantilevers. Dissertation zur Erlangung des akademischen Grades eines Doktors. Ph.D. Thesis, Universität Kassel, Kassel, Germany, 2008. [Google Scholar]
- Chiu, S.W.; Tang, K.T. Towards a chemiresistive sensor-integrated electronic nose: A review. Sensors 2013, 13, 14214–14247. [Google Scholar] [CrossRef] [PubMed]
- Tewari, J.C.; Irudayaraj, J.M.K. Floral classification of honey using mid-infrared spectroscopy and surface acoustic wave based z-Nose sensor. J. Agric. Food Chem. 2005, 53, 6955–6966. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, R.; Zhang, F.; Iliescu, D.D.; Hines, E.L.; Leeson, M.S.; Napier, R. Detection of Diseases and Volatile Discrimination of Plants: An Electronic Nose and Self-Organizing Maps Approach. In Intelligent Systems for Machine Olfaction: Tools and Methodologies; IGI Global: Hershey, PA, USA, 2011; pp. 214–230. [Google Scholar]
- Zhang, F.; Iliescu, D.D.; Hines, E.L.; Leeson, M.S. Tomato Plant Health Monitoring: An Electronic Nose Approach. In Intelligent Systems for Machine Olfaction: Tools and Methodologies; IGI Global: Hershey, PA, USA, 2011; pp. 231–248. [Google Scholar]
- Spinelli, F.; Noferini, M.; Vanneste, J.L.; Costa, G. Potential of the electronic-nose for the diagnosis of bacterial and fungal diseases in fruit trees. EPPO Bull. 2010, 40, 59–67. [Google Scholar] [CrossRef]
- Markom, M.A.; Shakaff, A.Y.M.; Adom, A.H.; Ahmad, M.N.; Hidayat, W.; Abdullah, A.H.; Fikri, N.A. Intelligent electronic nose system for basal stem rot disease detection. Comput. Electron. Agric. 2009, 66, 140–146. [Google Scholar] [CrossRef]
- Biondi, E.; Blasioli, S.; Galeone, A.; Spinelli, F.; Cellini, A.; Lucchese, C.; Braschi, I. Detection of potato brown rot and ring rot by electronic nose: From laboratory to real scale. Talanta 2014, 129, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Yangali-Quintanilla, V.; Verliefde, A.; Kim, T.U.; Sadmani, A.; Kennedy, M.; Amy, G. Artificial neural network models based on QSAR for predicting rejection of neutral organic compounds by polyamide nanofiltration and reverse osmosis membranes. J. Memb. Sci. 2009, 342, 251–262. [Google Scholar] [CrossRef]
- Wu, B.; Quilot, B.; Kervella, J.; Génard, M.; Li, S. Analysis of genotypic variation of sugar and acid contents in peaches and nectarines through the Principle Component Analysis. Euphytica 2003, 132, 375–384. [Google Scholar] [CrossRef]
- Belous, O.; Malyarovskaya, V.; Klemeshova, K. Diagnostics of subtropical plants functional state by cluster analysis. Potravinarstvo 2016, 10, 237–242. [Google Scholar] [CrossRef]
- Laothawornkitkul, J.; Moore, J.P.; Taylor, J.E.; Possell, M.; Gibson, T.D.; Hewitt, C.N.; Paul, N.D. Discrimination of plant volatile signatures by an electronic nose: A potential technology for plant pest and disease monitoring. Environ. Sci. Technol. 2008, 42, 8433–8439. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.H.; Shakaff, A.Y.M.; Zakaria, A.; Saad, F.S.A.; Shukor, S.A.A.; Mat, A. Application Specific Electronic Nose (ASEN) for Ganoderma boninense detection using artificial neural network. In Proceedings of the 2014 2nd International Conference on Electronic Design (ICED), Penang, Malaysia, 19–21 August 2014; pp. 148–152. [Google Scholar] [CrossRef]
- Xiaobo, Z.; Jiewen, Z. Comparative analyses of apple aroma by a tin-oxide gas sensor array device and GC/MS. Food Chem. 2008, 107, 120–128. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, J. The prediction of food additives in the fruit juice based on electronic nose with chemometrics. Food Chem. 2017, 230, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Adam, E.; Deng, H.; Odindi, J.; Abdel-Rahman, E.M.; Mutanga, O. Detecting the Early Stage of Phaeosphaeria Leaf Spot Infestations in Maize Crop Using in situ Hyperspectral Data and Guided Regularized Random Forest Algorithm. J. Spectrosc. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Knauer, U.; Matros, A.; Petrovic, T.; Zanker, T.; Scott, E.S.; Seiffert, U. Improved classification accuracy of powdery mildew infection levels of wine grapes by spatial-spectral analysis of hyperspectral images. Plant Methods 2017, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; De Moraes, C.M. Plant defense priming against herbivores: Getting ready for a different battle. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.T.; Lenardis, A.; Fuente de la, E.B. Insect assemblies related to volatile signals emitted by different soybean – weeds – herbivory combinations. Agric. Ecosyst. Environ. 2018, 255, 20–26. [Google Scholar] [CrossRef]
- Wilson, A.D.; Lester, D.G.; Oberle, C.S. Development of conductive polymer analysis for the rapid detection and identification of phytopathogenic microbes. Phytopathology 2004, 94, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Green, G.C.; Chan, A.D.C.; Dan, H.; Lin, M. Using a metal oxide sensor (MOS)-based electronic nose for discrimination of bacteria based on individual colonies in suspension. Sens. Actuators B Chem. 2011, 152, 21–28. [Google Scholar] [CrossRef]
- Cheng, S.-M.; Wang, J.; Wang, Y.-W.; Wei, Z.-B. Discrimination of Different Types Damage of Tomato Seedling by Electronic Nose. ITM Web Conf. 2017, 11, 1–8. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Z.; Lu, H.; Luo, X.; Lan, Y.; Zhang, Y.; Li, Y. Estimation of the age and amount of brown rice plant hoppers based on bionic electronic nose use. Sensors 2014, 14, 18114–18130. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; dos Santos, A.C.; Rossato, M.; Pansera, M.R.; dos Santos, P.L.; Serafini, L.A.; Molon, R.; Moyna, P. Essential oil yield and composition of Lamiaceae species growing in Southern Brazil. Braz. Arch. Biol. Technol. 2009, 52, 473–478. [Google Scholar] [CrossRef]
- Cerreta, M.M.; Furton, K.G. An assessment of detection canine alerts using flowers that release methyl benzoate, the cocaine odorant, and an evaluation of their behavior in terms of the VOCs produced. Forensic Sci. Int. 2015, 251, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Murfitt, L.M.; Mann, C.J.; Gorenstein, N.; Kolosova, N.; Kish, C.M.; Bonham, C.; Wood, K. Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell 2000, 12, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.N.; Reithel, J.S.; Irwin, R.E. A trade-off between the frequency and duration of bumblebee visits to flowers. Oecologia 1998, 117, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.S.; Kim, K.J.; Jung, I.H.; Lee, S.J.; Kim, J.C. Seasonal variations and emission fluxes of monoterpene emitted from coniferous trees in East Asia: Focused on Pinus rigida and Pinus koraiensis. J. Atmos. Chem. 2015, 72, 27–41. [Google Scholar] [CrossRef]
- Cui, S.; Wu, J.; Wang, J.; Wang, X. Discrimination of American ginseng and Asian ginseng using electronic nose and gas chromatography-mass spectrometry coupled with chemometrics. J. Ginseng Res. 2017, 41, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wang, J.; Yang, L.; Wu, J.; Wang, X. Qualitative and quantitative analysis on aroma characteristics of ginseng at different ages using E-nose and GC-MS combined with chemometrics. J. Pharm. Biomed. Anal. 2015, 102, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Al Makky, A.; Alaswad, A.; Gibson, D.; Olabi, A.G. Renewable energy scenario and environmental aspects of soil emission measurements. Renew. Sustain. Energy Rev. 2017, 68, 1157–1173. [Google Scholar] [CrossRef]
- Marć, M.; Zabiegała, B.; Namieśnik, J. Testing and sampling devices for monitoring volatile and semi-volatile organic compounds in indoor air. TrAC Trends Anal. Chem. 2012, 32, 76–86. [Google Scholar] [CrossRef]
- Vasiliev, A.A.; Pisliakov, A.V.; Sokolov, A.V.; Samotaev, N.N.; Soloviev, S.A.; Oblov, K.; Guarnieri, V.; Lorenzelli, L.; Brunelli, J.; Maglione, A.; et al. Non-silicon MEMS platform for gas sensor. Sens. Actuactor B Chem. 2016, 224, 700–713. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, F.; Liu, S.; Dang, L.; Peng, X.; Yin, X. Chaotic time series prediction of E-nose sensor drift in embedded phase space. Sens. Actuators, B Chem. 2013, 182, 71–79. [Google Scholar] [CrossRef]
- Sadeghifard, S.; Esmaeilani, L. A new embedded e-nose system to identify smell of smoke. In Proceedings of the 2012 7th International Conference on System of Systems Engineering (SoSE), Genova, Italy, 16–19 July 2012; pp. 253–257. [Google Scholar] [CrossRef]
- Kumbhar, A.; Gharpure, D.C.; Botre, B.A.; Sadistap, S.S. Embedded e-nose for food inspection. In Proceedings of the 2012 1st International Symposium on Physics and Technology of Sensors (ISPTS-1), Pune, India, 7–10 March 2012; pp. 311–314. [Google Scholar] [CrossRef]
| Techniques | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| PCR | Mature technology, easy to operate and portable | Subjected to DNA extraction, and inhibitors and polymerase activity | [13,14] |
| FISH | Highly sensitive | Auto-fluorescence | [15,16,17] |
| ELISH | Low-cost, rapid and visible results | Low-sensitivity to bacteria | [18] |
| Fluorescence imaging | Sensitive to abnormalities in photosynthesis | Limited in field setting | [19,20] |
| Hyperspectral Techniques | Rapid and highly robust | Affected by external factors, such as light, view angle; relatively expensive | [21] |
| GC-MS | Providing individual VOCs information | Expensive, not real-time, expertise skills needed | [22,23] |
| Enzymatic biosensor | Real-time and high specificity | Unstable, easily affected by pH, environment | [24] |
| DNA-based biosensor | Low cost, low limit of detection | Easily affected by DAN extraction, not real-time | [25] |
| Antibody-based biosensor | Low cost | Not real-time | [26] |
| Name | Advantage | Disadvantage |
|---|---|---|
| CP 1 | Wide range of available conducting polymers; room temperature operation; fast response; sensitive to polar compounds | High sensitivity to humidity and temperature; sensor response drift with time; short-life time |
| MOS 2 | Small size; easy to integrate into measurement circuitry; fast response and recovery time; high sensitivity | High-power-consumption; limited application on portable systems; blind with sulfur gas; limited coating materials; sensitive to humidity |
| SAW 3 | Broad applications; high sensitivity; fast response; diverse sensing materials; small size; | Relatively poor signal to noise performance; complex circuitry; unsatisfactory reproducibility |
| QCM 4 | Fast response time; easier fabrication compared to SAW; high sensitivity; diverse sensing materials; small | Unsatisfactory reproducibility; complex circuitry |
| CM 5 | High sensitivity; fast response; robustness in hazardous environment; disposable after use | Sensitive to humidity; complex supporting software and instrument; short life time; only sensitive to oxygen and VOCs |
| Name | Functions | Advantages | Disadvantages |
|---|---|---|---|
| CA | Classification | Reveal associations and structures in data which are not evident; results are easy to understand | Some methods are not clearly established; no satisfactory method for determining the appropriate number of clusters |
| ANN | Classification, regression and prediction | Require less formal statistical restrictions; able to model complex nonlinear relationships; able to train multiple algorithms | Big computation burden; tend to overfit |
| RF | Classification, regression and prediction | Efficient for large database; estimate the important variable in the classification; generate forests for further use | Overfitting for some datasets with noisy classification and regression tasks |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.; Ling, P.; Zhu, H.; Keener, H.M. Plant Pest Detection Using an Artificial Nose System: A Review. Sensors 2018, 18, 378. https://doi.org/10.3390/s18020378
Cui S, Ling P, Zhu H, Keener HM. Plant Pest Detection Using an Artificial Nose System: A Review. Sensors. 2018; 18(2):378. https://doi.org/10.3390/s18020378
Chicago/Turabian StyleCui, Shaoqing, Peter Ling, Heping Zhu, and Harold M. Keener. 2018. "Plant Pest Detection Using an Artificial Nose System: A Review" Sensors 18, no. 2: 378. https://doi.org/10.3390/s18020378
APA StyleCui, S., Ling, P., Zhu, H., & Keener, H. M. (2018). Plant Pest Detection Using an Artificial Nose System: A Review. Sensors, 18(2), 378. https://doi.org/10.3390/s18020378





